Abstract
In vitro studies have suggested that acidic pH may reduce and increase the efficacy of ciprofloxacin and fosfomycin, respectively, when used to treat Escherichia coli and Klebsiella pneumoniae infections. We assessed the effects of acidic, neutral, and alkaline urine pH on the efficacy of optimized ciprofloxacin and fosfomycin dosages in UTI murine model of E. coli and K. pneumoniae. Immunocompetent and immunocompromised mice with adjusted urine pH were inoculated with E. coli and K. pneumoniae strains, and the efficacy was assessed based on the bacterial concentrations in tissues and fluids at 72 h, with respect to untreated controls. At acidic urine pH, both antimicrobials were effective, achieving similar reductions in E. coli concentrations in the kidneys in immunocompetent and immunocompromised mice and in K. pneumoniae in immunocompetent mice. At a neutral urine pH, both therapies reduced the presence of E. coli in the kidneys of immunocompetent mice. However, in immunocompromised mice, antimicrobials were ineffective at treating E. coli infection in the kidneys at a neutral urine pH and showed reduced efficacy against K. pneumoniae at both acidic and neutral urine pH. The results showed no correlation between urine pH and antimicrobial efficacy, suggesting that the reduced effectiveness is associated with the animals’ immunocompetence status.
1. Introduction
Urinary tract infections (UTIs) are highly prevalent among kidney transplant recipients (KTRs), representing nearly half of all infectious complications in this population [1]. As is the case in the general population, Escherichia coli is the most frequent cause of UTI in KTRs [1,2], followed by Klebsiella pneumoniae [3]. Antimicrobial resistance among Gram-negative bacilli (GNB) has increased during the last decade, and this may be associated with risk factors commonly observed in the transplant setting [1,4,5]. Effective selection of antimicrobial agents is crucial for resolving these infections and mitigating the emergence of bacterial resistance [6]. The antibiotics most frequently used to treat Enterobacterales infections include β-lactams, β-lactams/β-lactamase inhibitors, fluoroquinolones, fosfomycin, and aminoglycosides, among others [7]. A recent study carried out in six European countries highlighted the highest resistance rates for antibiotics recommended for E. coli UTI treatment: 22.4% for trimethoprim/sulfamethoxazole, 16.7% for amoxicillin/clavulanic acid, and 15.1% for ciprofloxacin [8].
Ciprofloxacin is a second-generation fluoroquinolone antibiotic that interacts with DNA gyrase to influence transcription and DNA replication [9,10]. Its excellent bioavailability and high concentration in urine make it a good option for ambulatory management of UTIs [11,12]. However, in November 2018, the European Medicines Agency published a review detailing various serious, disabling, and potentially permanent side effects of fluoroquinolone antibiotics and recommended that they should not be used to treat bacterial infections in patients with kidney disease or in organ transplant recipients [13].
Fosfomycin, an antibiotic that is often prescribed to treat uncomplicated lower UTIs, has garnered attention due to its favorable pharmacokinetic and pharmacodynamics properties, including its high concentrations in urine [14,15]. Although E. coli’s resistance to fosfomycin remains relatively low (typically less than 3% [16]), recent reports indicate a concerning trend of rising resistance rates [17]. UTIs caused by extended-spectrum β-lactamase-producing Enterobacterales, such as E. coli, have similarly poor resistance to fosfomycin. Fosfomycin also exhibits activity against K. pneumoniae, with a susceptibility rate of 62% [18]. These findings underscore its potential as a valuable treatment for UTIs.
Information regarding the use of fosfomycin in immunocompromised patients, such as KTRs, is scarce. To our knowledge, thus far, only Rivera-Sánchez et al. have described fosfomycin as one of the main therapeutic options for treating UTIs in KTRs caused by ciprofloxacin-resistant Gram-negative isolates [19]. As such, there is a pressing need to assess alternative treatment options and understand the factors that affect antibiotic efficacy in the urinary tract microenvironment.
The microenvironment of the urinary tract may significantly influence the efficacy of antimicrobial agents [20,21,22,23]. While in vitro investigations have suggested that acidic pH or anaerobic conditions may adversely affect the bactericidal activity of ciprofloxacin [20,24], fosfomycin has demonstrated increased activity under such conditions [25]. However, there remains a notable gap in our understanding, as few in vivo studies have explored the impact of urine pH on the effectiveness of antibiotics in treating GNB UTIs [26,27]. In a recent observational study carried out in KTRs prescribed fosfomycin or ciprofloxacin therapy to treat UTI episodes caused by E. coli and K. pneumoniae, acidic urine pH was associated with symptomatic UTI episodes at one-month follow-up; this was particularly noticeable in patients who received fosfomycin therapy for E. coli episodes. Furthermore, at acidic pH, the minimum inhibitory concentration of ciprofloxacin against 90% of strains (MIC90) increased from 8 to >8 mg/L in E. coli and from 4 to >8 mg/L in K. pneumoniae, whereas the fosfomycin MIC90 decreased from 8 to 4 mg/L in E. coli and from 512 to 128 mg/L in K. pneumoniae [26]. In addition, acidic pH conditions increased the cells invasion by E. coli in human renal cell cultures, and such conditions were associated with enhanced bacterial concentrations and pyelonephritis-like symptoms in the kidneys in a lower UTI murine model of E. coli and K. pneumoniae infection [28].
To better characterize the association between different urine pHs, especially the acidic condition, and the antimicrobial efficacy, in the present study, we evaluated treatment with ciprofloxacin and fosfomycin in both immunocompetent and immunocompromised mice with E. coli or K. pneumoniae infections in their lower urinary tracts, considering the influence of different urinary pH conditions. By elucidating the impact of urinary pH on antibiotic efficacy, we aimed to provide valuable insights that will help to optimize strategies for treating UTIs.
2. Results
2.1. In Vitro Assays
2.1.1. Time–Kill Curve Assays
At maximum serum concentration (mice Cmax, Supplementary Table S1), both antibiotics showed bactericidal activity against all E. coli strains and K. pneumoniae strains from 2 to 24 h and from 6 to 24 h, respectively; these effects were independent of the pH and growth media (Müller–Hinton Broth (MHB) or urine) (Figure 1 and Figure 2 and Supplementary Figures S1 and S2). No differences were observed in strain control growths between MHB or urine across the different pH conditions, although at 24 h, they were approximately 1 log10 CFU/mL lower in the urine experiments than in MHB. Among the K. pneumoniae strains, HUVR5 (which is resistant to both antimicrobials) showed regrowth in the fosfomycin assay at alkaline broth pH at 24 h, while HUVR91 (which is resistant to ciprofloxacin) exhibited regrowth in the ciprofloxacin assay from 6 to 24 h at acidic broth pH.
2.1.2. Pharmacodynamics of Ciprofloxacin and Fosfomycin
The pharmacokinetics data, namely Cmax, t1/2, and AUC0–24, of ciprofloxacin and fosfomycin and their pharmacodynamics variables, namely AUC0–24/MIC, Cmax/MIC, and Δt/MIC, for the E. coli and K. pneumoniae strains are listed in Supplementary Table S1.
2.2. In Vivo Assays
2.2.1. Impact of Acidic Urine pH and Immunocompromise on the Bacterial Concentrations in the Kidneys in a Lower UTI Model of E. coli
In immunocompetent and immunocompromised, untreated control mice infected with the four E. coli strains, the bacterial concentrations in the kidneys after 72 h were greater in the groups with acidic urine pH than in those with a neutral urine pH in a range from +1.75 to +2.19 and from +3.41 to +5.51 log10 CFU/g, respectively, and in the Nu14 strain with alkaline urine pH in immunocompromised mice (+3.11 log10 CFU/g; Supplementary Figures S3 and S4).
The immunocompromised, untreated control mice had higher bacterial concentrations in the kidneys at acidic urine pH than the immunocompetent mice infected with the E. coli Nu 14 and HUVR 94 strains (+2.2 and +2.25 log10 CFU/g, respectively). Conversely, at a neutral urine pH, the bacterial concentrations in the kidneys were lower in immunocompromised than in immunocompetent mice infected with the E. coli Nu14 gyrA D87G and Nu14 glpT missense mutation strains (−2.99 and −2.59 log10 CFU/g, respectively). There were no differences in the bacterial concentrations in the kidneys with regard to the immunocompetence at alkaline urine pH or in the blood stream infection (BSI) and mortality rates at any pH condition (Supplementary Tables S2–S4).
2.2.2. Efficacy of Ciprofloxacin and Fosfomycin in an Immunocompetent Lower UTI Model of E. coli
At acidic urine pH, ciprofloxacin reduced the bacterial concentrations of E. coli Nu14, HUVR94, and Nu14 glpT strains in the kidneys by −1.08, −4.08, and −2.44 log10 CFU/g, respectively, whereas fosfomycin reduced the bacterial concentrations of E. coli Nu14, HUVR94, and Nu14 glpT strains in the kidneys by −0.97, −1.54, and −1.88 log10 CFU/g, respectively. Ciprofloxacin reduced the bacterial concentrations of E. coli Nu14, HUVR94, Nu14 gyrA, and Nu14 glpT strains in the urine by −2.70, −3.63, −5.05, and −3.62 log10 CFU/mL, respectively, compared with untreated controls, while fosfomycin also reduced the concentrations of E. coli HUVR94 and Nu14 gyrA in the urine by −4.15 and −4.28 log10 CFU/mL, respectively (Figure 3 and Supplementary Figure S3). Ultimately, ciprofloxacin was more effective than fosfomycin when it came to reducing the bacterial concentration of E. coli Nu14 (−1.64 log10 CFU/mL) in urine. No differences were observed in the BSI and mortality rates in the control groups after both treatments (Supplementary Figure S5).
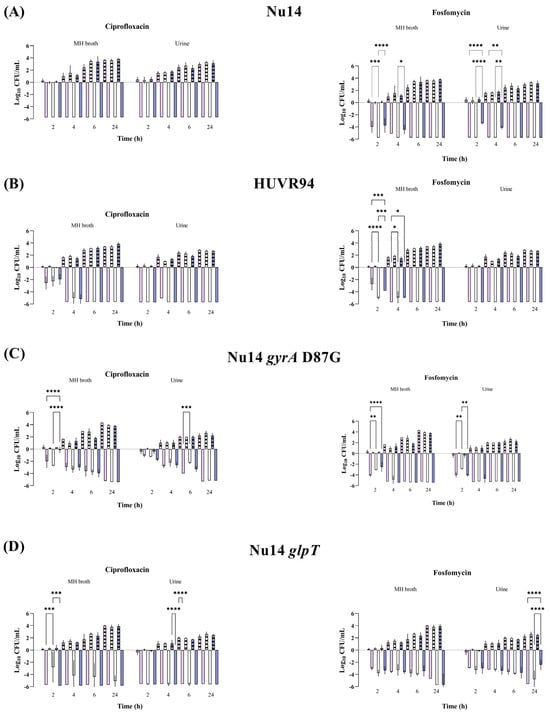
Figure 1.
Bactericidal activity of ciprofloxacin and fosfomycin at Cmax concentrations in MHB and urine, determined at pH 5 (pink bars), pH 7 (white bars), and pH 8 (purple bars), against Escherichia coli Nu14 (A), HUVR94 (B), Nu14 gyrA (C), and Nu14 glpT (D) strains. Results are represented as differences (log10 CFU/mL) relative to the initial timepoint (0 h). Striped bar: growth control; empty bar: antimicrobial condition at the Cmax levels reached in C57BL/6J mice plasma (13.22 mg/L for ciprofloxacin and 1354.09 mg/L for fosfomycin). Differences in bacterial concentrations across pH levels at the same time point were compared using analysis of variance (ANOVA) followed by Dunnett’s and Tukey’s post hoc tests. Significant differences were observed between the antimicrobial condition and its corresponding growth control at all time points; however, the figure highlights only the statistically significant differences in pH within each time point. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001. Growth control curves for each strain and pH condition are represented in Supplementary Figure S1.
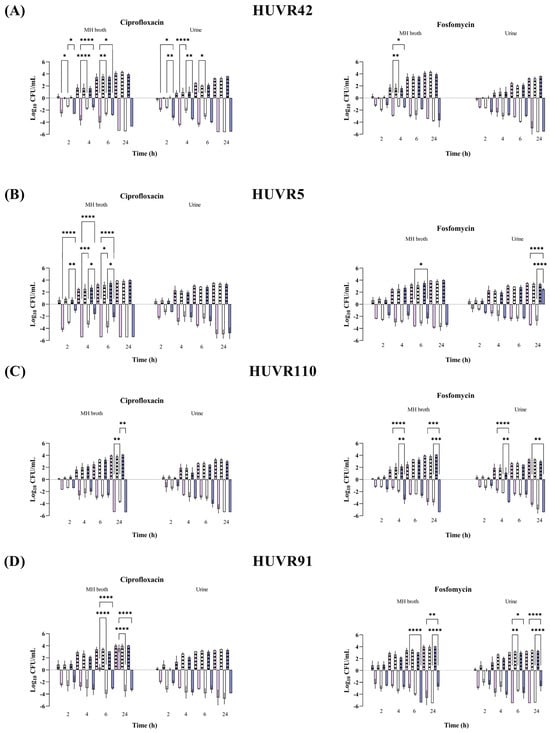
Figure 2.
Bactericidal activity of ciprofloxacin and fosfomycin at Cmax concentrations in MHB and urine, determined at pH 5 (pink bars), pH 7 (white bars), and pH 8 (purple bars), against Klebsiella pneumoniae HUVR42 (A), HUVR5 (B), HUVR110 (C), and HUVR91 (D) strains. Results are represented as differences (log10 CFU/mL) relative to the initial timepoint (0 h). Striped bar: growth control; empty bar: antimicrobial condition at the Cmax levels reached in C57BL/6J mice plasma (13.22 mg/L for ciprofloxacin and 1354.09 mg/L for fosfomycin). Differences in bacterial concentrations across pH levels at the same time point were compared using analysis of variance (ANOVA) followed by Dunnett’s and Tukey’s post hoc tests. Significant differences were observed between the antimicrobial condition and its corresponding growth control at all time points; however, the figure highlights only the statistically significant differences in pH within each time point. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001. Growth control curves for each strain and pH condition are represented in Supplementary Figure S2.
At neutral urine pH, ciprofloxacin decreased the bacterial concentrations of E. coli Nu14, HUVR94, and Nu14 gyrA strains in the kidneys by −1.94, −2.25, and −3.39 log10 CFU/g, respectively, and fosfomycin reduced the concentrations of E. coli HUVR94, Nu14 gyrA, and Nu14 glpT strains by −2.72, −3.39, and −1.50 log10 CFU/g, respectively. Moreover, ciprofloxacin outperformed fosfomycin in reducing the bacterial concentration in the kidneys of mice infected with E. coli Nu14 (−1.60 log10 CFU/g). Ciprofloxacin also reduced the bacterial concentrations of E. coli Nu14, HUVR94, Nu14 gyrA, and Nu14 glpT strains in the bladder by −4.57, −2.65, −1.21, and −2.67 log10 CFU/g, respectively, and it reduced the concentrations of E. coli strains in the urine by −3.35, −3.46, −4.69, and −4.21 log10 CFU/mL, respectively, compared with untreated controls. Fosfomycin reduced the bacterial concentrations of E. coli Nu14, Nu14 gyrA, and Nu14 glpT strains in the bladder by −4.39, −1.69, and −2.34 log10 CFU/g, respectively, compared to untreated controls, and it reduced E. coli Nu14 and Nu14 gyrA strains in the bladder by −4.66 and −3.55 log10 CFU/mL (Figure 3 and Supplementary Figure S3).
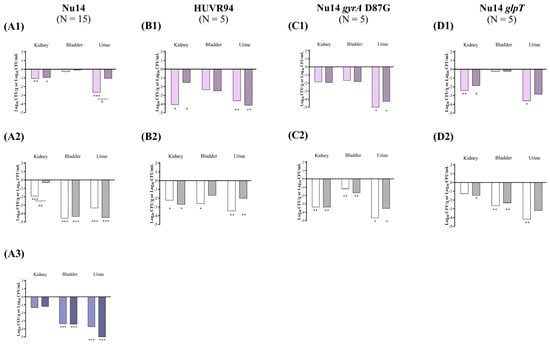
Figure 3.
In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompetent mice model by E. coli Nu14 (A), HUVR94 (B), Nu14 gyrA (C), and Nu14 glpT (D) strains at acidic (1), neutral (2), and alkaline (3) urine pH. Ciprofloxacin− and fosfomycin−treated groups are represented as difference with respect to mean of bacterial concentration in control mice groups (log10 CFU/mL; Supplementary Figure S3). Empty bar: ciprofloxacin−treated mice; striped bar: fosfomycin−treated mice. The Mann–Whitney U test was used to compare quantitative variables. *: p < 0.05 compared to their control mice group; **: p < 0.01 compared to their control mice group; ***: p < 0.001 compared to their control mice group; #: p < 0.05 compared to fosfomycin−treated mice group; ##: p < 0.01 compared to fosfomycin−treated mice group.
At alkaline pH, in mice infected with E. coli Nu14, both antibiotics effectively decreased the bacterial concentrations in the bladder and urine compared with those of the untreated control groups, from −3.37 to −3.42 log10 CFU/g and from −3.74 to −5.23 log10 CFU/mL, respectively (Figure 3 and Supplementary Figure S3). The BSI and mortality rates were 0% in both the untreated control and the ciprofloxacin− or fosfomycin−treated groups infected with E. coli Nu14 at alkaline pH.
2.2.3. Efficacy of Ciprofloxacin and Fosfomycin in an Immunocompromised Lower UTI Model of E. coli
At acidic urine pH, ciprofloxacin significantly reduced the bacterial concentrations of E. coli Nu14, HUVR94, Nu14 gyrA, and Nu14 glpT strains in the kidneys by −4.37, −3.15, −3.54, and −2.46 log10 CFU/g, respectively, while fosfomycin reduced the presence of E. coli Nu14, HUVR94, Nu14 gyrA, and Nu14 glpT strains in the kidneys by −5.58, −4.17, −5.49, and −3.13 log10 CFU/g, respectively. In immunocompromised mice, ciprofloxacin was less effective than fosfomycin at reducing the bacterial concentrations of E. coli HUVR94 in the kidneys, achieving a reduction of −1.02 log10 CFU/g. Ciprofloxacin also reduced the bacterial concentration of E. coli HUVR94 and Nu14 glpT in the bladder by −3.41 and −0.93 log10 CFU/g, respectively, and reduced the concentrations of E. coli Nu14 and HUVR94 strains in the urine by −3.47 and −4.51 log10 CFU/mL, respectively, compared with untreated controls. Fosfomycin reduced the concentrations of HUVR94, Nu14 gyrA, and Nu14 glpT in the bladder by −3.43, −0.72, and −1.08 log10 CFU/g, respectively, and reduced the bacterial concentration of Nu14 in the urine by −4.07 log10 CFU/mL (Figure 4 and Supplementary Figure S4). In accordance with the results of the immunocompetent mice, ciprofloxacin demonstrated better efficacy than fosfomycin in reducing the bacterial concentration of E. coli HUVR94 in the urine (−3.61 log10 CFU/mL). No differences were observed in the BSI and mortality rates after both treatments with respect to the control groups (Supplementary Figure S5).
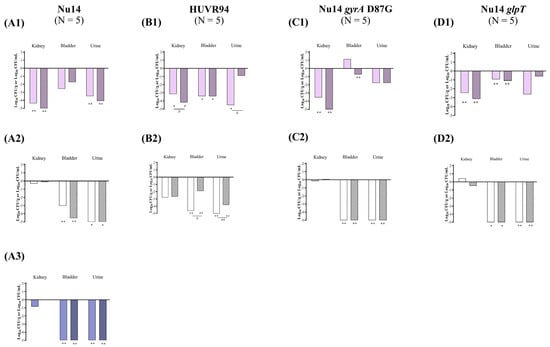
Figure 4.
In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompromised mice model by E. coli Nu14 (A), HUVR94 (B), Nu14 gyrA (C), and Nu14 glpT (D) strains at acidic (1), neutral (2), and alkaline (3) urine pH. Ciprofloxacin− and fosfomycin−treated groups are represented as difference with respect to mean bacterial concentration of control mice group (log10 CFU/mL; Supplementary Figure S4). Empty bar: ciprofloxacin−treated mice; striped bar: fosfomycin−treated mice. The Mann–Whitney U test was used to compare quantitative variables. *: p < 0.05 compared to their control mice group; **: p < 0.01 compared to their control mice group; #: p < 0.05 compared to fosfomycin−treated mice group; ##: p < 0.01 compared to fosfomycin−treated mice group.
At neutral urine pH, both antimicrobials were ineffective at reducing bacterial concentrations in the kidneys compared with untreated controls, which exhibited low bacterial concentrations in the kidneys, with means from 0.66 to 3.75 log10 CFU/g. However, both ciprofloxacin and fosfomycin effectively reduced the bacterial concentrations of the four E. coli strains in the bladder and the urine. Thus, ciprofloxacin reduced the bacterial concentrations of Nu14, HUVR94, Nu14 gyrA, and Nu14 glpT strains in the bladder by −3.03, −4.65, −5.26, and −6.75 log10 CFU/g, respectively, while fosfomycin reduced the concentrations by −4.54, −1.90, −6.60, and −6.46 log10 CFU/g, respectively. Additionally, ciprofloxacin decreased the bacterial concentrations of Nu14, HUVR94, Nu14 gyrA, and Nu14 glpT in the urine by −6.37, −8.74, −7.71, and −8.37 log10 CFU/mL, respectively, while fosfomycin reduced their concentrations by −5.52, −3.83, −6.14, and −6.19 log10 CFU/mL. Ciprofloxacin also outperformed fosfomycin in terms of bladder and urine clearance in mice infected with E. coli HUVR94 (−2.75 log10 CFU/g and −3.21 log10 CFU/mL, respectively).
At alkaline pH, in mice infected with E. coli Nu14, similar to the findings in the immunocompetent mice, both antimicrobials reduced the bacterial concentrations in the bladder (−5.41 and −5.31 log10 CFU/g) and urine (−5.37 and −5.28 log10 CFU/mL) with respect to those of the untreated mice (Figure 4 and Supplementary Figure S4). Additionally, the BSI and mortality rates were 0% for both the untreated control and the ciprofloxacin− or fosfomycin−treated groups, although BSI was observed in one out of the five mice in the ciprofloxacin−treated group.
2.2.4. Impact of Acidic Urine pH and Immunocompromise on the Bacterial Concentrations in the Kidneys in a Lower UTI Model of K. pneumoniae
In immunocompetent and immunocompromised, untreated control mice infected with K. pneumoniae, the bacterial concentrations in the kidneys after 72 h were greater in the groups with acidic pH urine than in those with neutral urine pH, in a range from +1.90 to +4.43 and from +3.95 to +4.28 log10 CFU/g, and in the HUVR42 strain group with alkaline urine pH from +3.48 to +4.84 log10 CFU/g (Supplementary Figures S7 and S8).
There were no differences in the bacterial concentrations in the kidneys at acidic and neutral urine pH when we compared the immunocompromised and immunocompetent, untreated control mice infected with K. pneumoniae strains. Only the HUVR42 strain differed, showing a higher bacterial concentration in the kidneys of immunocompromised mice (+1.64 log10 CFU/g) at neutral urine pH condition. In addition, at acidic urine pH, HUVR91 produced 100% BSI in immunocompromised mice vs. 0% in the immunocompetent mice. Immunocompetence did not affect the mortality rates of the mice at any pH condition (Supplementary Tables S2–S4).
2.2.5. Efficacy of Ciprofloxacin and Fosfomycin in an Immunocompetent Lower UTI Model of K. pneumoniae
At acidic urine pH, compared with untreated controls, ciprofloxacin reduced the bacterial concentrations of K. pneumoniae HUVR42, HUVR5, HUVR110, and HUVR91 strains in the kidneys by −2.94, −5.86, −4.54, and −4.69 log10 CFU/g, respectively, while fosfomycin reduced the concentrations of K. pneumoniae HUVR42, HUVR5, and HUVR91 by −3.57, −4.93, and −4.65 log10 CFU/g, respectively. Ciprofloxacin reduced the bacterial concentrations of HUVR42, HUVR5, and HUVR91 in the bladder by −1.57, −4.07, and −3.79 log10 CFU/g, respectively, and reduced the concentrations of HUVR42 and HUVR5 strains in the urine by −1.93 and −3.03 log10 CFU/mL, respectively. Fosfomycin reduced the bacterial concentrations of HUVR42, HUVR5, and HUVR91 in the urine by −2.20, −3.23, and −2.93 log10 CFU/g, respectively, and reduced the concentrations of K. pneumoniae HUVR42 in the urine by −1.58 log10 CFU/g (Figure 5 and Supplementary Figure S7). Additionally, ciprofloxacin showed superior efficacy to fosfomycin when it came to reducing the bacterial concentration of K. pneumoniae HUVR5 in the urine (−2.77 log10 CFU/mL). No differences were observed in the BSI and mortality rates after both treatments with respect to the control groups (Supplementary Figure S5).
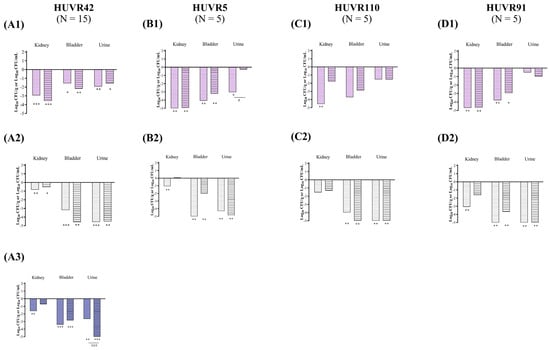
Figure 5.
In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompetent mice model by K. pneumoniae HUVR42 (A), HUVR5 (B), HUVR110 (C), and HUVR91 (D) strains at acidic (1), neutral (2), and alkaline (3) urine pH. Ciprofloxacin− and fosfomycin−treated groups are represented as difference with respect to mean bacterial concentration of control mice group (log10 CFU/mL; Supplementary Figure S7). Empty bar: ciprofloxacin−treated mice; striped bar: fosfomycin−treated mice. The Mann–Whitney U test was used to compare quantitative variables. *: p < 0.05 compared to their control mice group; **: p < 0.01 compared to their control mice group; ***: p < 0.001 compared to their control mice group; #: p < 0.05 compared to fosfomycin−treated mice group; ###: p < 0.001 compared to fosfomycin−treated mice group.
At neutral urine pH, ciprofloxacin reduced the bacterial concentrations of K. pneumoniae HUVR42 and HUVR91 in the kidneys by −0.84 and −3.12 log10 CFU/g, while fosfomycin achieved a similar reduction for K. pneumoniae HUVR42 alone (−0.55 log10 CFU/g). Both antimicrobials significantly decreased the bacterial concentrations of K. pneumoniae HUVR42, HUVR5, HUVR110, and HUVR91 in the bladder and in the urine. Ciprofloxacin reduced the bacterial concentrations in the bladder by −3.18, −5.01, −3.98, and −5.57 log10 CFU/g, respectively, and reduced the urine bacterial concentrations of the same four K. pneumoniae strains by −4.56, −4.29, −5.90, and −8.04 log10 CFU/mL, respectively, compared with untreated controls. Fosfomycin reduced the bacterial concentrations of HUVR42, HUVR5, HUVR110, and HUVR91 in the bladder by −4.59, −2.02, −5.66, and −3.69 log10 CFU/g, respectively, and in the bladder, it reduced the concentrations of the same four K. pneumoniae strains by −4.48, −4.89, −6.83, and −8.44 log10 CFU/mL, respectively, compared with untreated controls. Moreover, ciprofloxacin outperformed fosfomycin in reducing the bacterial concentration of K. pneumoniae HUVR5 in the bladders of infected mice (−2.99 log10 CFU/g).
At alkaline pH, ciprofloxacin reduced the bacterial concentration of HUVR42 in the kidneys with respect to the concentrations observed in untreated controls (−1.62 log10 CFU/g). Both antibiotics decreased the bacterial concentrations in the bladder (−2.85 to −3.40 log10 CFU/g) and urine (−2.65 to −5.94 log10 CFU/mL) compared with the concentrations observed in the untreated control groups. However, fosfomycin was more effective than ciprofloxacin at reducing the bacterial concentrations in urine (−3.29 log10 CFU/mL) (Figure 5 and Supplementary Figure S7). The BSI and mortality rates were 0% in the untreated control and ciprofloxacin- or fosfomycin-treated groups infected with K. pneumoniae HUVR42 at alkaline pH, though BSI was observed in two out of fifteen mice in the untreated control group.
2.2.6. Efficacy of Ciprofloxacin and Fosfomycin in an Immunocompromised Lower UTI Model of K. pneumoniae
At acidic urine pH, ciprofloxacin reduced the bacterial concentrations of K. pneumoniae HUVR5 and HUVR110 strains in the kidneys by −5.29 and −5.17 log10 CFU/g, respectively, and reduced the concentrations of HUVR5 in the bladder and urine by −4.49 log10 CFU/g and −4.07 log10 CFU/mL, respectively, compared with untreated controls. However, fosfomycin reduced the bacterial concentrations of K. pneumoniae HUVR5 in the kidneys (−4.72 log10 CFU/g), bladder (−3.14 log10 CFU/g), and urine (−4.49 log10 CFU/mL) (Figure 6 and Supplementary Figure S8). No differences were observed in the BSI and mortality rates after both treatments with respect to the control groups (Supplementary Figure S5).
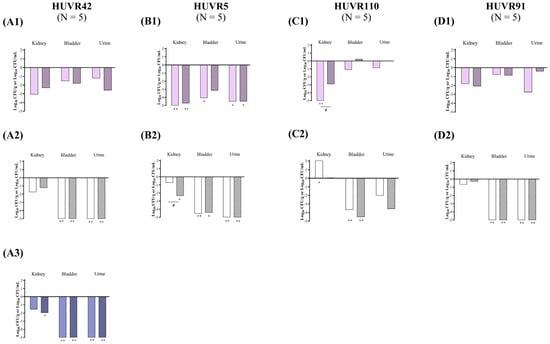
Figure 6.
In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompromised mice model by K. pneumoniae HUVR42 (A), HUVR5 (B), HUVR110 (C), and HUVR91 (D) strains at acidic (1), neutral (2), and alkaline (3) urine pH. Ciprofloxacin− and fosfomycin−treated groups are represented as difference with respect to mean bacterial concentration of control mice group (log10 CFU/mL; Supplementary Figure S8). Empty bar: ciprofloxacin−treated mice; striped bar: fosfomycin−treated mice. The Mann–Whitney U test was used to compare quantitative variables. *: p < 0.05 compared to their control mice group; **: p < 0.01 compared to their control mice group; #: p < 0.05 compared to fosfomycin−treated mice group.
At neutral urine pH, fosfomycin reduced the bacterial concentrations of K. pneumoniae HUVR5 in the kidneys (−2.35 log10 CFU/g). Both antimicrobials reduced the bacterial concentrations in the bladder and urine for all four studied strains, while the concentrations were similarly reduced for three strains in the untreated controls. Ciprofloxacin reduced the bacterial concentrations of K. pneumoniae HUVR42, HUVR5, HUVR110, and HUVR91 in the bladder by −6.59, −4.55, −3.68, and −6.25 log10 CFU/g, respectively, while in the urine, it reduced the concentrations of HUVR42, HUVR5, and HUVR91 by −7.53, −5.66, and −5.22 log10 CFU/mL, respectively. Fosfomycin proved to be an effective treatment for HUVR42, HUVR5, HUVR110, and HUVR91, reducing their concentrations by −6.18, −4.41, −4.53, and −5.02 log10 CFU/g, respectively, while in urine, it was effective for HUVR42, HUVR5, and HUVR91, reducing them by −7.67, −6.36, and −5.72 log10 CFU/mL.
Finally, at alkaline pH, fosfomycin significantly reduced the kidneys’ bacterial concentrations with respect to untreated mice infected with K. pneumoniae HUVR42 by −1.96 log10 CFU/g. Ciprofloxacin and fosfomycin also decreased the bacterial concentrations in the bladder (−5.00 and −5.09 log10 CFU/g) and urine (−7.18 and −7.98 log10 CFU/mL) with respect to the untreated controls (Figure 6 and Supplementary Figure S8). The BSI rates were 60%, 40%, and 0% in the untreated control, ciprofloxacin, and fosfomycin groups. No mortality was observed at alkaline urine pH in mice infected with the K. pneumoniae HUVR42 strain.
3. Discussion
At acidic urine pH, ciprofloxacin and fosfomycin demonstrated similar efficacy in reducing the bacterial concentrations in the kidneys in both immunocompetent and immunocompromised mice with E. coli in their lower urinary tracts in spite of the higher bacterial concentrations found in the kidneys in the untreated control groups at the end of the 72 h experimental period. Moreover, both antimicrobials successfully reduced kidney infection at neutral urine pH in the immunocompetent mouse model. Notably, both antimicrobials were ineffective at reducing kidney infection in immunocompromised mice at neutral and at alkaline urine pH.
Regarding the K. pneumoniae lower UTI model in the immunocompetent mice, both ciprofloxacin and fosfomycin effectively reduced the bacterial concentrations in the kidneys for most of the strains at acidic urine pH, successfully treating two of the four strains, but they showed reduced efficacy at neutral urine pH, at which only one strain was affected; moreover, ciprofloxacin achieved a greater reduction in the bacterial concentrations in the kidneys with regard to the strain tested at alkaline urine pH. In the immunocompromised mouse model, both ciprofloxacin and fosfomycin showed reduced efficacy at acidic urine pH, successfully treating two and one of the four strains, respectively. At neutral and alkaline urine pH, only fosfomycin reduced the level of infection for one out of the four strains and for the unique tested strain, respectively. The antimicrobial efficacy was similar to the results of the bacterial eradication from the bladder and urine at any urine pH and immunocompetence and in the E. coli and K. pneumoniae lower UTI models, though small differences were observed for specific strains.
In accordance with results previously reported [20,24] for E. coli strains, time–kill curves showed that both antibiotics exhibited bactericidal activity against E. coli and K. pneumoniae strains at Cmax concentrations independently of the pH and growth media. While no growth differences were noted between MHB or urine across pH conditions, the bacterial growth in urine was lower at 24 h compared to that in MHB, which was also consistent with previous reports [20,24]. Regarding the impact of pH of the medium on the in vitro antimicrobial activity, ciprofloxacin is less active at acidic pH and exhibits higher MIC concentrations [20,24], while fosfomycin remains marginally active independently of the media [26]. Martín-Gutiérrez et al. [25] observed a decrease in fosfomycin activity in MHB at alkaline pH in vitro, with an increase in the fosfomycin MIC against susceptible E. coli strains and strains with a low level of fosfomycin resistance. These findings are in agreement with previous studies suggesting that changes in media pH affect the activity of different antibiotics [29]. pH influences ciprofloxacin and fosfomycin activity, leading to changes in their molecular structure and ionization state [30]. The activity of ciprofloxacin, optimal at neutral pH, where it exists predominantly in zwitterionic and neutral forms, enhances bacterial permeability and absorption [31]. However, in acidic environments, typical in urine (pH ≤ 6.5) [32], ciprofloxacin is presented in its cationic form, reducing its ability to penetrate and decreasing its activity [33]. However, in the case of fosfomycin, the acidic environment enhances its activity, and being in an anaerobic environment also activates the expression of GlpT-binding and UhpT efflux pumps [34], increasing susceptibility to fosfomycin among uropathogenic bacteria. The antimicrobial activity of other antimicrobials also appears to be influenced by the pH. Thus, a recent in vitro study showed that a low pH reduces the activity of other antibiotics, such as ceftolozane/tazobactam and meropenem, against E. coli and K. pneumoniae in human urine medium [21]. These results were not completely reproduced in the present study when the assayed concentrations were increased to Cmax. At this concentration, scarce regrowth was observed during the experiments; interestingly, however, bacterial regrowth at 24 h after incubation with fosfomycin was observed at alkaline urine pH; likewise, bacterial regrowth occurred 24 h after incubation with ciprofloxacin at acidic urine pH, highlighting an interplay between antibiotic activity and urinary pH.
The in vivo efficacy of ciprofloxacin is associated with the AUC0–24/MIC pharmacodynamic (PD) parameter, and it has been reported to be static with a value of ~35 and with a maximal effect on values ≥ 100 [35]. In addition, data from an experimental pneumonia murine model infected with K. pneumoniae indicate that an AUC0–24/MIC ratio of >30 is bactericidal [36]. In the current study, the AUC0–24/MIC ratio for six of the E. coli and K. pneumoniae strains was >100; only two of them had ratios < 100 but higher than 30, without consistent differences in the efficacy of ciprofloxacin against the different strains, which supports the previously reported data. In addition, the volume of distribution of ciprofloxacin was approximately 3 L/kg, with tissues and urine concentrations higher than the serum levels [37]. In the case of fosfomycin, the results of neutropenic murine thigh models infected with E. coli and K. pneumoniae strains showed that the fAUC/MIC was the relevant PD parameter for these Gram-negative bacilli [38]. Based on these results, a population PD study in patients with E. coli bacteremia in the urinary tract showed that fosfomycin at a mean Cmax between 400 and 500 mg/L—which was lower than that in our experimental model—had a 93.9% probability of achieving a bacteriostatic effect for an MIC of 128 mg/L and between 89% and 96% probability of decreasing the 1-log bacterial burden for an MIC of 32 mg/L [39]. Fosfomycin achieved high urine concentrations; thus, after an oral dose of 3 g, the serum Cmax was 22–32 mg/L, and the urine concentration was 2000 mg/L [40]. Considering the higher Cmax achieved with the dosage administered in our study, the results are consistent with the fosfomycin PK/PD data.
This study is the first to use both immunocompetent and immunocompromised mice to investigate the effects of urine pH on the efficacy of ciprofloxacin and fosfomycin as treatments for UTIs caused by E. coli and K. pneumoniae strains. The lack of efficacy of both antimicrobials in immunocompromised mice at neutral urine pH in the lower UTI model of E. coli and their lesser efficacies in the immunocompromised mice at acidic urine pH in the UTI model of K. pneumoniae are in line with the higher symptomatic risk of symptomatic UTI at one-month follow-up in KTRs with acidic urine pH [26]. Despite its optimal PK/PD parameters [14,15,41] and enhanced activity at acidic pH [25], fosfomycin exhibited similar effectiveness to ciprofloxacin when it came to reducing the bacterial concentrations in the tissues and urine of mice with UTIs caused by E. coli or K. pneumoniae strains across the three studied pH conditions.
Differences in the treatment responses between immunocompetent and immunocompromised mice shed light on the intricate dynamics between antimicrobial therapy, host immune function, and bacterial pathogenesis in UTIs. While both antimicrobials were effective at different urinary pH conditions in immunocompetent mice, divergent responses in immunocompromised mice are significant in the context of vulnerable patient populations such as KTRs. These individuals, who are often immunosuppressed to prevent graft rejection, face increased risks of severe or recurrent UTIs due to their compromised immune defenses [42,43,44]. Furthermore, the contraindication of ciprofloxacin in KTRs due to its potential for nephrotoxicity underscores the urgency of identifying alternative treatment options [13]. Fosfomycin is a promising candidate for this patient population. However, further research is needed to elucidate its long-term safety profiles.
The present study has several limitations. Firstly, the findings provide valuable insights into antimicrobial efficacy, but they are based on murine models, which may not fully mimic the complexities of human UTIs. Thus, we must be cautious when extrapolating the results to clinical practice. Additionally, potential challenges associated with chronic or recurrent infections were not considered. Furthermore, short-term mouse models are unsuitable for assessing whether immunocompromised models exhibit any renal function impairment after ciprofloxacin treatment, which has previously been observed in humans. Moreover, the lack of fosfomycin susceptibility breakpoints in Enterobacterales other than E. coli highlights the difficulties in interpreting susceptibility data and informing treatment decisions for UTIs caused by K. pneumoniae. These limitations emphasize the need to update clinical guidelines to optimize antimicrobial therapy. Finally, in spite of these limitations, the in vivo results did not confirm an association of acidic pH with lower or higher antimicrobial efficacy of ciprofloxacin and fosfomycin, respectively, as suggested by the previous in vitro activity studies, suggesting that the urine pH is not clinically important when an optimized antimicrobial dosage is prescribed.
4. Materials and Methods
4.1. Bacterial Strains
Experiments were conducted using four uropathogenic strains of both E. coli and K. pneumoniae, with the susceptibility/resistance profiles (MICs) of ciprofloxacin and fosfomycin previously characterized by broth microdilution and agar dilution methods, respectively, in accordance with the guidelines issued by the European Committee on Antimicrobial Susceptibility Testing [45]. The strains and their MICs of ciprofloxacin and fosfomycin, respectively, were (i) E. coli Nu14 wild type [46], 0.03 mg/L and 2 mg/L; (ii) E. coli HUVR94, 0.03 mg/L and 0.50 mg/L; (iii) E. coli Nu14 with a point mutation in gyrA D87G with LLQR [47], 0.25 mg/L and 0.50 mg/L, where gyrA encodes DNA gyrase, the target enzyme of ciprofloxacin, and mutations are associated with resistance to fluoroquinolones; (iv) E. coli Nu14 with a glpT missense mutation [48], 0.01 mg/L and 32 mg/L, where fosfomycin is transported into the cell via the GlpT and UhpT transporters, and defects in transport systems can confer fosfomycin resistance; (v) K. pneumoniae HUVR42, 0.007 mg/L and 4 mg/L; (vi) K. pneumoniae HUVR5, 8 mg/L and 128 mg/L; (vii) K. pneumoniae HUVR110, 8 mg/L and 4 mg/L; and (viii) K. pneumoniae HUVR91, 0.06 mg/L and 64 mg/L [26].
4.2. Time–Kill Curve Assays
Bactericidal time-dependent studies were carried out in triplicate in MHB (Thermo Scientific, Oxford, UK) and urine obtained from healthy volunteers who had not undergone antibiotic treatment in the previous three months. The urine samples were pooled and sterilized by filtration (using polyethersulfone membrane filters, 0.22 µm, VWR; Leicestershire, UK) and stored at 4 °C prior to analysis. For each strain, the bactericidal activity of ciprofloxacin and fosfomycin was assessed at concentrations equivalent to the Cmax obtained in healthy mice with the doses used in the in vivo experiments: For ciprofloxacin, Cmax was 13.22 mg/L [49], whereas for fosfomycin, it was 1354.09 mg/L [50]. The MHB and/or urine were adjusted to obtain acidic pH (pH = 5) or alkaline pH values (pH = 8) by adding 0.012% (v/v) of 12 N HCl or 0.072% (v/v) of 2 N NaOH, respectively (Sigma-Aldrich, Madrid, Spain). The initial inoculum was 5 × 105 CFU/mL, and tubes were incubated at 37 °C with shaking; samples were taken at 0, 2, 4, 8, and 24 h and then serially diluted and seeded in 5% sheep blood plates. Bactericidal activity was defined as a decrease of ≥3 log10 CFU/mL from the initial inoculum [51].
4.3. In Vivo Assays
4.3.1. Animals
Immunocompetent C57BL/6J female mice weighing 20 g (Production and Experimentation Animal Centre, US, Seville, Spain) were used in this study. Male mice were not included to avoid potential urogenital trauma that could occur during catheterization. The mice were murine pathogen-free, and their genetic authenticity was assessed. The animals were housed in individually ventilated cages under specific pathogen-free conditions, with water and food supplied ad libitum. This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals [52]. The experiments were approved by the Committee on the Ethics of Animal Experiments of Consejería de Agricultura, Pesca y Desarrollo Rural, Spain (06/03/2018/022), and Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible (16/11/2020/134). The sample size adhered to the 3Rs for the ethical use of animals in scientific research [53]. All procedures were performed under ketamine/xylazine anesthesia (Pfizer, Spain/Bayer Hispania S.L.; Madrid, Spain), and sodium thiopental was used for the sacrifices (B. Braun Medical S.A.; Barcelona, Spain).
4.3.2. Efficacy of Ciprofloxacin and Fosfomycin Therapy in a Lower UTI Model with Different Urine pH in Immunocompetent and Immunocompromised Mice
A lower UTI murine model was previously characterized at different urine pHs [28]. Three days prior to inoculation, drinking water was replaced daily with (i) water containing 5% glucose for the neutral urine pH group; (ii) water containing 5% glucose and 0.5% NaHCO3 for the alkaline urine pH group [54]; and (iii) water containing 100 mM sucrose plus 0.56 M NH4Cl for the acidic urine pH group [55]. For the immunocompromised female C57BL/6J mouse model, the same protocol was followed when adjusting the urine pH, and 150 mg/kg/intraperitoneally (ip) and 100 mg/kg/ip of cyclophosphamide were administered 4 and 1 days prior to bacterial inoculation (day 0), respectively [56]. On the day of the inoculation, the mice were anesthetized with ketamine/xylazine (ip) and received transurethral inoculation with 50 μL of the previously characterized doses: 9 log10 CFU/mL for E. coli Nu14 and E. coli HUVR 94 and 8 log10 CFU/mL for all the other strains. The inoculum required to induce UTI in immunocompromised mice was one log10 CFU/mL fewer than that in wild-type mice [28]. Treatments were initiated 48 h post inoculation and lasted 24 h. Clinical formulations of ciprofloxacin (Spanish generics S.A.; Madrid, Spain) and fosfomycin (ERN; Madrid, Spain) were used. The animal weights and urine pH were recorded daily.
For both the immunocompetent and immunocompromised models and for the three conditions of urine pH, mice inoculated with each strain were randomly assigned to different groups (N = 5–15): (i) controls (untreated); (ii) ciprofloxacin, 20 mg/kg/12 h/ip; and (iii) fosfomycin, 500 mg/kg/8 h/ip. The antimicrobial dosages were determined based on pharmacokinetics and pharmacodynamics (PK/PD) data and their proven efficacy in previous murine models of infection [49,50]. Mortality was recorded for 24 h after the treatments began. Immediately after the animals’ death or when they were euthanized at the end of the experiment (sodium thiopental, ip), the kidneys, bladder, and urine were aseptically obtained and processed for quantitative cultures (log10 CFU/g and log10 CFU/mL). Blood samples were also recovered via cardiac puncture and studied for qualitative cultures.
4.4. Statistical Analysis
Differences in bacterial concentrations across pH levels at the same time point were compared using analysis of variance (ANOVA) followed by Dunnett’s and Tukey’s post hoc tests. Mortality and positive blood culture rates are expressed as percentages (%), while bacterial tissues and urine concentrations are presented as the mean ± standard deviation of log10 CFU/g and log10 CFU/mL, respectively. The chi-square or Fisher’s exact test was used to compare the mortality and BSI among groups. The Mann–Whitney U test was used to compare quantitative variables. A p < 0.05 was considered to be statistically significant. The statistical analysis was conducted using SPSS v24.0 software (SPSS Inc., Chicago, IL, USA).
5. Conclusions
Our results on the in vivo antimicrobial efficacy of ciprofloxacin and fosfomycin in murine lower UTI models do not support the in vitro data showing lower and higher activity of these antimicrobials, respectively, in media with acidic pH. In the lower UTI model at acidic urine pH, both ciprofloxacin and fosfomycin were similarly effective when reducing the bacterial concentrations of E. coli strains in the kidneys of both immunocompetent and immunocompromised mice and the concentrations of K. pneumoniae strains in kidneys of the immunocompetent mice. Moreover, at neutral urine pH, both antimicrobials effectively treated E. coli infections in the kidneys of the immunocompetent mice. However, the data suggest an association between reduced efficacy and immunocompromise. For immunocompromised mice infected with E. coli, both antimicrobials were ineffective at reducing infection in the kidneys at neutral urine pH; similarly, both ciprofloxacin and fosfomycin were less effective when treating K. pneumoniae at acidic and neutral urine pH.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics13090827/s1, Supplementary Figure S1: Growth curves of Escherichia coli strains in MHB and urine, at pH 5 (pink), pH 7 (black), and pH 8 (purple); Supplementary Figure S2: Growth curves of Klebsiella pneumoniae strains in MHB and urine, at pH 5 (pink), pH 7 (black), and pH 8 (purple); Supplementary Figure S3: In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompetent mice model by four Escherichia coli strains at acidic (pink), neutral (white), and alkaline (purple) urine pH; Supplementary Figure S4: In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompromised mice model by four Escherichia coli strains at acidic (pink), neutral (white), and alkaline (purple) urine pH; Supplementary Figure S5: Bloodstream infection and mortality rates in lower urinary tract infection models by Escherichia coli and Klebsiella pneumoniae strains in immunocompetent and immunocompromised mice with acidic urine pH at 72 h of infection and treated with ciprofloxacin and fosfomycin; Supplementary Figure S6: Bloodstream infection and mortality rates in lower urinary tract infection models by Escherichia coli and Klebsiella pneumoniae strains in immunocompetent and immunocompromised mice with neutral urine pH at 72 h of infection and treated with ciprofloxacin and fosfomycin; Supplementary Figure S7: In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompetent mice model by four Klebsiella pneumoniae strains at acidic (pink), neutral (white), and alkaline (purple) urine pH; Supplementary Figure S8: In vivo efficacy of ciprofloxacin and fosfomycin for the experimental urinary tract infection in immunocompromised mice model by four Klebsiella pneumoniae strains at acidic (pink), neutral (white), and alkaline (purple) urine pH; Supplementary Table S1: Pharmacokinetics of ciprofloxacin and fosfomycin in healthy C57BL/6J female mice following administration of a single intraperitoneal dose and pharmacodynamics for Escherichia coli Nu14, HUVR94, gyrA, and glpT and Klebsiella pneumoniae HUVR42, HUVR5, HUVR110, and HUVR91 strains; Supplementary Table S2: Bacterial concentration in tissues and urine and BSI and mortality rates at acidic urine pH conditions in immunocompromised and immunocompetent mice after 72 h of lower urinary tract inoculation by Escherichia coli and Klebsiella pneumoniae strains; Supplementary Table S3: Bacterial concentration in tissues and urine and BSI and mortality rates at neutral urine pH conditions in immunocompromised and immunocompetent mice after 72 h of lower urinary tract inoculation by Escherichia coli and Klebsiella pneumoniae strains; Supplementary Table S4: Bacterial concentration in tissues and urine and BSI and mortality rates at alkaline urine pH condition in immunocompromised and immunocompetent mice after 72 h of lower urinary tract inoculation by Escherichia coli Nu14 and Klebsiella pneumoniae HUVR42 strains.
Author Contributions
Conceptualization, J.P., E.C. and M.E.P.-I.; methodology, J.P., E.C. and M.E.P.-I.; formal analysis, J.P., E.C., M.E.P.-I. and S.H.-E.; investigation and resources, S.H.-E., M.C.-L., M.A.B.-G., J.P., E.C. and M.E.P.-I.; writing—original draft preparation, S.H.-E.; writing—review and editing, J.P., E.C. and M.E.P.-I.; visualization, S.H.-E., M.C.-L., M.A.B.-G., J.P., E.C. and M.E.P.-I.; supervision, J.P., E.C. and M.E.P.-I.; project administration, M.E.P.-I.; funding acquisition, E.C. and M.E.P.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Instituto de Salud Carlos III, Proyectos de Investigación en Salud (PI17/01405 and PI20/01255), Subdirección General de Redes y Centros de Investigación Cooperativa, Consejería de Economía, Industria y Competitividad US-1381501 US/JUNTA/FEDER, UE and co-financed by European Development Regional Fund: “A way to achieve Europe”, Operative program Intelligent Growth 2014–2020.
Institutional Review Board Statement
The animal study protocol was approved by the Committee on the Ethics of Animal Experiments of Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible, Spain (06/03/2018/022 and 16/11/2020/134).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
S.H.-E. is supported by the program PFIS (Contratos Predoctorales de Formación en Investigación en Salud), Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia e Innovación under grant (FI21/00280). M.E.P.-I. is a “Nicolás Monardes” researcher (RC1-0006-2023, Servicio Andaluz de Salud, Junta de Andalucía, Spain. M.E.P.-I. also received support from the CIBER de Enfermedades Infecciosas (CIBERINFEC, CB21/13/00006), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, co-financed by the European Development Regional Fund.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the analyses and interpretation of data; in the writing of the manuscript; or in the decision to publish the result.
References
- Vidal, E.; Torre-Cisneros, J.; Blanes, M.; Montejo, M.; Cervera, C.; Aguado, J.M.; Len, O.; Carratalá, J.; Cordero, E.; Bou, G.; et al. Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl. Infect. Dis. 2012, 14, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Plate, A.; Kronenberg, A.; Risch, M.; Mueller, Y.; Di Gangi, S.; Rosemann, T.; Senn, O. Active surveillance of antibiotic resistance patterns in urinary tract infections in primary care in Switzerland. Infection 2019, 47, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Tekkarışmaz, N.; Özelsancak, R.; Micozkadıoğlu, H.; Çalışkan, K.; Demiroğlu, Y.Z.; Arslan, A.H.; Haberal, M. Risk Factors for Urinary Tract Infection After Kidney Transplant: A Retrospective Analysis. Exp. Clin. Transplant. 2020, 18, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Valera, B.; Gentil, M.A.; Cabello, V.; Fijo, J.; Cordero, E.; Cisneros, J.M. Epidemiology of urinary infections in renal transplant recipients. Transplant. Proc. 2006, 38, 2414–2415. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Sanclemente, G.; Lipperheide, I.; Allali, M.; Marco, F.; Bosch, J.; Cofan, F.; Ricart, M.J.; Esforzado, N.; Oppenheimer, F.; et al. Impact of urinary tract infections on short-term kidney graft outcome. Clin. Microbiol. Infect. 2015, 21, 1104.e1–1104.e8. [Google Scholar] [CrossRef][Green Version]
- Kot, B. Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef]
- Vila, J.; Sáez-López, E.; Johnson, J.R.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.G.; Naas, T.; Carattoli, A.; Martínez-Medina, M.; et al. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 40, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Ny, S.; Edquist, P.; Dumpis, U.; Gröndahl-Yli-Hannuksela, K.; Hermes, J.; Kling, A.M.; Klingeberg, A.; Kozlov, R.; Källman, O.; Lis, D.O.; et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2019, 17, 25–34. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Malone, R.; Lilley, S.H. New classification and update on the quinolone antibiotics. Am. Fam. Physician 2000, 61, 2741–2748. [Google Scholar]
- Suaifan, G.; Mohammed, A.A.M. Fluoroquinolones structural and medicinal developments (2013–2018): Where are we now? Bioorg. Med. Chem. 2019, 27, 3005–3060. [Google Scholar] [CrossRef]
- Drusano, G.L.; Standiford, H.C.; Plaisance, K.; Forrest, A.; Leslie, J.; Caldwell, J. Absolute oral bioavailability of ciprofloxacin. Antimicrob. Agents Chemother. 1986, 30, 444–446. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Kinzig-Schippers, M.; Sörgel, F.; Weidner, W.; Naber, K.G. Concentrations in plasma, urinary excretion and bactericidal activity of levofloxacin (500 mg) versus ciprofloxacin (500 mg) in healthy volunteers receiving a single oral dose. Int. J. Antimicrob. Agents 2006, 28, 551–559. [Google Scholar] [CrossRef] [PubMed]
- EMA (European Medicines Agency). Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. 2018. Available online: https://www.ema.europa.eu/en/documents/referral/quinolone-and-fluoroquinolone-article-31-referral-disabling-and-potentially-permanent-side-effects-lead-suspension-or-restrictions-quinolone-and-fluoroquinolone-antibiotics_en.pdf (accessed on 20 March 2024).
- Zykov, I.N.; Samuelsen, Ø.; Jakobsen, L.; Småbrekke, L.; Andersson, D.I.; Sundsfjord, A.; Frimodt-Møller, N. Pharmacokinetics and Pharmacodynamics of Fosfomycin and Its Activity against Extended-Spectrum-β-Lactamase-, Plasmid-Mediated AmpC-, and Carbapenemase-Producing Escherichia coli in a Murine Urinary Tract Infection Model. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Rodríguez-Gascón, A.; Canut-Blasco, A. Deciphering pharmacokinetics and pharmacodynamics of fosfomycin. Rev. Esp. Quimioter. 2019, 32 (Suppl. 1), 19–24. [Google Scholar] [PubMed]
- Cattoir, V.; Pourbaix, A.; Magnan, M.; Chau, F.; de Lastours, V.; Felden, B.; Fantin, B.; Guérin, F. Novel Chromosomal Mutations Responsible for Fosfomycin Resistance in Escherichia coli. Front. Microbiol. 2020, 11, 575031. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Treier, A.; Schmitt, K.; Stephan, R. Mobile fosfomycin resistance genes in Enterobacteriaceae-An increasing threat. Microbiol. Open 2020, 9, e1135. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.S. Oral treatment options for patients with urinary tract infections caused by extended spectrum βeta-lactamase (ESBL) producing Enterobacteriaceae. J. Infect. Public. Health 2019, 12, 843–846. [Google Scholar] [CrossRef]
- Rivera-Sanchez, R.; Delgado-Ochoa, D.; Flores-Paz, R.R.; García-Jiménez, E.E.; Espinosa-Hernández, R.; Bazan-Borges, A.A.; Arriaga-Alba, M. Prospective study of urinary tract infection surveillance after kidney transplantation. BMC Infect. Dis. 2010, 10, 245. [Google Scholar] [CrossRef]
- Erdogan-Yildirim, Z.; Burian, A.; Manafi, M.; Zeitlinger, M. Impact of pH on bacterial growth and activity of recent fluoroquinolones in pooled urine. Res. Microbiol. 2011, 162, 249–252. [Google Scholar] [CrossRef]
- Nussbaumer-Pröll, A.K.; Eberl, S.; Reiter, B.; Stimpfl, T.; Dorn, C.; Zeitlinger, M. Low pH reduces the activity of ceftolozane/tazobactam in human urine, but confirms current breakpoints for urinary tract infections. J. Antimicrob. Chemother. 2020, 75, 593–599. [Google Scholar] [CrossRef]
- Giannakopoulos, X.; Evangelou, A.; Kalfakakou, V.; Grammeniatis, E.; Papandropoulos, I.; Charalambopoulos, K. Human bladder urine oxygen content: Implications for urinary tract diseases. Int. Urol. Nephrol. 1997, 29, 393–401. [Google Scholar] [CrossRef]
- Kottur, J.; Nair, D.T. Reactive Oxygen Species Play an Important Role in the Bactericidal Activity of Quinolone Antibiotics. Angew. Chem. Int. Ed. Engl. 2016, 55, 2397–2400. [Google Scholar] [CrossRef]
- Martín-Gutiérrez, G.; Rodríguez-Beltrán, J.; Rodríguez-Martínez, J.M.; Costas, C.; Aznar, J.; Pascual, Á.; Blázquez, J. Urinary Tract Physiological Conditions Promote Ciprofloxacin Resistance in Low-Level-Quinolone-Resistant Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gutiérrez, G.; Docobo-Pérez, F.; Rodriguez-Beltrán, J.; Rodríguez-Martínez, J.M.; Aznar, J.; Pascual, A.; Blázquez, J. Urinary Tract Conditions Affect Fosfomycin Activity against Escherichia coli Strains Harboring Chromosomal Mutations Involved in Fosfomycin Uptake. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Herrera-Espejo, S.; Fontserè, S.; Infante, C.; Suárez-Benjumea, A.; Carretero-Ledesma, M.; Suñer-Poblet, M.; González-Corvillo, C.; Bernal, G.; Martín-Gutiérrez, G.; Pérez-Cáceres, J.A.; et al. Acidic Urine pH and Clinical Outcome of Lower Urinary Tract Infection in Kidney Transplant Recipients Treated with Ciprofloxacin and Fosfomycin. Antibiotics 2024, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Fontserè, S.; Infante-Domínguez, C.; Suárez-Benjumea, A.; Suñer-Poblet, M.; González-Corvillo, C.; Martín-Gutiérrez, G.; Bernal, G.; Pachón, J.; Pachón-Ibáñez, M.E.; Cordero, E. Impact of Treating Asymptomatic Bacteriuria in Kidney Transplant Recipients: A Prospective Cohort Study. Antibiotics 2021, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Espejo, S.; Domínguez-Miranda, J.L.; Rodríguez-Mogollo, J.I.; Pachón, J.; Cordero, E.; Pachón-Ibáñez, M.E. Effects of pH on the Pathogenicity of Escherichia coli and Klebsiella pneumoniae on the Kidney: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 7925. [Google Scholar] [CrossRef] [PubMed]
- Burian, A.; Erdogan, Z.; Jandrisits, C.; Zeitlinger, M. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology 2012, 90, 281–287. [Google Scholar] [CrossRef]
- Völgyi, G.; Vizserálek, G.; Takács-Novák, K.; Avdeef, A.; Tam, K.Y. Predicting the exposure and antibacterial activity of fluoroquinolones based on physicochemical properties. Eur. J. Pharm. Sci. 2012, 47, 21–27. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Lachowicz, J.I.; Zoroddu, M.A.; Peana, M.; Medici, S.; Veclani, D.; Tolazzi, M.; Melchior, A. Fluoroquinolones: A micro-species equilibrium in the protonation of amphoteric compounds. Eur. J. Pharm. Sci. 2016, 93, 380–391. [Google Scholar] [CrossRef]
- So, W.; Crandon, J.L.; Nicolau, D.P. Effects of Urine Matrix and pH on the Potency of Delafloxacin and Ciprofloxacin against Urogenic Escherichia coli and Klebsiella pneumoniae. J. Urol. 2015, 194, 563–570. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- Kurabayashi, K.; Tanimoto, K.; Fueki, S.; Tomita, H.; Hirakawa, H. Elevated Expression of GlpT and UhpT via FNR Activation Contributes to Increased Fosfomycin Susceptibility in Escherichia coli under Anaerobic Conditions. Antimicrob. Agents Chemother. 2015, 59, 6352–6360. [Google Scholar] [CrossRef]
- Tran, J.H.; Jacoby, G.A.; Hooper, D.C. Interaction of the plasmid-encoded quinolone resistance protein QnrA with Escherichia coli topoisomerase IV. Antimicrob. Agents Chemother. 2005, 49, 3050–3052. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Martínez, J.M.; Pichardo, C.; García, I.; Pachón-Ibañez, M.E.; Docobo-Pérez, F.; Pascual, A.; Pachón, J.; Martínez-Martínez, L. Activity of ciprofloxacin and levofloxacin in experimental pneumonia caused by Klebsiella pneumoniae deficient in porins, expressing active efflux and producing QnrA1. Clin. Microbiol. Infect. 2008, 14, 691–697. [Google Scholar] [CrossRef]
- Hooper, D.C.; Strahilevitz, J. Quinolones. In Mandell, Douglas, Bennett. Principles and Practice of Infectious Diseases, 9th ed.; Bennet, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; Volume 1, pp. 426–448. Available online: https://fama.us.es/discovery/fulldisplay?docid=alma991012946389704987&context=L&vid=34CBUA_US:VU1&lang=es&search_scope=all_data_not_idus&adaptor=Local%20Search%20Engine (accessed on 16 April 2024).
- Lepak, A.J.; Zhao, M.; VanScoy, B.; Taylor, D.S.; Ellis-Grosse, E.; Ambrose, P.G.; Andes, D.R. In Vivo Pharmacokinetics and Pharmacodynamics of ZTI-01 (Fosfomycin for Injection) in the Neutropenic Murine Thigh Infection Model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Merino-Bohórquez, V.; Docobo-Pérez, F.; Sojo, J.; Morales, I.; Lupión, C.; Martín, D.; Cameán, M.; Hope, W.; Pascual, Á.; Rodríguez-Baño, J. Population pharmacokinetics and pharmacodynamics of fosfomycin in non-critically ill patients with bacteremic urinary infection caused by multidrug-resistant Escherichia coli. Clin. Microbiol. Infect. 2018, 24, 1177–1183. [Google Scholar] [CrossRef]
- Horton, J.M. Urinary tract agents: Nitrofurantoin, fosfomycin and methenamine. In Mandell, Douglas, Bennett. Principles and Practice of Infectious Diseases, 9th ed.; Bennet, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; Volume 1, pp. 461–465. [Google Scholar]
- Chavan, R.; Naphade, B.; Waykar, B.; Bhagwat, S. Investigations on In Vivo Pharmacokinetic/Pharmacodynamic Determinants of Fosfomycin in Murine Thigh and Kidney Infection Models. Microb. Drug Resist. 2023, 29, 18–27. [Google Scholar] [CrossRef]
- Van, V.T.H.; Liu, Z.S.; Hsieh, Y.J.; Shiu, W.C.; Chen, B.Y.; Ku, Y.W.; Chen, P.W. Therapeutic effects of orally administration of viable and inactivated probiotic strains against murine urinary tract infection. J. Food Drug Anal. 2023, 31, 583–598. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, M.P.; Afanador Rubio, D.C.; Luna, I.M.; García Padilla, P.K.; Contreras Villamizar, K.M.; González González, C.A.; Patiño Trejos, J.A. Impact of Complicated Urinary Tract Infection on Renal Graft Function. Transplant. Proc. 2020, 52, 1173–1177. [Google Scholar] [CrossRef]
- Al Midani, A.; Elands, S.; Collier, S.; Harber, M.; Shendi, A.M. Impact of Urinary Tract Infections in Kidney Transplant Recipients: A 4-Year Single-Center Experience. Transplant. Proc. 2018, 50, 3351–3355. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 14.0, 2024. Available online: http://www.eucast.org (accessed on 19 March 2024).
- Mehershahi, K.S.; Chen, S.L. Complete Genome Sequence of the Uropathogenic Escherichia coli Strain NU14. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Komp Lindgren, P.; Marcusson, L.L.; Sandvang, D.; Frimodt-Møller, N.; Hughes, D. Biological cost of single and multiple norfloxacin resistance mutations in Escherichia coli implicated in urinary tract infections. Antimicrob. Agents Chemother. 2005, 49, 2343–2351. [Google Scholar] [CrossRef]
- Nilsson, A.I.; Berg, O.G.; Aspevall, O.; Kahlmeter, G.; Andersson, D.I. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob. Agents Chemother. 2003, 47, 2850–2858. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Herrera, J.; Velasco, C.; Docobo-Pérez, F.; Rodríguez-Martínez, J.M.; López-Rojas, R.; Briales, A.; Pichardo, C.; Díaz-de-Alba, P.; Rodríguez-Baño, J.; Pascual, A.; et al. Impact of qnrA1, qnrB1 and qnrS1 on the efficacy of ciprofloxacin and levofloxacin in an experimental pneumonia model caused by Escherichia coli with or without the GyrA mutation Ser83Leu. J. Antimicrob. Chemother. 2013, 68, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Cebrero-Cangueiro, T.; Labrador-Herrera, G.; Pascual, Á.; Díaz, C.; Rodríguez-Baño, J.; Pachón, J.; Del Palacio, J.P.; Pachón-Ibáñez, M.E.; Conejo, M.C. Efficacy of Fosfomycin and Its Combination with Aminoglycosides in an Experimental Sepsis Model by Carbapenemase-Producing Klebsiella pneumoniae Clinical Strains. Front. Med. 2021, 8, 615540. [Google Scholar] [CrossRef]
- Werth, B.J.; Rybak, M.J. Ceftaroline plus avibactam demonstrates bactericidal activity against pathogenic anaerobic bacteria in a one-compartment in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2014, 58, 559–562. [Google Scholar] [CrossRef]
- NRC (National Research Council). Guide for the Care and Use of Laboratory Animals; The National Academies Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Russell, W.; Burch, R. The Principles of Humane Experimental Technique.
- Robey, I.F.; Nesbit, L.A. Investigating mechanisms of alkalinization for reducing primary breast tumor invasion. Biomed. Res. Int. 2013, 2013, 485196. [Google Scholar] [CrossRef]
- Vasandani, V.M.; Burris, J.A.; Sung, C. Reversible nephrotoxicity of onconase and effect of lysine pH on renal onconase uptake. Cancer Chemother. Pharmacol. 1999, 44, 164–169. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, M.; Qian, Y.; Cong, Z.; Chen, S.; Zhang, W.; Jiang, W.; Dai, C.; Shao, N.; Ji, Z.; et al. Addressing MRSA infection and antibacterial resistance with peptoid polymers. Nat. Commun. 2021, 12, 5898. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).