Unveiling a New Antimicrobial Peptide with Efficacy against P. aeruginosa and K. pneumoniae from Mangrove-Derived Paenibacillus thiaminolyticus NNS5-6 and Genomic Analysis
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Investigation of Bacterial Isolates from Mangrove Sediments
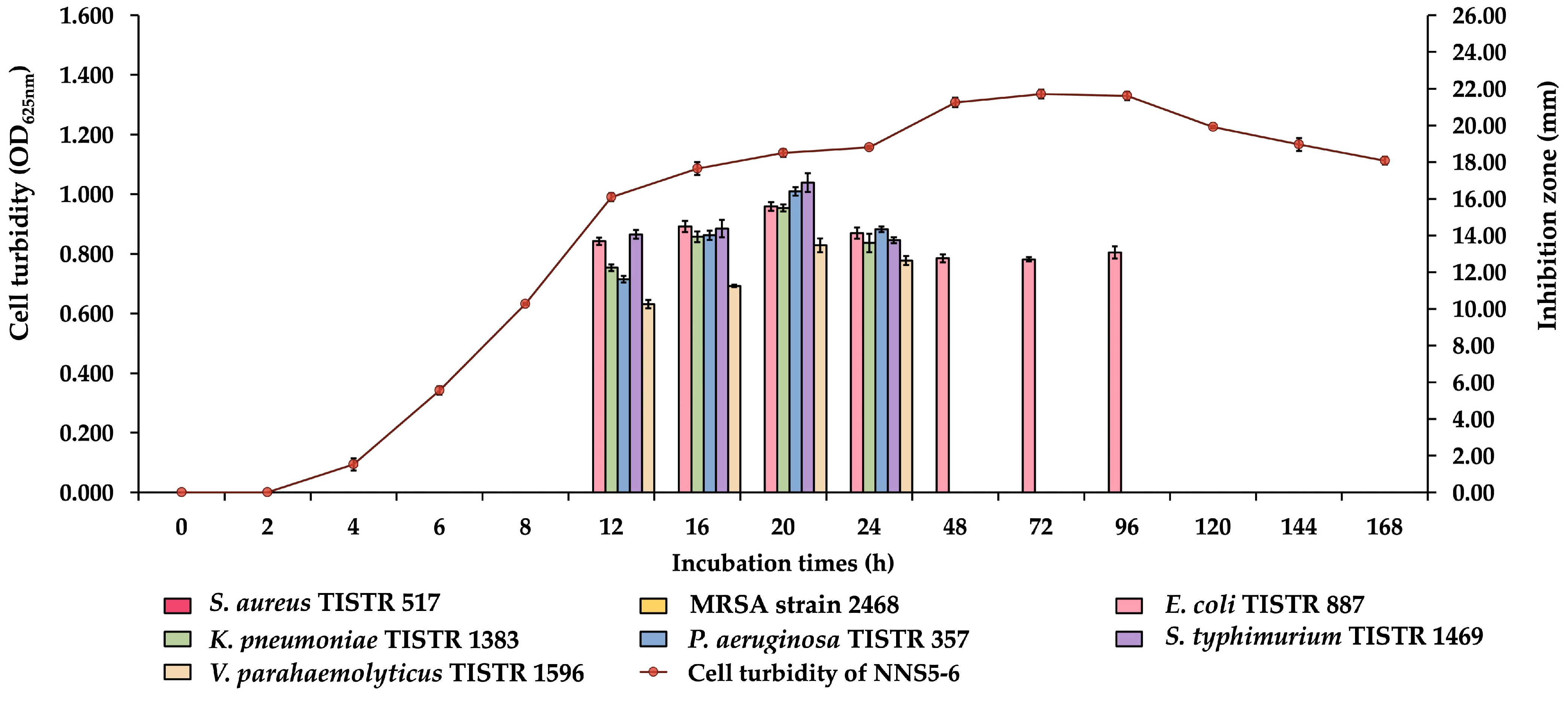
2.2. Production Kinetics of Antibacterial Compounds of NNS5-6
2.3. Purification of Antibacterial Compounds of NNS5-6
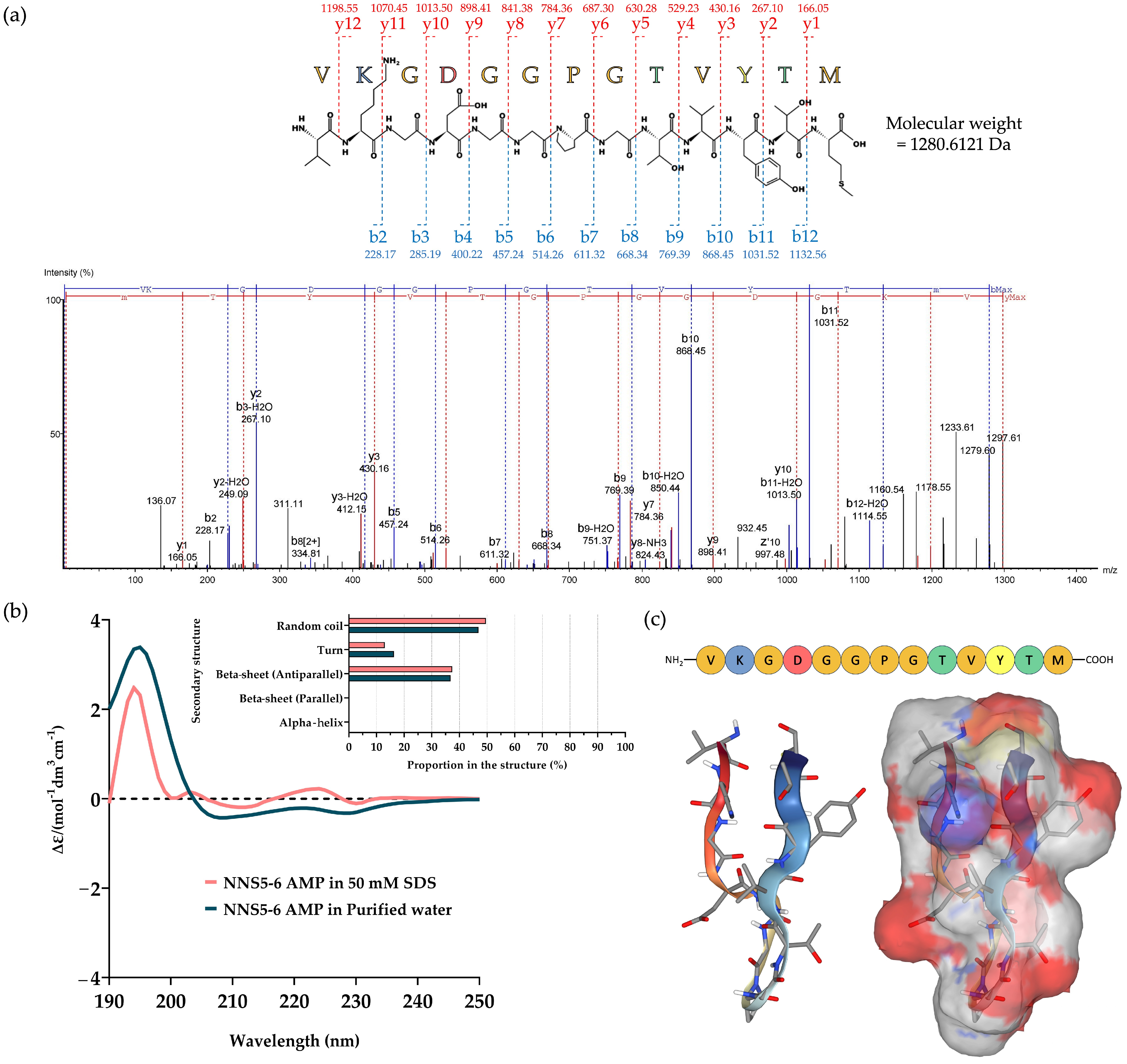
2.4. De Novo Amino Acid Sequence of the Purified AMP and Determination of Its Secondary Structure
2.5. Investigation of the Antibacterial Activities of the AMP
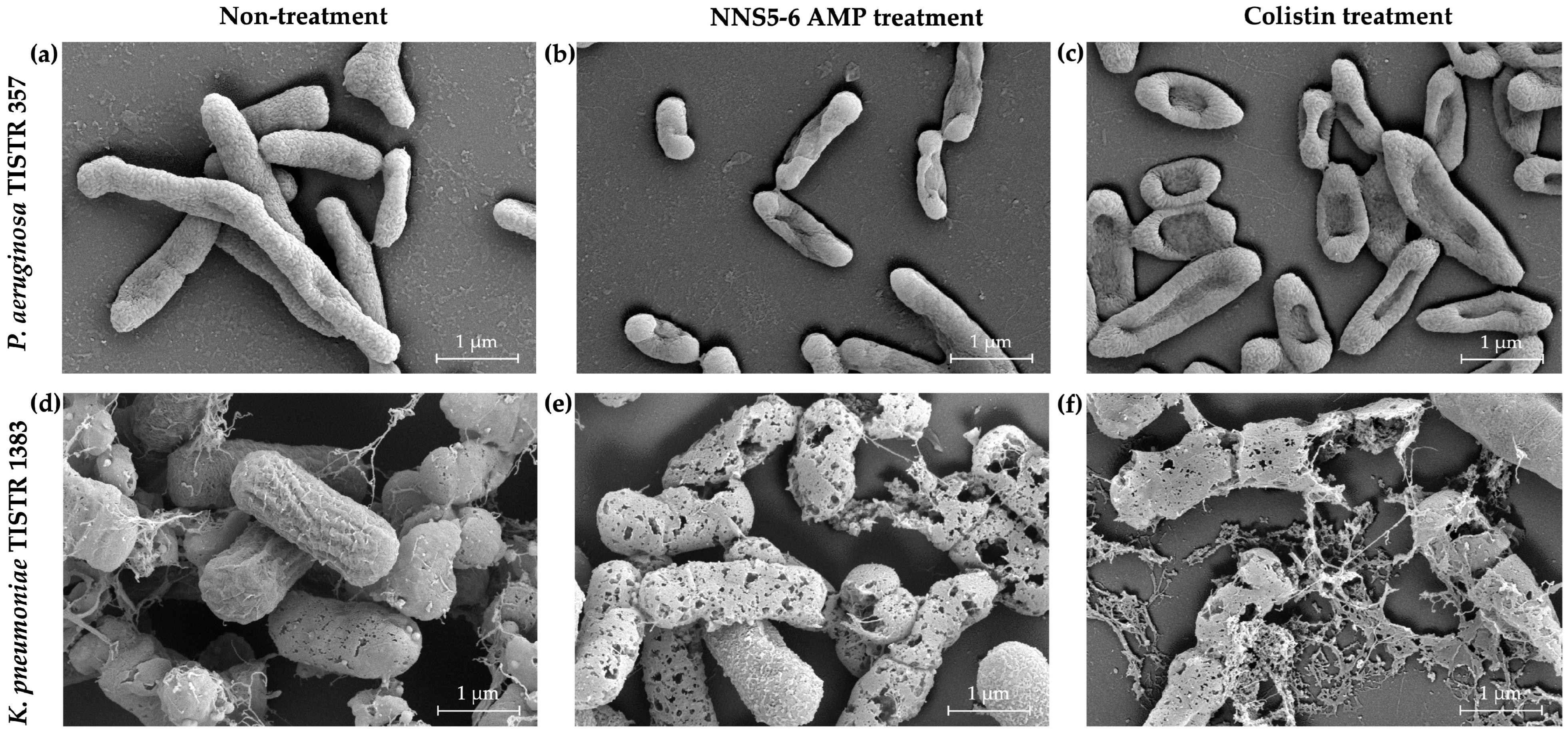
2.6. The Antibacterial Activity of NNS5-6 Derived AMP on Bacterial Pathogens Observed Using Scanning Electron Microscopy (SEM) Analysis
2.7. Time-Kill Assay of NNS5-6 AMP
2.8. Studies of Cell Permeability
2.9. Stability Studies of NNS5-6 AMP under Various Conditions
2.10. Phenotypic Characterization of NNS5-6
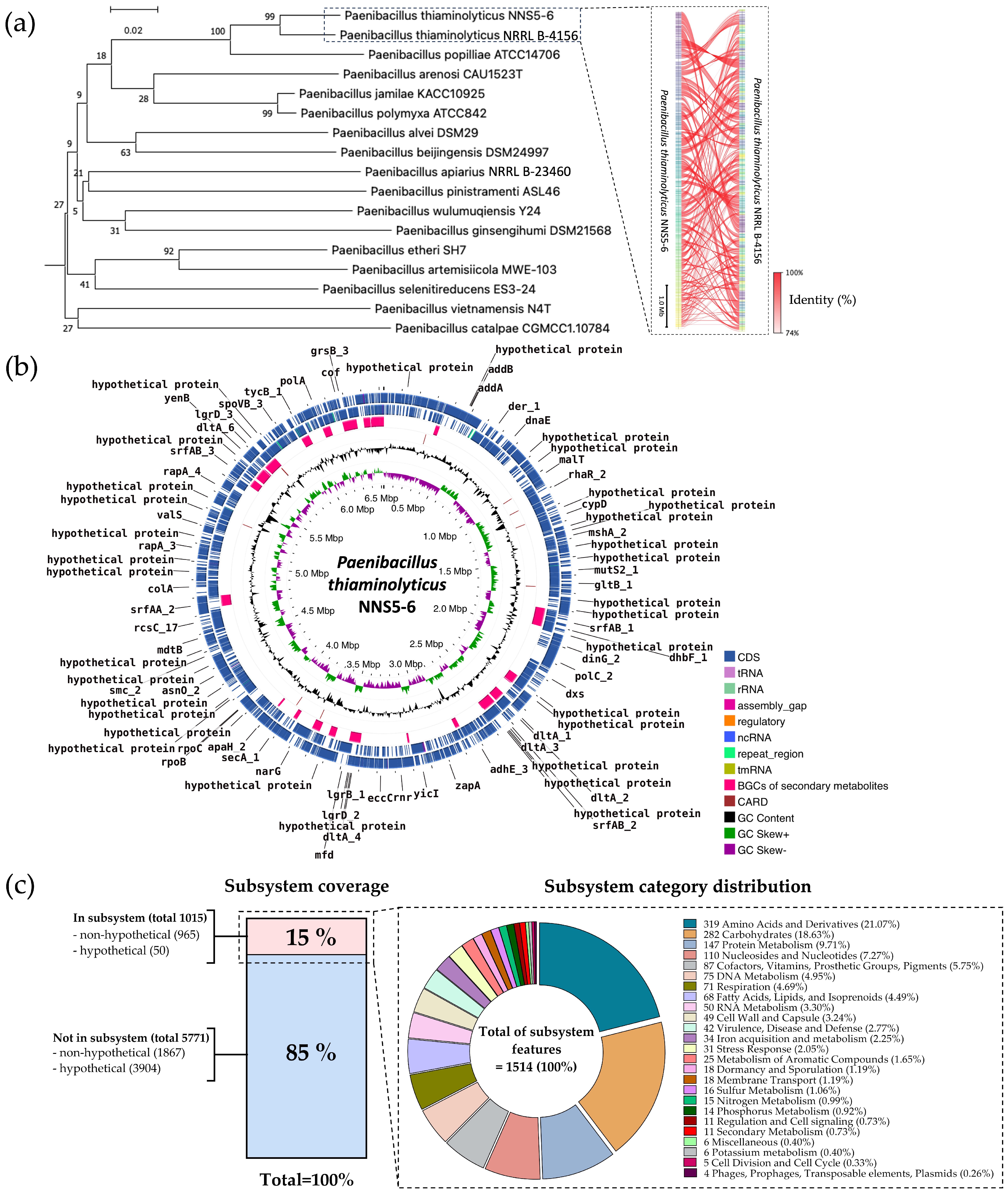
2.11. Genome Insight for Coding Sequence Annotation and Whole-Genome Phylogenetic Analysis
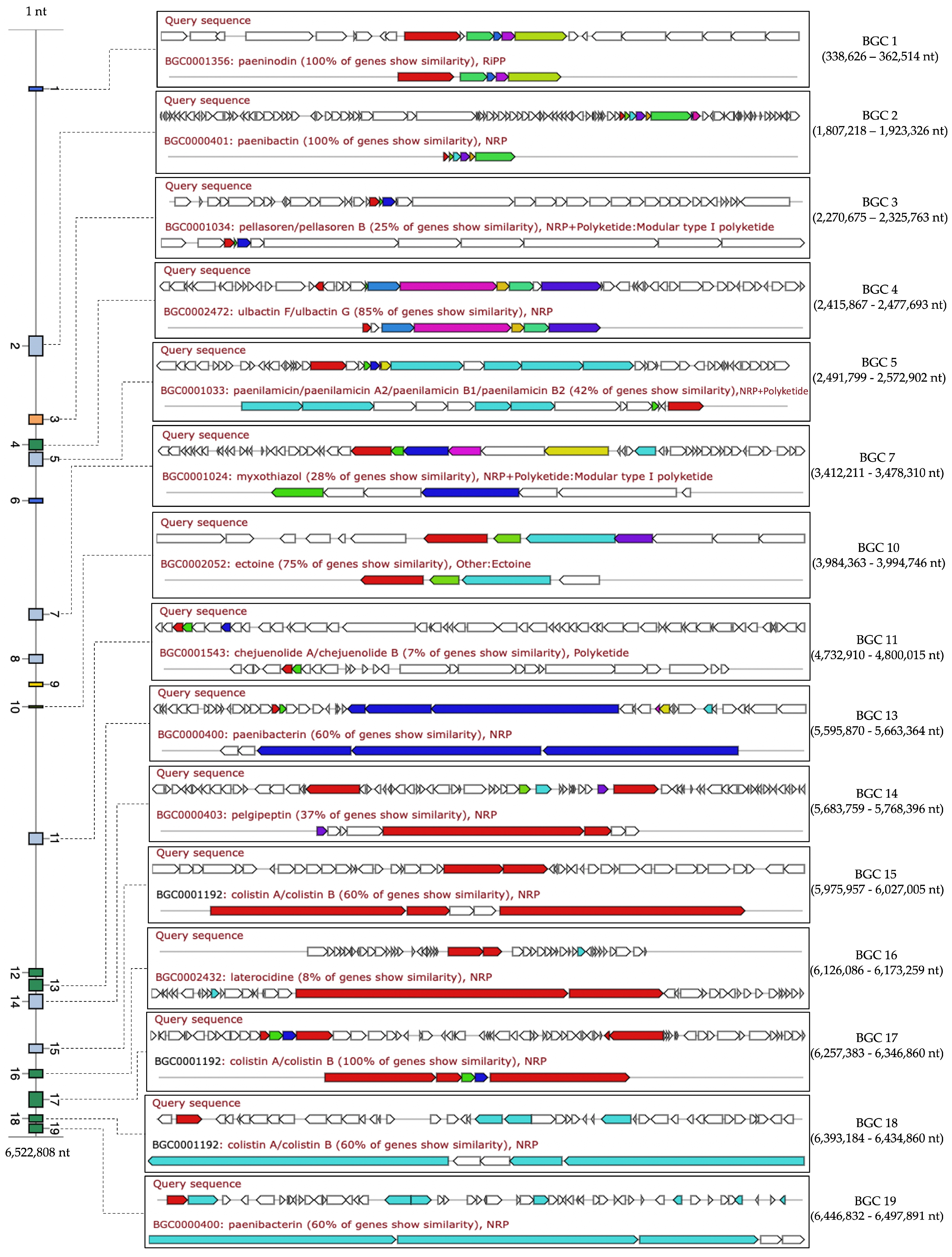
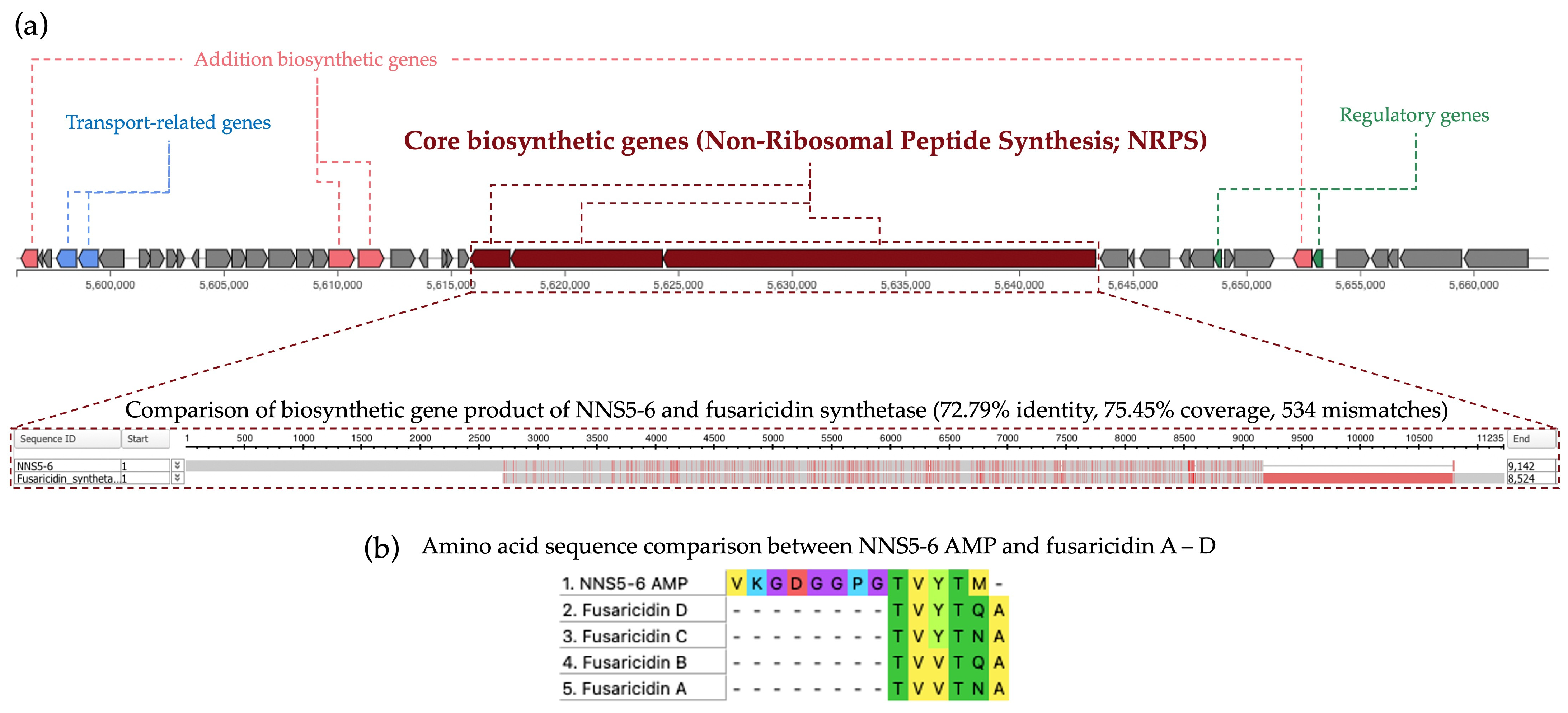
2.12. Comparative Analysis of Biosynthetic Gene Clusters in NNS5-6
2.13. Prediction of Antibiotic Resistance Genes in the NNS5-6 Genome and Determination of Antibiotic Susceptibility
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Bacterial Isolation
4.2. Antibacterial Screening Using the Soft Agar Overlay Method against P. aeruginosa TISTR 357
4.3. Verification of Antibacterial Activity Using the Agar Well Diffusion Technique
4.4. Investigation of the Production Kinetics of Antimicrobial Compounds of NNS5-6
4.5. Purification of the Antimicrobial Peptide
4.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Agar Overlay Assay
4.7. Peptide Sequencing
4.8. Determination of the Peptide Secondary Structure
4.9. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of NNS5-6 AMP
4.10. Scanning Electron Microscopy (SEM) of Cells Treated with NNS5-6 AMP
4.11. Time-Kill Kinetics of NNS5-6 AMP
4.12. Stability Studies of NNS-5-6 AMP
4.13. Effect of the AMP on Cell Membrane Permeability
4.14. Characterization of Bacterial Morphology
4.15. Whole Genome Sequencing and Bioinformatic Analysis
4.16. Antibiotic Susceptibility Studies of NNS5-6
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Review on Antimicrobial Resistance, Tackling Drug-resistant Infections Globally: Final Report and Recommendations; Wellcome Trust and HM Government: London, UK, 2016; pp. 10–16.
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa–Mechanisms, epidemiology and evolution. Drug Resist. Updat. 2019, 44, 100640. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Activity of antimicrobial peptides and ciprofloxacin against Pseudomonas aeruginosa biofilms. Molecules 2020, 25, 3843. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Karthik, Y.; Ishwara Kalyani, M.; Krishnappa, S.; Devappa, R.; Anjali Goud, C.; Ramakrishna, K.; Wani, M.A.; Alkafafy, M.; Hussen Abduljabbar, M.; Alswat, A.S.; et al. Antiproliferative activity of antimicrobial peptides and bioactive compounds from the mangrove Glutamicibacter mysorens. Front. Microbiol. 2023, 14, 1096826. [Google Scholar] [CrossRef]
- Karthik, Y.; Kalyani, M.I.; Sheetal, K.; Rakshitha, D.; Bineesha, B.K. Cytotoxic and antimicrobial activities of microbial proteins from mangrove soil actinomycetes of Mangalore, Dakshina Kannada. Biomedicine 2020, 40, 59–67. [Google Scholar]
- Núñez-Montero, K.; Barrientos, L. Advances in Antarctic research for antimicrobial discovery: A comprehensive narrative review of bacteria from Antarctic environments as potential sources of novel antibiotic compounds against human pathogens and microorganisms of industrial importance. Antibiotics 2018, 7, 90. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Ben Hur, D.; Kapach, G.; Wani, N.A.; Kiper, E.; Ashkenazi, M.; Smollan, G.; Keller, N.; Efrati, O.; Shai, Y. Antimicrobial peptides against multidrug-resistant Pseudomonas aeruginosa biofilm from cystic fibrosis patients. J. Med. Chem. 2022, 65, 9050–9062. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Poot, M.; Yue, S.T.; Millard, P.J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 1997, 63, 2421–2431. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Liu, D.M.; Liang, M.H.; Huang, Y.Y.; Lin, J.; Xiao, L.F. Genome-guided purification and characterization of polymyxin A1 from Paenibacillus thiaminolyticus SY20: A rarely explored member of polymyxins. Front. Microbiol. 2022, 13, 962507. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, Y.; Kaneda, M. Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation and biological activity. J. Antibiot. 1996, 49, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, Y.E.; Park, S.H.; Balaraju, K.; Park, J.W.; Lee, S.W.; Park, K. An antibiotic fusaricidin: A cyclic depsipeptide from Paenibacillus polymyxa E681 induces systemic resistance against Phytophthora blight of red-pepper. Phytoparasitica 2013, 41, 49–58. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, S.K.; Ryu, C.M.; Park, S.H. Chronicle of a soil bacterium: Paenibacillus polymyxa E681 as a tiny guardian of plant and human health. Front. Microbiol. 2019, 10, 467. [Google Scholar] [CrossRef]
- Vater, J.; Niu, B.; Dietel, K.; Borriss, R. Characterization of novel fusaricidins produced by Paenibacillus polymyxa-M1 using MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2015, 26, 1548–1558. [Google Scholar] [CrossRef]
- Caporale, A.; Adorinni, S.; Lamba, D.; Saviano, M. Peptide-protein interactions: From drug design to supramolecular biomaterials. Molecules 2021, 26, 1219. [Google Scholar] [CrossRef]
- Choi, U.; Lee, C.R. Antimicrobial agents that inhibit the outer membrane assembly machines of Gram-negative bacteria. J. Microbiol. Biotechnol. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Chai, H.; Allen, W.E.; Hicks, R.P. Spectroscopic investigations of the binding mechanisms between antimicrobial peptides and membrane models of Pseudomonas aeruginosa and Klebsiella pneumoniae. Bioorg. Med. Chem. 2014, 22, 4210–4222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Hale, J.D.; Elliott, M.; Hancock, R.E.; Straus, S.K. The importance of bacterial membrane composition in the structure and function of aurein 2.2 and selected variants. Biochim. Biophys. Acta 2011, 1808, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Panayi, T.; Diavoli, S.; Nicolaidou, V.; Papaneophytou, C.; Petrou, C.; Sarigiannis, Y. Short-chained linear scorpion peptides: A pool for novel antimicrobials. Antibiotics 2024, 13, 422. [Google Scholar] [CrossRef]
- Uggerhøj, L.E.; Poulsen, T.J.; Munk, J.K.; Fredborg, M.; Sondergaard, T.E.; Frimodt-Moller, N.; Hansen, P.R.; Wimmer, R. Rational design of alpha-helical antimicrobial peptides: do’s and don’ts. Chembiochem 2015, 16, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Feijoo-Coronel, M.L.; Mendes, B.; Ramírez, D.; Peña-Varas, C.; de Los Monteros-Silva, N.Q.E.; Proaño-Bolaños, C.; de Oliveira, L.C.; Lívio, D.F.; da Silva, J.A.; da Silva, J.M.S.F.; et al. Antibacterial and antiviral properties of chenopodin-derived synthetic peptides. Antibiotics 2024, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Hincapié, O.; Giraldo, P.; Orduz, S. In silico design of polycationic antimicrobial peptides active against Pseudomonas aeruginosa and Staphylococcus aureus. Antonie. Van Leeuwenhoek 2018, 111, 1871–1882. [Google Scholar] [CrossRef]
- Hua, B.; Feng, H.; Han, J.; Qiao, Z.; Wang, X.; Zhang, Q.; Liu, Z.; Wu, Z. Isolation and characterization of a new fusaricidin-type antibiotic produced by Paenibacillus bovis sp. nov BD3526. Curr. Microbiol. 2020, 77, 3990–3999. [Google Scholar] [CrossRef]
- Sannino, D.; Angert, E.R. Genomic insights into the thiamin metabolism of Paenibacillus thiaminolyticus NRRL B-4156 and P. apiarius NRRL B-23460. Stand. Genomic Sci. 2017, 12, 59. [Google Scholar] [CrossRef]
- Sáez-Nieto, J.A.; Medina-Pascual, M.J.; Carrasco, G.; Garrido, N.; Fernandez-Torres, M.A.; Villalón, P.; Valdezate, S. Paenibacillus spp. isolated from human and environmental samples in Spain: Detection of 11 new species. New Microbes. New Infect. 2017, 19, 19–27. [Google Scholar] [CrossRef]
- Shida, O. Transfer of Bacillus alginolyticus, Bacillus chondroitinus, Bacillus curdlanolyticus, Bacillus glucanolyticus, Bacillus kobensis, and Bacillus thiaminolyticus to the genus Paenibacillus and emended description of the genus Paenibacillus. Int. J. Syst. Bacteriol. 1997, 47, 289–298. [Google Scholar] [CrossRef]
- Huang, E.; Yousef, A.E. Draft genome sequence of Paenibacillus polymyxa OSY-DF, which coproduces a lantibiotic, paenibacillin, and polymyxin E1. J. Bacteriol. 2012, 194, 4739–4740. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Kisla, D.; Zhang, L.; Yuan, C.; Green-Church, K.B.; Yousef, A.E. Isolation and identification of a Paenibacillus polymyxa strain that coproduces a novel lantibiotic and polymyxin. Appl. Environ. Microbiol. 2007, 73, 168–178. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl. Environ. Microbiol. 2012, 78, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Negrel, J.; Govaerts, C.; Gianinazzi, S.; van Tuinen, D. Isolation and partial characterization of antagonistic peptides produced by Paenibacillus sp. strain B2 isolated from the sorghum mycorrhizosphere. Appl. Environ. Microbiol. 2005, 71, 6501–6507. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Pastar, I.; Houghten, R.A.; Padhee, S.; Higa, A.; Solis, M.; Valdez, J.; Head, C.R.; Michaels, H.; Lenhart, B.; et al. Novel cyclic lipopeptides fusaricidin analogs for treating wound infections. Front. Microbiol. 2021, 12, 708904. [Google Scholar] [CrossRef]
- Lebedeva, J.; Jukneviciute, G.; Čepaitė, R.; Vickackaite, V.; Pranckutė, R.; Kuisiene, N. Genome mining and characterization of biosynthetic gene clusters in two cave strains of Paenibacillus sp. Front. Microbiol. 2021, 11, 612483. [Google Scholar] [CrossRef]
- Bionda, N.; Fleeman, R.M.; Shaw, L.N.; Cudic, P. Effect of ester to amide or N-methylamide substitution on bacterial membrane depolarization and antibacterial activity of novel cyclic lipopeptides. Chem. Med. Chem. 2013, 8, 1394–1402. [Google Scholar] [CrossRef]
- Yu, W.B.; Yin, C.Y.; Zhou, Y.; Ye, B.C. Prediction of the mechanism of action of fusaricidin on Bacillus subtilis. PLoS ONE 2012, 7, e50003. [Google Scholar] [CrossRef]
- Lu, J.; Sha, Y.; Gao, M.; Shi, W.; Lin, X.; Li, K.; Bao, Q.; Feng, C. Identification and characterization of a novel aminoglycoside O-nucleotidyltransferase ANT(6)-If from Paenibacillus thiaminolyticus PATH554. Front. Microbiol. 2023, 14, 1184349. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial peptides: Challenging journey to the pharmaceutical, biomedical, and cosmeceutical use. Int. J. Mol. Sci. 2023, 24, 9031. [Google Scholar] [CrossRef]
- Kashfi, R.; Kelsey, C.; Gang, D.J.; Call, D.R.; Gang, D.R. Metabolomic diversity and identification of antibacterial activities of bacteria isolated from marine sediments in Hawai’i and Puerto Rico. Front. Mol. Biosci. 2020, 7, 23. [Google Scholar] [CrossRef]
- Hockett, K.L.; Baltrus, D.A. Use of the soft-agar overlay technique to screen for bacterially produced inhibitory compounds. J. Vis. Exp. 2017, 14, 55064. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Regmi, S.; Choi, Y.S.; Choi, Y.H.; Kim, Y.K.; Cho, S.S.; Yoo, J.C.; Suh, J.W. Antimicrobial peptide from Bacillus subtilis CSB138: Characterization, killing kinetics, and synergistic potency. Int. Microbiol. 2017, 20, 43–53. [Google Scholar] [PubMed]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus 1.0320. LWT 2021, 137, 110338. [Google Scholar] [CrossRef]
- Sermkaew, N.; Atipairin, A.; Wanganuttara, T.; Krobthong, S.; Aonbangkhen, C.; Yingchutrakul, Y.; Uchiyama, J.; Songnaka, N. A Novel bacitracin-like peptide from mangrove-isolated Bacillus paralicheniformis NNS4-3 against MRSA and its genomic insights. Antibiotics 2024, 13, 716. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the Expasy Server. In The Proteomics Protocols Handbook, 1st ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Murail, S.; de Vries, S.; Derreumaux, P.; Tuffery, P. PEP-FOLD4: A pH-dependent force field for peptide structure prediction in aqueous solution. Nucleic Acids Res. 2023, 51, W432–W437. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 15–52. [Google Scholar]
- Songnaka, N.; Lertcanawanichakul, M.; Hutapea, A.M.; Krobthong, S.; Yingchutrakul, Y.; Atipairin, A. Purification and characterization of novel anti-MRSA peptides produced by Brevibacillus sp. SPR-20. Molecules 2022, 27, 8452. [Google Scholar] [CrossRef]
- Uzair, B.; Menaa, F.; Khan, B.A.; Mohammad, F.V.; Ahmad, V.U.; Djeribi, R.; Menaa, B. Isolation, purification, structural elucidation and antimicrobial activities of kocumarin, a novel antibiotic isolated from actinobacterium Kocuria marina CMG S2 associated with the brown seaweed Pelvetia canaliculata. Microbiol. Res. 2018, 206, 186–197. [Google Scholar] [CrossRef]
- Choyam, S.; Jain, P.M.; Kammara, R. Characterization of a potent new-generation antimicrobial peptide of Bacillus. Front. Microbiol. 2021, 12, 710741. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Sapra, A. Gram Staining. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562156/ (accessed on 18 May 2024).
- Hussey, M.A.; Zayaitz, A. Endospore stain protocol. Am. Soc. Microbiol. 2007, 8, 1–11. [Google Scholar]
- Galaxy Community. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res. 2024, 52, gkae410. [Google Scholar]
- Babraham Bioinformatics: FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 18 June 2024).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2209. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Terlouw, B.R.; Blin, K.; Navarro-Muñoz, J.C.; Avalon, N.E.; Chevrette, M.G.; Egbert, S.; Lee, S.; Meijer, D.; Recchia, M.J.J.; Reitz, Z.L.; et al. MIBiG 3.0: A community-driven effort to annotate experimentally validated biosynthetic gene clusters. Nucleic Acids Res. 2023, 51, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing, 13th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; pp. 58–66. [Google Scholar]


| Isolate | Zone of Inhibition (mm ± SD; n = 3) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus TISTR 517 | MRSA Strain 2468 | E. coli TISTR 887 | K. pneumoniae TISTR 1383 | P. aeruginosa TISTR 357 | S. typhimurium TISTR 1469 | V. parahaemolyticus TISTR 1596 | |
| NNS5-6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 14.24 ± 0.15 | 13.34 ± 0.53 | 14.84 ± 0.15 | 12.70 ± 0.25 | 13.09 ± 0.64 |
| Vancomycin (30 µg) | 21.59 ± 0.51 | 21.76 ± 0.78 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Colistin (1 µg) | 0.00 ± 0.00 | 0.00 ± 0.00 | 18.13 ± 0.88 | 18.81 ± 0.39 | 18.05 ± 0.67 | 20.4 ± 0.89 | 18.8 ± 0.76 |
| Purification Procedure | Volume (mL) | Total Dried Weight (mg) | Activity (AU/mL) | Total Activity (AU) | Specific Activity (AU/mg) | Purification Factor | %Yield |
|---|---|---|---|---|---|---|---|
| Crude product | 976.50 | 453.30 | 20.00 | 19,530.00 | 43.08 | 1.00 | 100.00 |
| Salt precipitation | 72.78 | 102.40 | 80.00 | 5822.40 | 56.86 | 1.32 | 29.81 |
| Cation-exchange chromatography | 42.67 | 37.92 | 80.00 | 3413.60 | 90.02 | 2.09 | 17.48 |
| Size-exclusion chromatography | 12.88 | 3.63 | 160.00 | 2060.80 | 567.40 | 13.17 | 10.55 |
| Active Compounds | Tested Strains | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|
| NNS5-6 AMP | P. aeruginosa TISTR 357 | 4 | 4 |
| K. pneumoniae TISTR 1383 | 4 | 8 | |
| Colistin | P. aeruginosa TISTR 357 | 1 | 1 |
| K. pneumoniae TISTR 1383 | 1 | 1 |
| Conditions | % Residual Activity of NNS5-6 AMP against P. aeruginosa TISTR 357 | ||
|---|---|---|---|
| 1 h | 6 h | 12 h | |
| Effect of Temperatures | |||
| Non-treated NNS5-6 AMP | 100.00 ± 1.23 | 100.00 ± 0.70 | 100.00 ± 0.62 |
| 37 °C | 99.39 ± 1.98 | 99.39 ± 0.61 | 99.69 ± 0.95 |
| 40 °C | 99.80 ± 0.94 | 99.19 ± 0.93 | 99.07 ± 0.72 |
| 50 °C | 65.16 ± 3.07 * | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| 60 °C | 0.00 ± 0.00 * | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| 80 °C | 0.00 ± 0.00 * | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| 100 °C | 0.00 ± 0.00 * | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| 121 °C, 15 psi, 15 min | 0.00 ± 0.00 * | ||
| 121 °C, 15 psi, 30 min | 0.00 ± 0.00 * | ||
| Effect of Proteolytic enzymes | |||
| Non-treated NNS5-6 AMP | 100.00 ± 0.95 | 100.00 ± 0.34 | 100.00 ± 0.58 |
| NNS5-6 AMP with Proteinase K (1 mg/mL) | 96.08 ± 3.41 * | 86.58 ± 1.22 * | 83.62 ± 1.34 * |
| NNS5-6 AMP with Trypsin (1 mg/mL) | 99.38 ± 1.99 | 90.47 ± 2.02 * | 88.90 ± 1.46 * |
| NNS5-6 AMP with α-chymotrypsin (1 mg/mL) | 99.38 ± 0.95 | 99.42 ± 1.22 | 98.46 ± 0.88 |
| Effect of Surfactants | |||
| Non-treated NNS5-6 AMP | 100.00 ± 0.60 | 100.00 ± 0.93 | 100.00 ± 0.61 |
| NNS5-6 AMP with 1% SDS | 116.77 ± 1.80 * | 122.52 ± 1.86 * | 119.19 ± 1.53 * |
| NNS5-6 AMP with 1% Triton X-100 | 116.17 ± 0.60 * | 120.28 ± 1.53 * | 120.81 ± 1.53 * |
| 1% SDS alone | 121.76 ± 2.42 * | ||
| 1% Triton X-100 alone | 126.83 ± 2.72 * | ||
| Effect of pH variation | |||
| Non-treated NNS5-6 AMP | 100.00 ± 1.19 | 100.00 ± 1.28 | 100.00 ± 0.72 |
| pH 1.2 | 89.62 ± 1.85 * | 83.83 ± 1.72 * | 81.14 ± 1.71 * |
| pH 4.5 | 97.31 ± 0.33 | 95.69 ± 1.75 | 95.62 ± 1.19 |
| pH 6.8 | 97.31 ± 3.33 | 96.46 ± 0.57 | 96.00 ± 0.57 |
| pH 7.4 | 98.65 ± 0.58 | 98.18 ± 1.15 | 98.48 ± 0.66 |
| pH 8.0 | 99.23 ± 1.15 | 97.22 ± 0.98 | 98.48 ± 0.87 |
| pH 10.0 | 98.85 ± 1.20 | 97.42 ± 0.82 | 98.10 ± 1.44 |
| pH 12.0 | 94.31 ± 0.68 * | 93.40 ± 0.78 * | 90.10 ± 2.38 * |
| pH 14.0 | 90.77 ± 0.88 * | 84.40 ± 1.15 * | 79.05 ± 1.75 * |
| Antibiotic Resistance Gene | Antibiotic Resistance Gene Family | Resistance Mechanism | Position in Genome | Identity (%) | Coverage Length (%) |
|---|---|---|---|---|---|
| vanY gene in vanB cluster | vanY, glycopeptide resistance gene cluster | antibiotic target alteration | 291,432 to 292,277 | 33.04 | 104.85 |
| Otr(A) | tetracycline-resistant ribosomal protection protein of tetracycline antibiotics | antibiotic target protection | 1,037,370 to 1,039,346 | 45.40 | 99.25 |
| vanW gene in vanI cluster | vanW, glycopeptide resistance gene cluster | antibiotic target alteration | 1,132,154 to 1,133,269 | 34.43 | 99.46 |
| qacG | small multidrug resistance (SMR) antibiotic efflux pump | antibiotic efflux | 1,236,341 to 1,236,751 | 42.86 | 127.10 |
| potxA | Miscellaneous ABC-F subfamily ATP-binding cassette ribosomal protection proteins of oxazolidinones antibiotics | antibiotic target protection | 1,668,816 to 1,670,789 | 36.48 | 121.22 |
| norC | major facilitator superfamily (MFS) antibiotic efflux pump of fluoroquinolone antibiotics | efflux pump complex or subunit conferring antibiotic resistance | 3,712,623 to 3,714,038 | 59.69 | 101.95 |
| vanW gene in vanI cluster | vanW, glycopeptide resistance gene cluster | antibiotic target alteration | 3,898,660 to 3,900,096 | 36.69 | 128.15 |
| vanY gene in vanB cluster | vanY, glycopeptide resistance gene cluster | antibiotic target alteration | 5,289,399 to 5,290,199 | 33.56 | 99.25 |
| vanT gene in vanG cluster | vanT, glycopeptide resistance gene cluster | antibiotic target alteration | 5,756,379 to 5,758,274 | 48.63 | 88.62 |
| vanXY gene in vanG cluster | vanXY, glycopeptide resistance gene cluster | antibiotic target alteration | 5,758,264 to 5,759,118 | 43.70 | 111.81 |
| vanG | Van ligase, glycopeptide resistance gene cluster | antibiotic target alteration | 5,759,115 to 5,760,176 | 53.47 | 101.15 |
| Antibiotics | Zone of Inhibition (mm ± SD); n = 3 |
|---|---|
| Ciprofloxacin (5 µg) | 36.24 ± 0.63 |
| Piperacillin (100 µg) and Tazobactam (10 µg) | 50.29 ± 0.41 |
| Imipenem (10 µg) | 38.52 ± 0.52 |
| Ceftriaxone (30 µg) | 42.50 ± 0.48 |
| Cefoxitin (30 µg) | 25.40 ± 0.36 |
| Doxycycline (30 µg) | 31.07 ± 0.73 |
| Vancomycin (30 µg) | 21.51 ± 1.25 |
| Erythromycin (15 µg) | 34.88 ± 0.73 |
| Gentamicin (10 µg) | 25.57 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sermkaew, N.; Atipairin, A.; Krobthong, S.; Aonbangkhen, C.; Yingchutrakul, Y.; Uchiyama, J.; Songnaka, N. Unveiling a New Antimicrobial Peptide with Efficacy against P. aeruginosa and K. pneumoniae from Mangrove-Derived Paenibacillus thiaminolyticus NNS5-6 and Genomic Analysis. Antibiotics 2024, 13, 846. https://doi.org/10.3390/antibiotics13090846
Sermkaew N, Atipairin A, Krobthong S, Aonbangkhen C, Yingchutrakul Y, Uchiyama J, Songnaka N. Unveiling a New Antimicrobial Peptide with Efficacy against P. aeruginosa and K. pneumoniae from Mangrove-Derived Paenibacillus thiaminolyticus NNS5-6 and Genomic Analysis. Antibiotics. 2024; 13(9):846. https://doi.org/10.3390/antibiotics13090846
Chicago/Turabian StyleSermkaew, Namfa, Apichart Atipairin, Sucheewin Krobthong, Chanat Aonbangkhen, Yodying Yingchutrakul, Jumpei Uchiyama, and Nuttapon Songnaka. 2024. "Unveiling a New Antimicrobial Peptide with Efficacy against P. aeruginosa and K. pneumoniae from Mangrove-Derived Paenibacillus thiaminolyticus NNS5-6 and Genomic Analysis" Antibiotics 13, no. 9: 846. https://doi.org/10.3390/antibiotics13090846
APA StyleSermkaew, N., Atipairin, A., Krobthong, S., Aonbangkhen, C., Yingchutrakul, Y., Uchiyama, J., & Songnaka, N. (2024). Unveiling a New Antimicrobial Peptide with Efficacy against P. aeruginosa and K. pneumoniae from Mangrove-Derived Paenibacillus thiaminolyticus NNS5-6 and Genomic Analysis. Antibiotics, 13(9), 846. https://doi.org/10.3390/antibiotics13090846








