Evaluation of the Diagnosis and Antibiotic Prescription Pattern in Patients Hospitalized with Urinary Tract Infections: Single-Center Study from a University-Affiliated Hospital
Abstract
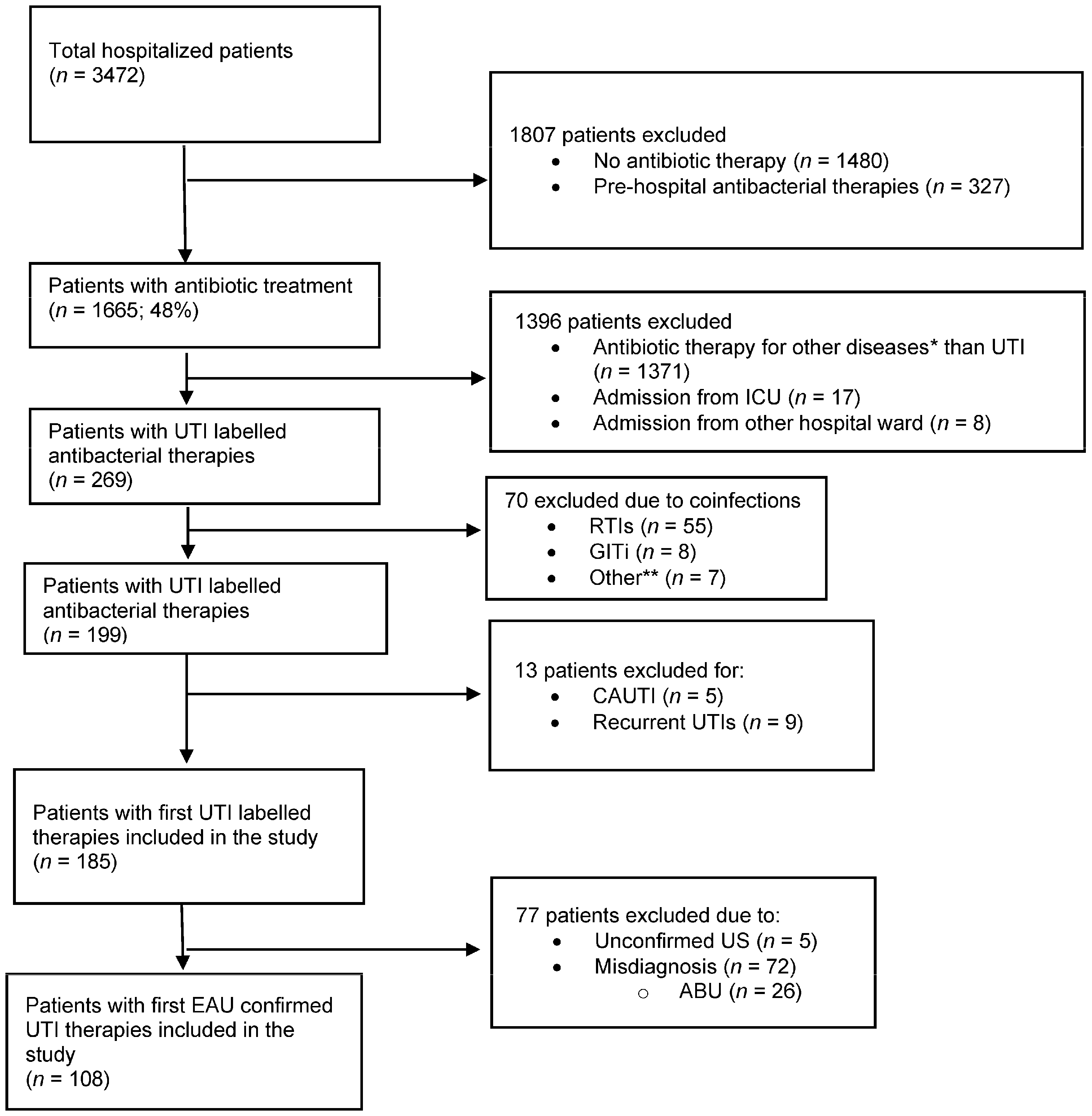
:1. Introduction
2. Results
2.1. Patient Characteristics and Main Outcomes
2.2. Diagnosis of UTIs
2.3. EAU-Confirmed UTIs and Most Frequently Isolated Pathogen Species
2.4. Antibiotic Therapy for EAU-Confirmed UTIs
2.5. Guideline Adherence in EAU-Confirmed UTIs
3. Discussion
3.1. Diagnosis
3.2. Guideline Adherent Empirical Antibiotic Therapy
3.2.1. Guideline Adherence: Agent Selection
3.2.2. Guideline Adherence: Dosage and Duration of Antibiotic Therapy
3.3. Changes in the First Empirical Therapy
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Setting
4.2. Data Collection
4.3. Main Outcome Measures
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Types of UTIs | uc-Cystitis | uc-Pyelonephritis | cUTI without Pyelonephriti | cUTI with Pyelonephritis | Urosepsis |
|---|---|---|---|---|---|
| Risk Factors | |||||
| No known relevant anatomical and functional abnormalities within the urinary tract, indwelling urinary catheters, renal diseases, obstruction | ✓ | ✓ | |||
| No comorbidities 1 | ✓ | ✓ | |||
| Age (over 65 years old) | ✓ | ✓ | |||
| Pregnancy | ✓ | ✓ | |||
| Men | ✓ | ✓ | |||
| Known relevant anatomical and functional abnormalities within the urinary tract, indwelling urinary catheters, renal diseases, obstruction | ✓ | ✓ | ✓ | ||
| Comorbidities 1 | ✓ | ✓ | ✓ | ||
| Recent history of HAI 2 | ✓ | ✓ | ✓ | ||
| SIRS, life-threatening organ dysfunction 3 | ✓ | ||||
| Potential signs and symptoms | |||||
| Lower urinary tract: dysuria, frequency, urgency | ✓ | ✓ | ✓ | ✓ | |
| Absence of vaginal discharge or irritation | ✓ | ||||
| Fever (>38 °C) | ✓ | ✓ | ✓ | ✓ | |
| Hypothermia | ✓ | ||||
| Chills, suprapubic pain | ✓ | ✓ | ✓ | ||
| Chills, flank pain | ✓ | ||||
| Nausea, vomiting | ✓ | ✓ | ✓ | ||
| Costovertebral angle tenderness | ✓ | ✓ | ✓ | ||
| Bacteriuria, pyuria | ✓ | ✓ | ✓ | ||
| Tachycardia and tachypnoea | ✓ | ||||
| Differential diagnosis | |||||
| Imaging technique 4 | ✓ | ✓ | |||
| Laboratory diagnosis | |||||
| Urinalysis (assessment of white and red blood cells and nitrite) 5 | ✓ | ✓ | |||
| Urine culture and antimicrobial susceptibility testing 5 | ✓ | ✓ | ✓ | ✓ | |
| Blood culture | ✓ | ||||
| CBC (leukocytosis or leukopenia) | ✓ | ||||
| Rise in the level of PCT | ✓ | ||||
| Rise in the level of Serum lactate | ✓ | ||||
Appendix B
| Antimicrobial Agent | Appropriate (Recommended) Dose | Recommended Dose Adjustment by SPC | Debatable Dose | Underdose/Overdose | |
|---|---|---|---|---|---|
| EAU UTI Guideline 1 | eGFR (mL/min) | Body Weight (kg) | |||
| Fosfomycin trometamol | oral 3 g SD | <10 | <50% deviation from the recommended dose and/or absence of loading dose | ≥50% deviation from the recommended dose and/or no dose adjustment in renal impairment and in extremes 2 for body weight | |
| Nitrofurantoin monohydrate | oral 100 mg b.i.d | ≤50 | |||
| Amoxicillin/clavulanic acid | oral 375 mg t.i.d | <10 | <50 kg | ||
| Amoxicillin/clavulanic acid | oral 625 mg t.i.d | <10 | <50 kg | ||
| Amoxicillin/clavulanic acid | iv 1.2 g t.i.d | <10 | <50 kg | ||
| Cefuroxim axetil | oral 500 mg b.i.d | <20 | |||
| Cefuroxim sodium | iv 0.75–1.5 g b.i.d-t.i.d | <20 | |||
| Trimethoprim/sulphametoxazole | oral 160/800 mg b.i.d | <30 | |||
| Ofloxacin | oral 200 mg b.i.d | <50 | |||
| Norfloxacin | oral 400 mg b.i.d | <20 | |||
| Ciprofloxacin | oral 500–750 mg b.i.d or iv 400 mg b.i.d | <30 | |||
| Levofloxacin | oral 750 mg q.d iv 500 mg q.d | <50 | |||
| Cefotaxime | iv 2 g t.i.d | <5 | |||
| Ceftriaxone | iv 1–2 g q.d | <10 | <50 kg | ||
| Cefepime | iv 1–2 g q.d | <50 | <40 kg | ||
| Piperacillin/tazobactam | iv 2.5–4.5 g t.i.d | <50 | |||
| Gentamicin | iv 5 mg/kg q.d | <40 | 5 mg/bodyweight | ||
| Amikacin | iv 15 mg/kg q.d | <70 | 5 mg/bodyweight | ||
| Imipenem/cilastatin | iv 0.5 g t.i.d | <70 | |||
| Meropenem | iv 1 g t.i.d | <50 | |||
References
- Ghosh, D.; Veeraraghavan, B.; Elangovan, R.; Vivekanandan, P. Antibiotic Resistance and Epigenetics: More to It than Meets the Eye. Antimicrob. Agents Chemother. 2020, 64, e02225-19. [Google Scholar] [CrossRef] [PubMed]
- Allel, K.; Day, L.; Hamilton, A.; Lin, L.; Furuya-Kanamori, L.; Moore, C.E.; Van Boeckel, T.; Laxminarayan, R.; Yakob, L. Global antimicrobial-resistance drivers: An ecological country-level study at the human-animal interface. Lancet Planet. Health 2023, 7, e291–e303. [Google Scholar] [CrossRef]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Mello, M.S.; Oliveira, A.C. Overview of the actions to combat bacterial resistance in large hospitals. Rev. Lat. Am. Enferm. 2021, 29, e3407. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Vogelaers, D.; Blot, S. The rising problem of antimicrobial resistance in the intensive care unit. Ann. Intensive Care 2011, 1, 47. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.W.; Trautner, B.W.; Jump, R.L.P. Urinary Tract Infection and Asymptomatic Bacteriuria in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 673–688. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef]
- ECDC. Antimicrobial Consumption Database (ESAC-Net). Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database (accessed on 19 January 2022).
- ECDC. European Centre for Disease Prevention and Control, Antimicrobial Use in European Hospitals. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-use-european-hospitals (accessed on 11 December 2022).
- ECDC. Diagnosis Site of Antimicrobial Treatment. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections-acute-care-hospitals/database/indications-antimicrobial-use/diagnosis-site (accessed on 2 July 2023).
- Grabe, M.; Bjerklund-Johansen, T.E.; Botto, H.; Çek, M.; Naber, K.G.; Tenke, P.; Wagenlehner, F. Guidelines on Urological Infections. Available online: https://d56bochluxqnz.cloudfront.net/documents/EAU-Guidelines-Urological-Infections-2015.pdf (accessed on 7 October 2023).
- Ostrow, O.; Prodanuk, M.; Foong, Y.; Singh, V.; Morrissey, L.; Harvey, G.; Campigotto, A.; Science, M. Decreasing Misdiagnoses of Urinary Tract Infections in a Pediatric Emergency Department. Pediatrics 2022, 150, e2021055866. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.C.S.; Thum, L.W.; Sadun, T.; Markowitz, M.; Maliski, S.L.; Ackerman, A.L.; Anger, J.T.; Kim, J.H. Fear and Frustration among Women with Recurrent Urinary Tract Infections: Findings from Patient Focus Groups. J. Urol. 2021, 206, 688–695. [Google Scholar] [CrossRef]
- Gupta, A.; Petty, L.; Gandhi, T.; Flanders, S.; Hsaiky, L.; Basu, T.; Zhang, Q.; Horowitz, J.; Masood, Z.; Chopra, V.; et al. Overdiagnosis of urinary tract infection linked to overdiagnosis of pneumonia: A multihospital cohort study. BMJ Qual. Saf. 2022, 31, 383–386. [Google Scholar] [CrossRef]
- Tomas, M.E.; Getman, D.; Donskey, C.J.; Hecker, M.T. Overdiagnosis of Urinary Tract Infection and Underdiagnosis of Sexually Transmitted Infection in Adult Women Presenting to an Emergency Department. J. Clin. Microbiol. 2015, 53, 2686–2692. [Google Scholar] [CrossRef]
- Childers, R.; Liotta, B.; Brennan, J.; Wang, P.; Kattoula, J.; Tran, T.; Montilla-Guedez, H.; Castillo, E.M.; Vilke, G. Urine testing is associated with inappropriate antibiotic use and increased length of stay in emergency department patients. Heliyon 2022, 8, e11049. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Flanders, S.A.; Snyder, A.; Conlon, A.; Rogers, M.A.M.; Malani, A.N.; McLaughlin, E.; Bloemers, S.; Srinivasan, A.; Nagel, J.; et al. Excess Antibiotic Treatment Duration and Adverse Events in Patients Hospitalized with Pneumonia: A Multihospital Cohort Study. Ann. Intern. Med. 2019, 171, 153–163. [Google Scholar] [CrossRef]
- Benko, R.; Matuz, M.; Juhasz, Z.; Bognar, J.; Bordas, R.; Soos, G.; Hajdu, E.; Peto, Z. Treatment of Cystitis by Hungarian General Practitioners: A Prospective Observational Study. Front. Pharmacol. 2019, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, Z.; Benko, R.; Matuz, M.; Viola, R.; Soos, G.; Hajdu, E. Treatment of acute cystitis in Hungary: Comparison with national guidelines and with disease-specific quality indicators. Scand. J. Infect. Dis. 2013, 45, 612–615. [Google Scholar] [CrossRef]
- ECDC. Indications for Antimicrobial Use. European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections-acute-care-hospitals/database/indications-antimicrobial-use (accessed on 10 January 2023).
- Bonkat, G.R.B.; Bruyère, F.; Cai, T. EAU Guideline on Urological Infections. Available online: https://uroweb.org/guidelines/urological-infections (accessed on 7 October 2023).
- Rousham, E.; Cooper, M.; Petherick, E.; Saukko, P.; Oppenheim, B. Overprescribing antibiotics for asymptomatic bacteriuria in older adults: A case series review of admissions in two UK hospitals. Antimicrob. Resist. Infect. Control 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N. Overdiagnosis and overtreatment as a quality problem: Insights from healthcare improvement research. BMJ Qual. Saf. 2018, 27, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Woodford, H.J.; George, J. Diagnosis and management of urinary tract infection in hospitalized older people. J. Am. Geriatr. Soc. 2009, 57, 107–114. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gupta, A.; Petty, L.A.; Gandhi, T.N.; Flanders, S.A.; Swaminathan, L.; Hsaiky, L.; Ratz, D.; Horowitz, J. Misdiagnosis of Urinary Tract Infection Linked to Misdiagnosis of Pneumonia: A Multihospital Cohort Study. Infect. Control Hosp. Epidemiol. 2020, 41, s488–s489. [Google Scholar] [CrossRef]
- Walker, S.; McGeer, A.; Simor, A.E.; Armstrong-Evans, M.; Loeb, M. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians’ and nurses’ perceptions. CMAJ 2000, 163, 273–277. [Google Scholar]
- Kistler, C.E.; Sloane, P.D.; Platts-Mills, T.F.; Beeber, A.S.; Khandelwal, C.; Weber, D.J.; Mitchell, C.M.; Reed, D.; Chisholm, L.; Zimmerman, S. Challenges of antibiotic prescribing for assisted living residents: Perspectives of providers, staff, residents, and family members. J. Am. Geriatr. Soc. 2013, 61, 565–570. [Google Scholar] [CrossRef]
- Kistler, C.E.; Wretman, C.J.; Zimmerman, S.; Enyioha, C.; Ward, K.; Farel, C.E.; Sloane, P.D.; Boynton, M.H.; Beeber, A.S.; Preisser, J.S. Overdiagnosis of urinary tract infections by nursing home clinicians versus a clinical guideline. J. Am. Geriatr. Soc. 2022, 70, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.; Bowden, A.; Sundvall, P.D.; Gunnarsson, R. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing—A systematic literature review. BMC Geriatr. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.D.; Adepoju, O.; Stone, N.; Moudouni, D.K.; Nwaiwu, O.; Zhao, H.; Frentzel, E.; Mehr, D.; Garfinkel, S. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, M.; Kim, N.H.; Kim, C.J.; Song, K.H.; Choe, P.G.; Park, W.B.; Bang, J.H.; Kim, E.S.; Park, S.W.; et al. Why is asymptomatic bacteriuria overtreated?: A tertiary care institutional survey of resident physicians. BMC Infect. Dis. 2015, 15, 289. [Google Scholar] [CrossRef]
- Illiano, E.B.R.; Li Marzi, V.; Mancini, V.; Finazzi Agrò, E.; De Rienzo, G.; Natale, F.; Pastore, A.; Palleschi, G.; Balzarro, M.; Rubilotta, E.; et al. Urinary tract infection still a challenge to fight: A real setting study. Neurourol. Urodyn. 2018, 37, S364–S365. [Google Scholar]
- Zhang, L.; Zhang, F.; Xu, F.; Wang, Z.; Ren, Y.; Han, D.; Lyu, J.; Yin, H. Construction and Evaluation of a Sepsis Risk Prediction Model for Urinary Tract Infection. Front. Med. 2021, 8, 671184. [Google Scholar] [CrossRef]
- NCE. Results of National Microbiological Surveillance Results on Antibiotic Resistance. Available online: http://www.oek.hu/oek.web?nid=666&pid=12 (accessed on 24 July 2023).
- Alrosan, S.; Al Mse’adeen, M.; Alkhawaldeh, I.M.; Mishael, J.; Aljarab’ah, N.; Aljarajreh, M.; Yamin, M.; Abu-Jeyyab, M. An Audit to Reevaluate the Adherence to the Guidelines in Patients with Urinary Tract Infection at the Al-Karak Hospital in Jordan. Cureus 2023, 15, e39509. [Google Scholar] [CrossRef]
- Ramdani, A.; Rebaudet, S.; Beni-Chougrane, N.; Penaranda, G.; Coquet, E. A review of urinary tract infection management for patients admitted to the emergency department: Assessment of adherence to guidelines and identification of hospitalization criteria. Pharm. Hosp. Clin. 2017, 52, e38. [Google Scholar] [CrossRef]
- Chen, A.K.; Duffy, E.J.; Ritchie, S.R.; Thomas, M.G. Diagnostic accuracy and adherence to treatment guidelines in adult inpatients with urinary tract infections in a tertiary hospital. J. Pharm. Pract. Res. 2019, 49, 246–253. [Google Scholar] [CrossRef]
- Appaneal, H.J.; Shireman, T.I.; Lopes, V.V.; Mor, V.; Dosa, D.M.; LaPlante, K.L.; Caffrey, A.R. Poor clinical outcomes associated with suboptimal antibiotic treatment among older long-term care facility residents with urinary tract infection: A retrospective cohort study. BMC Geriatr. 2021, 21, 436. [Google Scholar] [CrossRef] [PubMed]
- Shafrin, J.; Marijam, A.; Joshi, A.V.; Mitrani-Gold, F.S.; Everson, K.; Tuly, R.; Rosenquist, P.; Gillam, M.; Ruiz, M.E. Impact of suboptimal or inappropriate treatment on healthcare resource use and cost among patients with uncomplicated urinary tract infection: An analysis of integrated delivery network electronic health records. Antimicrob. Resist. Infect. Control 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Sigler, M.; Leal, J.E.; Bliven, K.; Cogdill, B.; Thompson, A. Assessment of Appropriate Antibiotic Prescribing for Urinary Tract Infections in an Internal Medicine Clinic. South. Med. J. 2015, 108, 300–304. [Google Scholar] [CrossRef]
- Katchman, E.A.; Milo, G.; Paul, M.; Christiaens, T.; Baerheim, A.; Leibovici, L. Three-day vs longer duration of antibiotic treatment for cystitis in women: Systematic review and meta-analysis. Am. J. Med. 2005, 118, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.; Verreault, R.; Gourdeau, M.; Morin, M.; Grenier-Gosselin, L.; Rochette, L. Optimal duration of antibiotic therapy for uncomplicated urinary tract infection in older women: A double-blind randomized controlled trial. CMAJ 2004, 170, 469–473. [Google Scholar] [PubMed]
- Lutters, M.; Vogt-Ferrier, N.B. Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women. Cochrane Database Syst. Rev. 2008, 3, CD001535. [Google Scholar] [CrossRef] [PubMed]
- Drekonja, D.M.; Trautner, B.; Amundson, C.; Kuskowski, M.; Johnson, J.R. Effect of 7 vs 14 Days of Antibiotic Therapy on Resolution of Symptoms among Afebrile Men with Urinary Tract Infection: A Randomized Clinical Trial. JAMA 2021, 326, 324–331. [Google Scholar] [CrossRef]
- Goebel, M.C.; Trautner, B.W.; Grigoryan, L. The Five Ds of Outpatient Antibiotic Stewardship for Urinary Tract Infections. Clin. Microbiol. Rev. 2021, 34, e00003-20. [Google Scholar] [CrossRef]
- Rieger, K.L.; Bosso, J.A.; MacVane, S.H.; Temple, Z.; Wahlquist, A.; Bohm, N. Intravenous-only or Intravenous Transitioned to Oral Antimicrobials for Enterobacteriaceae-Associated Bacteremic Urinary Tract Infection. Pharmacotherapy 2017, 37, 1479–1483. [Google Scholar] [CrossRef]
- Gamble, K.C.; Rose, D.T.; Sapozhnikov, J. Intravenous to Oral Antibiotics Versus Intravenous Antibiotics: A Step-Up or a Step-Down for Extended Spectrum Beta-Lactamase Producing Urinary Tract Infections? Open Forum Infect. Dis. 2021, 8, S118–S119. [Google Scholar] [CrossRef]
- Alshareef, H.; Alfahad, W.; Albaadani, A.; Alyazid, H.; Talib, R.B. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J. Infect. Public Health 2020, 13, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Iffat Shafiq, J.M.; Schweighardt, A.; Zak, M.; Evans, C. Incidence of Antibiotic De-Escalation in Hospitalized Internal Medicine Patients Diagnosed with Cystitis or Pyelonephritis. Infect. Dis. 2016, 3 Suppl. 1, 1323. [Google Scholar] [CrossRef]
- Fesus, A.; Benko, R.; Matuz, M.; Kungler-Goracz, O.; Fesus, M.A.; Bazso, T.; Csernatony, Z.; Kardos, G. The Effect of Pharmacist-Led Intervention on Surgical Antibacterial Prophylaxis (SAP) at an Orthopedic Unit. Antibiotics 2021, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Fésüs, A.; Benkő, R.; Matuz, M.; Engi, Z.; Ruzsa, R.; Hambalek, H.A.; Illés, Á.; Kardos, G. Impact of Guideline Adherence on Outcomes in Patients Hospitalized with Community-Acquired Pneumonia (CAP) in Hungary: A Retrospective Observational Study. Antibiotics 2022, 11, 468. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- WHO. Collaborating Centre for Drug Statistics Methodology, Definition and General Considerations. 2023. Available online: https://www.whocc.no/ddd/definition_and_general_considera/ (accessed on 13 July 2023).
| Parameters | N | % |
|---|---|---|
| 185 | 100 | |
| Gender (female) | 125 | 67.6 |
| Age (years) (mean ± SD, range) | 71.87 ± 15.04 (21–101) | |
| 20–64 years | 52 | 28.1 |
| 65+ years | 133 | 71.9 |
| CCI (Charlson Comorbidity Index) (mean ± SD, range) | 4.71 ± 1.96 (0–11) | |
| 0 | 7 | 3.78 |
| 1 | 1 | 0.5 |
| 2 | 12 | 6.5 |
| 3 | 28 | 15.1 |
| 4 | 33 | 17.8 |
| >4 | 104 | 56.2 |
| Comorbidities | ||
| Cardiovascular disease * | 46 | 24.9 |
| Diabetes mellitus | 45 | 24.3 |
| Chronic kidney disease (moderate to severe) | 25 | 13.5 |
| Chronic liver disease (moderate to severe) | 24 | 13.0 |
| Hematological malignant diseases | 23 | 12.4 |
| Solid tumor | 18 | 9.7 |
| Localized | 16 | 8.6 |
| Metastatic | 2 | 1.1 |
| Dementia | 17 | 9.2 |
| Cerebrovascular accident or transient ischemic attack | 15 | 8.1 |
| Peptic ulcer disease | 14 | 7.6 |
| Chronic obstructive pulmonary disease | 8 | 4.3 |
| Peripheral vascular disease | 1 | 0.5 |
| Discharge types | ||
| Discharged home | 160 | 86.5 |
| Other hospital ward | 4 | 2.2 |
| ICU (intensive care unit) | 7 | 3.8 |
| LTCF (long-term care facility) | 1 | 0.5 |
| Outcome | ||
| In-hospital mortality | 13 | 7.0 |
| 30-day mortality | 17 | 9.2 |
| Recorded Diagnosis | EAU-Confirmed Diagnosis |
|---|---|
| Total (n = 185), 100% | |
| Cystitis (n = 10), 5.4% | ucUTI (n = 3) cUTI (n = 5) ABU (n = 1) Misdiagnosis (n = 1) |
| Pyelonephritis (n = 9), 3.8% | ucUTI (n = 4) cUTI (n = 3) Misdiagnosis (n = 2) |
| Urosepsis (n = 18), 9.7% | ucUTI (n = 7) cUTI (n = 4) ABU (n = 2) Unconfirmed urosespsis (n = 5) |
| UTI (n = 148), 80.0% | ucUTI (n = 46) * cUTI (n = 36) ABU (n = 23) Misdiagnosis (n = 43) |
| Total: ucUTI (n = 60) *, 32.4% cUTI (n = 48), 25.9% ABU (n =26), 14.1% Unconfirmed urosepsis (n = 5), 2.7% Misdiagnosis (n = 46), 24.9% | |
| Pathogens | ucUTI (Cystitis) N = 1 | ucUTI (Pyelonephritis) N = 59 (%) | cUTI (Pyelonephritis) N = 48 (%) | Total N = 108 (%) |
|---|---|---|---|---|
| Total urine cultures | - | 30 (50.8%) | 25 (52.1%) | 55 (50.9%) |
| Positive urine cultures | - | 23 (39.0%) | 20 (41.7%) | 43 (39.8%) |
| Contaminated sample | - | 2 (3.4%) | 1 (2.1%) | 3 (2.8%) |
| From positive urine cultures (%) | ||||
| More than one pathogen per sample | - | 6 (26.12%) | 2 (10.0%) | 8 (18.6%) |
| Esherchia coli | - | 10 (43.5%) | 9 (45.0%) | 19 (44.2%) |
| Klebsiella spp. | - | 4 (17.4%) | 2 (10.0%) | 6 (14.0%) |
| Other Enterobacteriaceae spp. | - | 4 (17.4%) | 1 (5.0%) | 5 (11.6%) |
| Enterococcus faecalis | - | 1 (4.3%) | 4 (20.0%) | 5 (11.6%) |
| Pseudomonas spp. | - | 1 (4.3%) | 3 (15.0%) | 4 (9.3%) |
| Proteus spp. | - | 1 (4.3%) | - | 1 (2.3%) |
| Streptococcus spp. | - | 1 (4.3%) | - | 1 (2.3%) |
| Candida spp. | - | 4 (17.4%) | 3 (15.0%) | 7 (16.3%) |
| MDR bacteria | - | 3 (13.0%) | - | 3 (7.0%) |
| Parameters | N | % |
|---|---|---|
| 108 | 100 | |
| EAU guideline-adherent agent(s) | 49 | 45.4 |
| EAU guideline adherent agent(s) and route of administration | 49 | 45.4 |
| EAU guideline adherent agent(s), route of administration, and dose | 39 | 36.1 |
| EAU guideline adherent agent(s), route of administration, dose, and duration | 11 | 10.2 |
| Type of the first antibiotic therapy | ||
| Monotherapies | 82 | 75.9 |
| Combination therapies | 26 | 24.1 |
| Route of administration of the first antibiotic therapy | ||
| intravenous | 66 | 61.1 |
| oral | 42 | 38.9 |
| Dosage of the first antibiotic therapy for guideline adherent agent selection | ||
| appropriate | 39 | 36.1 |
| overdosed (compared to SPC due to lack of guideline recommended dose) | 0 | 0.0 |
| underdosed (compared to EAU guideline and due to body weight) | 10 | 9.3 |
| Duration of total antibiotic therapies (Mean ± SD, range) 6.59 ± 5.21 (1–35) | ||
| Total antibiotic exposure (Mean ± SD, DDD/patient) 9.84 ± 14.99 (0.50–72.47) | ||
| Number of consecutive antibiotic therapies | ||
| 1 | 69 | 63.9 |
| >1 (2–5) | 39 | 36.1 |
| Changes in the first empirical therapy | ||
| Sequential antibiotic therapy * | 7 | 6.5 |
| De-escalation | 6 | 5.6 |
| Escalation | 28 | 25.9 |
| No change | 67 | 62.0 |
| Antibiotics | Route of Administration 4 | Doses/Day | Frequency (N) | Total % | EAU Guideline Adherence 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Guideline Adherence % | uc-Cystitis (n = 1) | uc-Pyelonephritis (n = 59) | cUTI without Pyelonephritis (n = 0) | cUTI with Pyelonephritis (n = 48) | Rosepsis (n = 0) | |||||
| Monotherapies (N = 82; 100%) | ||||||||||
| Fosfomycin trometamol | po | 3 g q.d | 1 | 1.2 | 0.0 | 1 | ||||
| Nitrofurantoin monohydrate | po | 100 mg b.i.d-t.i.d | 2 | 2.4 | 0.0 | 1 | 1 | |||
| Amoxicillin/clavulanic acid | po | 625 mg b.i.d-t.i.d | 12 | 14.6 | 11.0 | 1 | 9 | 2 | ||
| Amoxicillin/clavulanic acid | iv | 1.2 g b.i.d-t.i.d | 24 | 29.3 | 29.3 | 14 | 10 | |||
| Ceftibuten | po | - | 0 | 0.0 | 0.0 | |||||
| Cefuroxim axetil | po | - | 0 | 0.0 | 0.0 | |||||
| Cefuroxim sodium | iv | - | 0 | 0.0 | 0.0 | |||||
| Trimethoprim-sulphamethoxazole 1 | po | 400–800/80–160 mg b.i.d | 3 | 3.7 | 0.0 | 3 | ||||
| Ofloxacin | po | - | 0 | 0.0 | 0.0 | |||||
| Norfloxacin | po | 400 mg b.i.d | 3 | 3.7 | 1.2 | 1 | 2 | |||
| Ciprofloxacin 2 | po | 500 mg b.i.d | 12 | 14.6 | 14.6 | 7 | 5 | |||
| Ciprofloxacin 2 | iv | 400 mg b.i.d | 3 | 3.7 | 3.7 | 1 | 2 | |||
| Levofloxacin 2 | po | 500 mg q.d | 2 | 2.4 | 2.4 | 2 | ||||
| Levofloxacin 2 | iv | - | 0 | 0.0 | 0.0 | |||||
| Cefotaxime | iv | - | 0 | 0.0 | 0.0 | |||||
| Ceftriaxone | iv | 2 g q.d | 12 | 14.6 | 14.6 | 5 | 7 | |||
| Cefepime | iv | - | 0 | 0.0 | 0.0 | |||||
| Piperacillin/tazobactam | iv | - | 0 | 0.0 | 0.0 | |||||
| Imipenem/cilastatin | iv | - | 0 | 0.0 | 0.0 | |||||
| Meropenem | iv | 500 mg t.i.d- 1 g b.i.d | 4 | 4.9 | 2.4 | 2 | 2 | |||
| Clarithromycin | po | 500 mg b.i.d | 1 | 1.2 | 0.0 | 1 | ||||
| Gentamicin monotherapy | iv | 80–120 mg q.d | 2 | 2.4 | 0.0 | 2 | ||||
| Moxifloxacin | po | 400 mg q.d | 1 | 1.2 | 0.0 | 1 | ||||
| Combination therapies (N = 26; 100%) | ||||||||||
| Amoxicillin-clavulanic acid + amikacin/gentamicin 3 | iv | 1.2 g t.i.d + 80 mg b.i.d | 1 | 3.8 | 0.0 | 1 | ||||
| Ceftriaxone + amikacin/gentamicin 3 | iv | - | 0 | 0.0 | 0.0 | |||||
| Piperacillin/tazobactam + amikacin/gentamicin 3 | iv | - | 0 | 0.0 | 0.0 | |||||
| Meropenem + amikacin/gentamicin 3 | iv | - | 0 | 0.0 | 0.0 | |||||
| Imipenem-cilastatin + amikacin/gentamicin 3 | iv | - | 0 | 0.0 | 0.0 | |||||
| Amoxicillin-clavulanic acid + clarithromycin | iv | 1.2 g t.i.d + 500 mg b.i.d | 1 | 3.8 | 0.0 | 1 | ||||
| Amoxicillin-clavulanic acid + metronidazole | iv + po | 1.2 g b.i.d-t.i.d + 500 mg b.i.d | 4 | 15.4 | 0.0 | 1 | 3 | |||
| Amoxicillin-clavulanic acid + metronidazole | po | 625 mg b.i.d-t.i.d + 500 mg b.i.d | 2 | 7.7 | 0.0 | 2 | ||||
| Amoxicillin-clavulanic acid + Trimethoprim-sulphamethoxazole | iv + po | 1.2 g t.i.d + 400/80 mg b.i.d | 1 | 3.8 | 0.0 | 1 | ||||
| Ceftriaxone + metronidazole | iv | 2 g q.d + 250–500 mg b.i.d | 5 | 19.2 | 0.0 | 4 | 1 | |||
| Ceftriaxone + metronidazole | iv + po | 2 g q.d + 500 mg b.i.d | 2 | 7.7 | 0.0 | 2 | ||||
| Ceftriaxone + trimethoprim-sulphamethoxazole | iv + po | 500 mg b.i.d–2 g q.d + 400/80 mg b.i.d | 2 | 7.7 | 0.0 | 1 | 1 | |||
| Ciprofloxacin + metronidazole | po | 500 mg b.i.d + 500 mg b.i.d | 2 | 7.7 | 0.0 | 1 | 1 | |||
| Ciprofloxacin + moxifloxacin | iv + po | 500 mg b.i.d + 400 mg q.d | 1 | 3.8 | 0.0 | 1 | ||||
| Ciprofloxacin + tobramycin | iv | 500 mg b.i.d + 80 mg b.i.d | 1 | 3.8 | 0.0 | 1 | ||||
| Nitrofurantoin + metronidazole | po | 100 mg t.i.d + 500 mg b.i.d | 2 | 7.7 | 0.0 | 1 | 1 | |||
| Imipenem + metronidazole | iv + po | 1 g t.i.d + 500 mg b.i.d | 1 | 3.8 | 0.0 | 1 | ||||
| Tygecyclin + colomycin | iv | 400 mg q.d + 1 IU t.i.d | 1 | 3.8 | 0.0 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fésüs, A.; Matuz, M.; Papfalvi, E.; Hambalek, H.; Ruzsa, R.; Tánczos, B.; Bácskay, I.; Lekli, I.; Illés, Á.; Benkő, R. Evaluation of the Diagnosis and Antibiotic Prescription Pattern in Patients Hospitalized with Urinary Tract Infections: Single-Center Study from a University-Affiliated Hospital. Antibiotics 2023, 12, 1689. https://doi.org/10.3390/antibiotics12121689
Fésüs A, Matuz M, Papfalvi E, Hambalek H, Ruzsa R, Tánczos B, Bácskay I, Lekli I, Illés Á, Benkő R. Evaluation of the Diagnosis and Antibiotic Prescription Pattern in Patients Hospitalized with Urinary Tract Infections: Single-Center Study from a University-Affiliated Hospital. Antibiotics. 2023; 12(12):1689. https://doi.org/10.3390/antibiotics12121689
Chicago/Turabian StyleFésüs, Adina, Mária Matuz, Erika Papfalvi, Helga Hambalek, Roxána Ruzsa, Bence Tánczos, Ildikó Bácskay, István Lekli, Árpád Illés, and Ria Benkő. 2023. "Evaluation of the Diagnosis and Antibiotic Prescription Pattern in Patients Hospitalized with Urinary Tract Infections: Single-Center Study from a University-Affiliated Hospital" Antibiotics 12, no. 12: 1689. https://doi.org/10.3390/antibiotics12121689
APA StyleFésüs, A., Matuz, M., Papfalvi, E., Hambalek, H., Ruzsa, R., Tánczos, B., Bácskay, I., Lekli, I., Illés, Á., & Benkő, R. (2023). Evaluation of the Diagnosis and Antibiotic Prescription Pattern in Patients Hospitalized with Urinary Tract Infections: Single-Center Study from a University-Affiliated Hospital. Antibiotics, 12(12), 1689. https://doi.org/10.3390/antibiotics12121689








