The Profile of Bacterial Infections in a Burn Unit during and after the COVID-19 Pandemic Period
Abstract
1. Introduction
2. Results
2.1. Clinical Samples
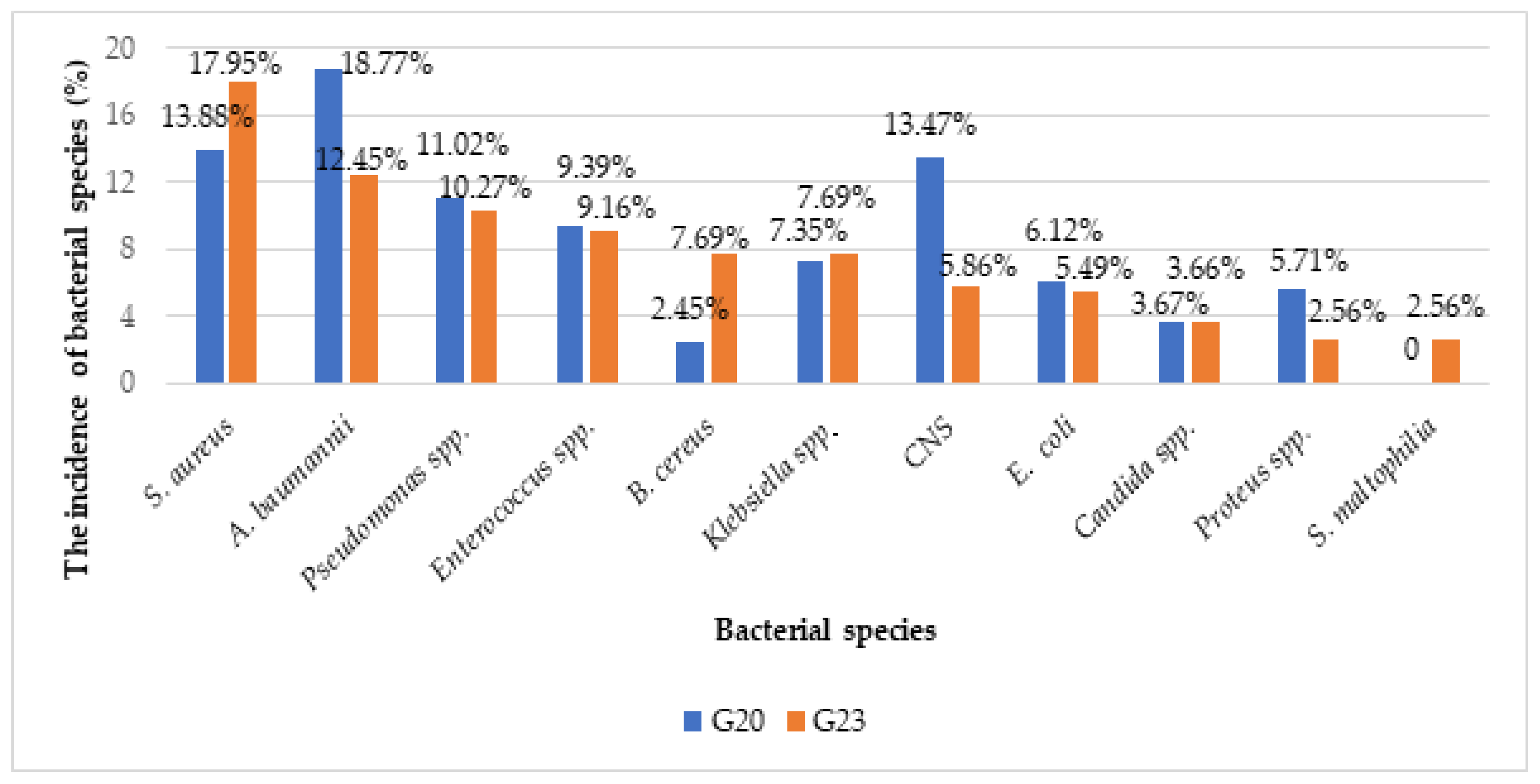
2.2. Clinical Isolates
3. Discussion
4. Materials and Methods
4.1. Microbiology Procedures
4.2. Statistical Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Branski, L.K.; Al-Mousawi, A.; Rivero, H.; Jeschke, M.G.; Sanford, A.P.; Herndon, D.N. Emerging infections in burns. Surg. Infect. 2009, 10, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Vindenes, H.; Bjerknes, R. Microbial colonization of large wounds. Burns 1995, 21, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ravat, F.; Le-Floch, R.; Vinsonneau, C.; Ainaud, P.; Bertin-Maghit, M.; Carsin, H.; Perro, G. Antibiotics and the burn patient. Burns 2011, 37, 16–26. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Ducret, V.; Leoni, S.; Fleuchot, B.; Jafari, P.; Raffoul, W.; Applegate, L.A.; Que, Y.-A.; Perron, K. Transcriptome Analysis of Pseudomonas aeruginosa Cultured in Human Burn Wound Exudates. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef]
- Moser, C.; Jensen, P.Ø.; Thomsen, K.; Kolpen, M.; Rybtke, M.; Lauland, A.S.; Trøstrup, H.; Tolker-Nielsen, T. Immune Responses to Pseudomonas aeruginosa Biofilm Infections. Front. Immunol. 2021, 12, 625597. [Google Scholar] [CrossRef]
- Kirketerp-Møller, K.; Stewart, P.S.; Bjarnsholt, T. The zone model: A conceptual model for understanding the microenvironment of chronic wound infection. Wound Repair Regen. 2020, 28, 593–599. [Google Scholar] [CrossRef]
- Maslova, E.; Eisaiankhongi, L.; Sjöberg, F.; McCarthy, R.R. Burns and biofilms: Priority pathogens and in vivo models. NPJ Biofilms Microbiomes 2021, 7, 73. [Google Scholar] [CrossRef]
- Brandenburg, K.S.; Weaver, A.J.; Karna, S.L.R.; Leung, K.P. The impact of simultaneous inoculation of Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans on rodent burn wounds. Burns 2021, 47, 1818–1832. [Google Scholar] [CrossRef]
- Brandenburg, K.S.; Weaver, A.J.; Karna, S.L.R.; You, T.; Chen, P.; Van Stryk, S.; Qian, L.; Pineda, U.; Abercrombie, J.J.; Leung, K.P. Formation of Pseudomonas aeruginosa Biofilms in Full-thickness Scald Burn Wounds in Rats. Sci. Rep. 2019, 9, 13627. [Google Scholar] [CrossRef]
- Kennedy, P.; Brammah, S.; Wills, E. Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010, 36, 49–56. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Foncerrada, G.; Culnan, D.M.; Capek, K.D.; González-Trejo, S.; Cambiaso-Daniel, J.; Woodson, L.C.; Herndon, D.N.; Finnerty, C.C.; Lee, J.O. Inhalation Injury in the Burned Patient. Ann. Plast. Surg. 2018, 80, S98–S105. [Google Scholar] [CrossRef]
- Boehm, D.; Menke, H. Sepsis in Burns—Lessons Learnt from Developments in the Management of Septic Shock. Medicina 2022, 58, 26. [Google Scholar] [CrossRef]
- Laura, P.; José, A.; Nikki, A.; Khaled, A.; Barret, J.; Jeffery, C.; Shobha, C.; Jack, C.S.; Scott, C.; Nadia, D.; et al. Impact of COVID-19 on global burn care. Burns 2022, 48, 1301–1310. [Google Scholar] [CrossRef]
- Kumar, S.; Kain, R.; More, A.; Sheth, S.; Arumugam, P.K. Burns and COVID-19-Initial Experience and Challenges. J. Burn Care Res. 2021, 42, 794–800. [Google Scholar] [CrossRef]
- Walters, E.T.; Palackic, A.; Franco-Mesa, C.; Shah, N.R.; Erickson, M.J.; Wolf, S.E. The impact of COVID-19 on clinical outcomes of burn patients. Burn. Trauma 2023, 11, tkad042. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022–2020 data, Surveill Rep [Internet]. 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data (accessed on 20 September 2023).
- Lima, K.M.; Davis, R.R.; Liu, S.Y.; Greenhalgh, D.G.; Tran, N.K. Longitudinal profiling of the burn patient cutaneous and gastrointestinal microbiota: A pilot study. Sci. Rep. 2021, 11, 10667. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Surveillance of Antimicrobial resistance in Europe Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2016. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AMR-surveillance-Europe-2016.pdf (accessed on 16 August 2020).
- Boccabella, L.; Palma, E.G.; Abenavoli, L.; Scarlata, G.G.M.; Boni, M.; Ianiro, G.; Santori, P.; Tack, J.F.; Scarpellini, E. Post-Coronavirus Disease 2019 Pandemic Antimicrobial Resistance. Antibiotics 2024, 13, 233. [Google Scholar] [CrossRef]
- Rossato, M.; Andrisani, A.; Zabeo, E.; Di Vincenzo, A. Men with COVID-19 die. Women survi at any age! Maturitas 2022, 163, 88. [Google Scholar] [CrossRef]
- Muntean, D.; Horhat, F.-G.; Bădițoiu, L.; Dumitrașcu, V.; Bagiu, I.-C.; Horhat, D.-I.; Coșniță, D.A.; Krasta, A.; Dugăeşescu, D.; Licker, M. Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study of Trends in a Tertiary Healthcare Unit. Medicina 2018, 54, 92. [Google Scholar] [CrossRef] [PubMed]
- Licker, M.; Musuroi, C.; Muntean, D.; Crainiceanu, Z. Updates in the Management of Multidrug-Resistant Bacterial Infections in Burn Patients. Proceedings [Internet]. 2023. Available online: https://www.srm.ro/media/2023/11/volum-rezumate-cnme-1.pdf (accessed on 15 January 2024).
- Gupta, N.; Haque, A.; Mukhopadhyay, G.; Narayan, R.P.; Prasad, R. Interactions between bacteria and Candida in the burn wound. Burns 2005, 31, 375–378. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef]
- Nazik, H.; Bektöre, B.; Öngen, B.; Ilktaç, M.; Özyurt, M.; Kuvat, N.; Baylan, O.; Keküllüoglu, H.; Haznedaroglu, T.; Kelesoglu, F.M. Plasmid-Mediated Quinolone Resistance Genes in Escherichia coli Urinary Isolates from Two Teaching Hospitals in Turkey: Coexistence of TEM, SHV, CTX-M and VEB-1 Type β-lactamases. Trop. J. Pharm. Res. 2011, 10, 325–333. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.C.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; Yeom, J.-S.; Song, Y.G. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia and clinical impact of quinolone-resistant strains. BMC Infect. Dis. 2019, 19, 754. [Google Scholar] [CrossRef]
- Kumwenda, G.P.; Kasambara, W.; Chizani, K.; Phiri, A.; Banda, A.; Choonara, F.; Lichapa, B. A multidrug-resistant Stenotrophomonas maltophilia clinical isolate from Kamuzu Central Hospital, Malawi. Malawi Med. J. 2021, 33, 82–84. [Google Scholar] [CrossRef]
- Brooke, J.S. New strategies against Stenotrophomonas maltophilia: A serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev. Anti-Infect. Ther. 2014, 12, 1–4. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Stenström, T.A.; Okoh, A.I. Stenotrophomonas maltophilia as an Emerging Ubiquitous Pathogen: Looking Beyond Contemporary Antibiotic Therapy. Front. Microbiol. 2017, 8, 2276. [Google Scholar] [CrossRef]
- Pogson, K.; Nurczyk, K.; Sljivic, S.; Jones, S.W.; Cairns, B.; Nizamani, R.; Chrisco, L.; Williams, F.N. 716 Increased Mortality in Burn Center Admissions with Stenotrophomonas Maltophilia. J. Burn Care Res. 2020, 41 (Suppl. S1), S189–S190. [Google Scholar] [CrossRef]
- Brooke, J.S. Advances in the Microbiology of Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 2021, 34, e0003019. [Google Scholar] [CrossRef]
- Panghal, M.; Singh, K.; Kadyan, S.; Chaudary, U.; Yadav, J.P. The analysis of distribution of multidrug resistant Pseudomonas and Bacillus species from burn patients and burn ward environment. Burns 2015, 41, 812–819. [Google Scholar] [CrossRef]
- CDC. COVID-19 Impacts on Antimicrobial Resistance. 2024. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/COVID-19.html (accessed on 7 June 2024).
- Abdelaziz Abdelmoneim, S.; Mohamed Ghazy, R.; Anwar Sultan, E.; Hassaan, M.A.; Anwar Mahgoub, M. Antimicrobial resistance burden pre and post-COVID-19 pandemic with mapping the multidrug resistance in Egypt: A comparative cross-sectional study. Sci. Rep. 2024, 14, 7176. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Rossolini, G.M.; Schultsz, C.; Tacconelli, E.; Murthy, S.; Ohmagari, N.; Holmes, A.; Bachmann, T.; Goossens, H.; Canton, R.; et al. Antimicrobial resistance research in a post-pandemic world: Insights on antimicrobial resistance research in the COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2021, 25, 5–7. [Google Scholar] [CrossRef]
- Institutul Național de Sănătate Publică. Infectii Asociate Asistentei Medicale, Rezistenta Microbiana, Consum Antibiotice-CARMIAAN. Available online: https://insp.gov.ro/centrul-national-de-supraveghere-si-control-al-bolilor-transmisibile-cnscbt/analiza-date-supraveghere/ (accessed on 23 June 2024).
- Chaïbi, K.; de Ponfilly, G.; Dortet, L.; Zahar, J.-R.; Pilmis, B. Empiric Treatment in HAP/VAP: “Don’t You Want to Take a Leap of Faith”? Antibiotics 2022, 11, 359. [Google Scholar] [CrossRef]
- Novacescu, A.N.; Buzzi, B.; Bedreag, O.; Papurica, M.; Rogobete, A.F.; Sandesc, D.; Sorescu, T.; Baditoiu, L.; Musuroi, C.; Vlad, D.; et al. Bacterial and Fungal Superinfections in COVID-19 Patients Hospitalized in an Intensive Care Unit from Timișoara, Romania. Infect. Drug Resist. 2022, 15, 7001–7014. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Bianco, G.; Llorente, L.I.; Acero, L.A.; Nunes, D.; Seruca, M.; Mendes, V.S.; Almeida, A.; Bastos, P.; Rodríguez-Villodres, Á.; et al. Enterobacterales carrying chromosomal AmpC β-lactamases in Europe (EuESCPM): Epidemiology and antimicrobial resistance burden from a cohort of 27 hospitals, 2020–2022. Int. J. Antimicrob. Agents 2024, 63, 107115. [Google Scholar] [CrossRef]
- Bittner, E.A.; Shank, E.; Woodson, L.; Martyn, J.A.J. Acute and Perioperative Care of the Burn-injured Patient. Anesthesiology 2015, 122, 448–464. [Google Scholar] [CrossRef]
- Tobiasen, J.; Hiebert, J.M.; Edlich, R.F. The abbreviated burn severity index. Ann. Emerg. Med. 1982, 11, 260–262. [Google Scholar] [CrossRef]
- Usmani, A.; Pipal, D.K.; Bagla, H.; Verma, V.; Kumar, P.; Yadav, S.; Garima, G.; Rani, V.; Pipal, R.K. Prediction of Mortality in Acute Thermal Burn Patients Using the Abbreviated Burn Severity Index Score: A Single-Center Experience. Cureus 2022, 14, 26161. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 13.0. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 17 January 2024).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st ed.; 2021. Available online: https://clsi.org/media/z2uhcbmv/m100ed31_sample.pdf (accessed on 25 February 2020).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
| Variable | 2020 | 2023 | |||
|---|---|---|---|---|---|
| Nr. (%) | 95%CI * | Nr. (%) | 95%CI | p | |
| Patients | N1 = 48 | N2 = 69 | |||
| Women patients, F (%) | 16 (33.33) | [20.40–48.41%] | 20 (28.99) | [18.69–41.16%] | 0.685 |
| Male patients, M (%) | 32 (66.67) | [51.59–79.60%] | 49 (71.01) | [58.84–81.31%] | |
| Median Age [IQR] | 59.00 [47.50–70.50] | / | 49.00 [35.00–61.00] | / | 0.005 |
| Patients aged 18–19 (%) | 1 (2.08) | [0.05–11.07%] | 1 (1.45) | [0.04–7.81%] | 1.00 |
| Patients aged 20–29 (%) | 0 (0) | / | 10 (14.49) | [7.17–25.04%] | 0.005 |
| Patients aged 30–39 (%) | 5 (10.42) | [3.47–22.66%] | 12 (17.39) | [9.32–28.41%] | 0.424 |
| Patients aged 40–49 (%) | 8 (16.67) | [7.48–30.22%] | 12 (17.39) | [9.32–28.41%] | 1.00 |
| Patients aged 50–59 (%) | 10 (20.83) | [10.47–34.99%] | 15 (21.74) | [12.71–33.31%] | 1.00 |
| Patients aged 60–69 (%) | 11 (22.92) | [12.03–37.31%] | 9 (13.04) | [6.14–23.32%] | 0.212 |
| Patients aged 70–79 (%) | 5 (10.42) | [3.47–22.66%] | 7 (10.14) | [4.18–19.79%] | 1.00 |
| Patients aged 80–89 (%) | 7 (14.58) | [6.07–27.76%] | 2 (2.90) | [0.35–10.08%] | 0.031 |
| Patients aged 90–99 (%) | 1 (2.08) | [0.05–11.07%] | 1 (1.45) | [0.04–7.81%] | 1.00 |
| Median length of stay [IQR] | 20.00 [12.00–52.00] | / | 19 [8.00–41.00] | / | 0.745 |
| Median fatality [IQR] | 3.00 [2.00–4.00] | / | 2.00 [1.00–3.00] | / | 0.023 |
| Fatality Rate | 0–1% | 2% | 10–20% | 30–50% | 60–80% | >80% |
|---|---|---|---|---|---|---|
| G23 nr. (%) | 5 (7.25) | 17 (24.64) | 16 (23.19) | 16 (23.19) | 6 (8.69) | 9 (13.04) |
| G20 nr. (%) | 0 | 7 (14.58) | 8 (16.67) | 15 (31.25) | 11 (22.92) | 7 (14.58) |
| Variable | Fatal Outcome | Positive Evolution | ||||||
|---|---|---|---|---|---|---|---|---|
| N1 = 33 | 95%CI * | N2 = 80 | 95%CI | p | OR | p | HR | |
| Median age [IQR] | 64.00 [49.00–71.00] | / | 50.00 [35.00–62.00] | / | 0.005 | 1.04 [1.01–1.06] | 0.002 | 1.12 [1.04–1.21] |
| Body surface [IQR] | 40.00 [16.00–65.00] | / | 14.50 [5.50–24.50] | / | <0.001 | 1.06 [1.03–1.08] | 0.001 | 1.12 [1.05–1.21] |
| Burn dressing ≥10% of BS (%) | 23 (69.70) | [51.29–84.41%] | 38 (47.50) | [36.21–58.98%] | 0.039 | 2.54 [1.07–6.02] | 0.008 | 0.02 [0.00–0.37] |
| Ventilatory support (%) | 27 (81.82) | [64.54–93.02%] | 13 (16.25) | [8.95–26.18%] | <0.001 | 23.19 [7.99–67.31] | 0.002 | 37.32 [3.65–381.05] |
| Haemo-dialysis (%) | 13 (39.39) | [22.91–57.86%] | 2 (2.50) | [0.30–8.74%] | <0.001 | 25.35 [5.29–121.57] | 0.006 | 47.11 [2.99–740.36] |
| Patients infected with | ||||||||
| Acinetobacter spp. (%) | 21 (63.64) | 19 (23.75) | <0.001 | 5.61 [2.34–13.49] | 0.019 | 9.02 [1.44–56.62] | ||
| Species | 2020 | 2023 | |||
|---|---|---|---|---|---|
| Nr. (%) | 95%CI * | Nr. (%) | 95%CI | p | |
| Wound secretions | |||||
| S. aureus | 23 (16.31) | [10.63–23.46%] | 31 (18.45) | [12.90–25.16%] | 0.654 |
| Enterococcus spp. | 21 (14.89) | [9.46–21.86%] | 22 (13.10) | [8.39–19.15] | 0.366 |
| Pseudomonas spp. | 19 (13.48) | [8.31–20.24%] | 21 (12.50) | [7.91–18.47%] | 0.865 |
| Bacillus cereus | 5 (3.55) | [1.16–8.08%] | 20 (11.90) | [7.43–17.79%] | 0.010 |
| Acinetobacter spp. | 19 (13.48) | [8.31–20.24%] | 19 (11.31) | [6.95–17.10%] | 0.604 |
| Klebsiella spp. | 13 (9.22%) | [5.00–15.25%] | 16 (9.52) | [5.54–15.01%] | 1.00 |
| CNS | 10 (7.09) | [3.45–12.66%] | 1 (0.60) | [0.02–3.27%] | 0.032 |
| Blood cultures | |||||
| CNS | 21 (36.21) | [23.99–49.88%] | 15 (28.85) | [17.13–43.08%] | 0.424 |
| S. aureus | 7 (12.07) | [4.99–23.30%] | 12 (23.08) | [12.53–36.84%] | 0.139 |
| A. baumannii | 12 (20.69) | [11.17–33.35%] | 8 (15.38) | [6.88–28.08%] | 0.621 |
| Klebsiella spp. | 2 (3.45) | [0.42–11.91%] | 3 (5.77) | [1.21–15.95%] | 0.665 |
| Enterococcus spp. | 2 (3.45) | [0.42–11.91%] | 3 (5.77) | [1.21–15.95%] | 0.665 |
| Bronchial aspirates | |||||
| A. baumannii | 12 (35.29) | [19.75–53.51%] | 7 (23.33) | [9.93–42.28%] | 0.412 |
| S. maltophilia 1 | 0 (0) | / | 4 (13.33) | [3.76–30.72%] | 0.043 |
| Pseudomonas spp. | 4 (11.76) | [3.30–27.45%] | 3 (10.00) | [2.11–26.53%] | 1.00 |
| S. Paucimobilis 2 | 0 (0) | / | 2 (6.67) | [0.82–22.07%] | 0.216 |
| Klebsiella spp. | 2 (5.88) | [0.72–19.68%] | 2 (6.67) | [0.82–22.07%] | 1.00 |
| Strains | 2020 | 2023 | |||
|---|---|---|---|---|---|
| N = 245 (%) | 95%CI * | N = 245 (%) | 95%CI | p | |
| GNB of which: | 134 (54.69) | [48.23–61.04%] | 140 (51.28) | [45.18–57.35%] | 0.480 |
| Non-fermenting | 74 (30.20) | [24.52–36.37%] | 76 (27.84) | [22.61–33.56%] | 0.562 |
| GPC | 101 (41.22) | [35.00–47.67%] | 123 (45.05) | [39.05–51.17%] | 0.424 |
| Fungi | 10 (4.08) | [1.97–7.38%] | 10 (3.66) | [1.77–6.63%] | 0.823 |
| 2020 | 2023 | ||||
|---|---|---|---|---|---|
| PHENOTYPE | Nr. (%) | 95%CI * | Nr. (%) | 95%CI | p |
| Species: Acinetobacter baumannii TOTAL = 46/34 | |||||
| MDR | 45 (97.83) | [88.47–99.94%] | 34 (100) | [89.72–100.00%] | 1.00 |
| XDR | 28 (60.87) | [45.37–74.91%] | 26 (76.47) | [58.83–89.25%] | 0.156 |
| PDR | 0 (0) | / | 0 (0) | / | / |
| CR-GNB | 42 (91.30) | [79.21–97.58%] | 30 (88.24) | [72.55–96.70%] | 0.717 |
| R-SXT | 40 (86.96) | [73.74–95.06%] | 30 (88.24) | [72.55–96.70%] | 1.00 |
| Species: Staphylococcus aureus TOTAL = 34/49 | |||||
| MRSA | 14 (41.18) | [24.65–59.30%] | 22 (44.90) | [30.67–59.77%] | 0.823 |
| MDR | 20 (58.82) | [40.70–75.35%] | 25 (51.02) | [36.34–65.58%] | 0.510 |
| MLSB | 10 (29.41) | [15.10–47.48%] | 22 (44.90) | [30.67–59.77%] | 0.176 |
| R-FQ | 7 (20.59) | [8.70–37.90%] | 16 (32.65) | [19.95–47.54%] | 0.319 |
| R-SXT | 8 (23.53) | [10.75–41.17%] | 4 (8.16) | [2.27–19.60%] | 0.062 |
| Species: Coagulase-negative staphylococci TOTAL = 33/16 | |||||
| MRCNS | 11 (33.33) | [17.96–51.83%] | 10 (62.50) | [35.43–84.80%] | 0.069 |
| MLSB | 0 (0) | / | 2 (12.50) | [1.55–38.35%] | 0.102 |
| Species: Pseudomonas spp. TOTAL = 27/29 | |||||
| MDR | 5 (18.52) | [6.30–38.08%] | 13 (44.83) | [26.45–64.31%] | 0.047 |
| CR-GNB | 7 (25.93) | [11.11–46.28%] | 11 (37.93) | [20.69–57.74%] | 0.399 |
| XDR | 4 (14.81) | [4.19–33.73%] | 8 (27.59) | [12.73–47.24%] | 0.334 |
| PDR | 0 (0) | / | 0 (0) | / | / |
| Species: Enterococcus spp. TOTAL = 23/25 | |||||
| VRE | 1 (4.35) | [0.11–21.95%] | 1 (4.00) | [0.10–20.35%] | 1.00 |
| Species: Klebsiella spp. TOTAL = 18/22 | |||||
| MDR | 2 (11.11) | [1.38–34.71%] | 5 (22.73) | [7.82–45.37%] | 0.427 |
| XDR | 3 (16.67) | [3.58–41.42%] | 1 (4.55) | [0.12–22.84%] | 0.333 |
| PDR | 1 (5.55) | [0.14–27.29%] | 9 (40.91) | [20.71–63.65%] | 0.013 |
| ESBL | 8 (44.44) | [21.53–69.24%] | 11 (50) | [28.22–71.78%] | 0.761 |
| CR-GNB | 6 (33.33) | [13.34–59.01%] | 12 (54.55) | [32.21–75.61%] | 0.216 |
| R-AG | 4 (22.22) | [6.41–47.64%] | 11 (50) | [28.22–71.78%] | 0.104 |
| R-FQ | 5 (27.78) | [9.69–53.48%] | 13 (59.09) | [36.35–79.29%] | 0.062 |
| R-SXT | 3 (16.67) | [3.58–41.42%] | 11 (50) | [28.22–71.78%] | 0.045 |
| ABSI | Life Threat | Survival Probability (%) | Fatality Rate (%) |
|---|---|---|---|
| 2–3 | Very low | ≥99% | ≤1% |
| 4–5 | Moderate | 98% | 2% |
| 6–7 | Moderately severe | 80–90% | 10–20% |
| 8–9 | Serious | 50–70% | 30–50% |
| 10–11 | Severe | 20–40% | 60–80% |
| ≥12 | Maximal | ≤10% | ≥90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musuroi, C.; Musuroi, S.-I.; Baditoiu, L.; Crainiceanu, Z.; Muntean, D.; Voinescu, A.; Izmendi, O.; Sirmon, A.; Licker, M. The Profile of Bacterial Infections in a Burn Unit during and after the COVID-19 Pandemic Period. Antibiotics 2024, 13, 823. https://doi.org/10.3390/antibiotics13090823
Musuroi C, Musuroi S-I, Baditoiu L, Crainiceanu Z, Muntean D, Voinescu A, Izmendi O, Sirmon A, Licker M. The Profile of Bacterial Infections in a Burn Unit during and after the COVID-19 Pandemic Period. Antibiotics. 2024; 13(9):823. https://doi.org/10.3390/antibiotics13090823
Chicago/Turabian StyleMusuroi, Corina, Silvia-Ioana Musuroi, Luminita Baditoiu, Zorin Crainiceanu, Delia Muntean, Adela Voinescu, Oana Izmendi, Alexandra Sirmon, and Monica Licker. 2024. "The Profile of Bacterial Infections in a Burn Unit during and after the COVID-19 Pandemic Period" Antibiotics 13, no. 9: 823. https://doi.org/10.3390/antibiotics13090823
APA StyleMusuroi, C., Musuroi, S.-I., Baditoiu, L., Crainiceanu, Z., Muntean, D., Voinescu, A., Izmendi, O., Sirmon, A., & Licker, M. (2024). The Profile of Bacterial Infections in a Burn Unit during and after the COVID-19 Pandemic Period. Antibiotics, 13(9), 823. https://doi.org/10.3390/antibiotics13090823








