Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Hope, W.W.; Roberts, J.A. Applying Pharmacokinetic/Pharmacodynamic Principles in Critically Ill Patients: Optimizing Efficacy and Reducing Resistance Development. Semin. Respir. Crit. Care Med. 2015, 36, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Dhaese, S.; Van Vooren, S.; Boelens, J.; De Waele, J. Therapeutic drug monitoring of β-lactam antibiotics in the ICU. Expert Rev. Anti-infective Ther. 2020, 18, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.C.; Alfaro, L.A.; Reinoso, J.C.; Mestre, M.J.G.; Rodríguez-Gascón, A. Análisis Farmacocinético-Farmacodinámico en Microbiología: Herramienta para Evaluar el Tratamiento Antimicrobiano Enfermedades Infecciosas y Microbiologia Clinica; Elsevier Doyma: Amsterdam, The Netherlands, 2015; Volume 33, pp. 48–57. [Google Scholar]
- Rizk, M.L.; Bhavnani, S.M.; Drusano, G.; Dane, A.; Eakin, A.E.; Guina, T.; Jang, S.H.; Tomayko, J.F.; Wang, J.; Zhuang, L.; et al. Considerations for Dose Selection and Clinical Pharmacokinetics/Pharmacodynamics for the Development of Antibacterial Agents. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Scaglione, F.; Paraboni, L. Influence of pharmacokinetics/pharmacodynamics of antibacterials in their dosing regimen selection. Expert Rev. Anti-infective Ther. 2006, 4, 479–490. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Antimicrobial Resistance: Pharmacokinetics-Pharmacodynamics of Antimicrobial Therapy: It’s Not Just for Mice Anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef]
- Scharf, C.; Liebchen, U.; Paal, M.; Taubert, M.; Vogeser, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J. Intensive Care 2020, 8, 86. [Google Scholar] [CrossRef]
- Tängdén, T.; Martín, V.R.; Felton, T.W.; Nielsen, E.I.; Marchand, S.; Brüggemann, R.J.; Bulitta, J.B.; Bassetti, M.; Theuretzbacher, U.; Tsuji, B.T.; et al. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017, 43, 1021–1032. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.-H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper#. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with be-ta-lactam antibiotics in critically ill patients—Guidelines from the French Society of Pharmacology and Therapeutics (Société Fran-çaise de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 2019, 23, 1–20. [Google Scholar]
- Stašek, J.; Keller, F.; Kočí, V.; Klučka, J.; Klabusayová, E.; Wiewiorka, O.; Strašilová, Z.; Beňovská, M.; Škardová, M.; Maláska, J. Update on Therapeutic Drug Monitoring of Beta-Lactam Antibiotics in Critically Ill Patients—A Narrative Review. Antibiotics 2023, 12, 568. [Google Scholar] [CrossRef]
- Lin, B.; Hu, Y.; Xu, P.; Xu, T.; Chen, C.; He, L.; Zhou, M.; Chen, Z.; Zhang, C.; Yu, X.; et al. Expert consensus statement on therapeutic drug monitoring and individuali-zation of linezolid. Front. Public Health 2022, 10, 967311. [Google Scholar] [CrossRef]
- Wong, G.; Taccone, F.; Villois, P.; Scheetz, M.H.; Rhodes, N.J.; Briscoe, S.; McWhinney, B.; Nunez-Nunez, M.; Ungerer, J.; Lipman, J.; et al. β-Lactam pharmacodynamics in Gram-negative bloodstream infections in the critically ill. J. Antimicrob. Chemother. 2019, 75, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P.; Furlanut, M. Antimicrobial Therapy in Critically Ill Patients A Review of Pathophysiological Conditions Responsible for Altered Disposition and Pharmacokinetic Variability. Clin. Pharmacokinet. 2005, 44, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Paraboni, L. Pharmacokinetics/pharmacodynamics of antibacterials in the Intensive Care Unit: Setting appropriate dosing regimens. Int. J. Antimicrob. Agents 2008, 32, 294–301.e7. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.-A. Pharmacokinetics–pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Nuvials, X.; Palomar, M.; Masclans, J.R.; Rello, J. Appropriateness is Critical. Crit. Care Clin. 2011, 27, 35–51. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient — Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Cotta, M.O.; Roberts, J.A.; Lipman, J. Antibiotic Dose Optimization in Critically Ill Patients. Med. Intensive 2015, 39, 563–572. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The Effects of Hypoalbuminaemia on Optimizing Antibacterial Dosing in Critically Ill Patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef]
- Contejean, A.; Maillard, A.; Canouï, E.; Kernéis, S.; Fantin, B.; Bouscary, D.; Parize, P.; Garcia-Vidal, C.; Charlier, C. Advances in antibacterial treatment of adults with high-risk febrile neutropenia. J. Antimicrob. Chemother. 2023, 78, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Alobaid, A.S.; Hites, M.; Lipman, J.; Taccone, F.S.; Roberts, J.A. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: A structured review. Int. J. Antimicrob. Agents 2016, 47, 259–268. [Google Scholar] [CrossRef]
- Miglis, C.; Rhodes, N.J.; Kuti, J.L.; Nicolau, D.P.; Van Wart, S.A.; Scheetz, M.H. Defining the impact of severity of illness on time above the MIC threshold for cefepime in Gram-negative bacteraemia: A ‘Goldilocks’ window. Int. J. Antimicrob. Agents 2017, 50, 487–490. [Google Scholar] [CrossRef]
- Ewoldt, T.M.; Abdulla, A.; Hunfeld, N.; Li, L.; Smeets, T.J.; Gommers, D.; Koch, B.C.; Endeman, H. The impact of sepsis on hepatic drug metabolism in critically ill patients: A narrative review. Expert Opin. Drug Metab. Toxicol. 2022, 18, 413–421. [Google Scholar] [CrossRef]
- Pistolesi, V.; Morabito, S.; Mario, F.; Di Regolisti, G.; Cantarelli, C.; Fiaccadori, E. A Guide to Understanding Antimicrobial Drug Dosing in Critically Ill Patients on Renal Replacement Therapy [Internet]. 2019. Available online: http://aac.asm.org/ (accessed on 18 July 2024).
- Li, Z.; Bai, J.; Wen, A.; Shen, S.; Duan, M.; Li, X. Pharmacokinetic and Pharmacodynamic Analysis of Critically Ill Patients Under-going Continuous Renal Replacement Therapy with Imipenem. Clin. Ther. 2020, 42, 1564–1577.e8. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Peluso, L.; Taccone, F.S.; Hites, M. The impact of continuous renal replacement therapy on antibiotic pharmacokinetics in critically ill patients. Expert Opin. Drug Metab. Toxicol. 2021, 17, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Pea, F. Antimicrobial Dose Reduction in Continuous Renal Replacement Therapy: Myth or Real Need? A Practical Ap-proach for Guiding Dose Optimization of Novel Antibiotics. Clin. Pharmacokinet. 2021, 60, 1271–1289. [Google Scholar]
- Jang, S.M.; Lewis, S.J.; Rhie, S.J. Optimal antipseudomonal β-lactam drug dosing recommendations in critically-ill Asian patients receiving CRRT. J. Crit. Care 2022, 72, 154172. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Z.; Sun, L.; Zhang, X.; Hou, G.; Han, Q.; Li, X.; Liu, G.; Gao, Y.; Ye, M.; et al. Pharmacokinetics and Pharmacodynamics of Linezolid in Patients With Sepsis Receiving Continuous Venovenous Hemofiltration and Extended Daily Hemofiltration. J. Infect. Dis. 2020, 221, S279–S287. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Witebolle, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Bréchot, N.; Trouillet, J.-L.; Chastre, J. Antibiotic stewardship in the intensive care unit. Crit. Care 2014, 18, 480. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Lipman, J.; Akova, M.; Bassetti, M.; Dimopoulos, G.; Kaukonen, M.; Koulenti, D.; Martin, C.; Montravers, P.; Rello, J.; et al. Risk factors for target non-attainment during empirical treatment with β-lactam antibiotics in critically ill patients. Intensive Care Med. 2014, 40, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current ß-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Charmillon, A.; Novy, E.; Agrinier, N.; Leone, M.; Kimmoun, A.; Levy, B.; Demoré, B.; Dellamonica, J.; Pulcini, C. The ANTIBIOPERF study: A nationwide cross-sectional survey about practices for β-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin. Microbiol. Infect. 2016, 22, 625–631. [Google Scholar] [CrossRef]
- Williams, P.G.; Tabah, A.; Cotta, M.O.; Sandaradura, I.; Kanji, S.; Scheetz, M.H.; Imani, S.; Elhadi, M.; Luque-Pardos, S.; Schellack, N.; et al. International survey of antibiotic dosing and monitoring in adult intensive care units. Crit. Care 2023, 27, 241. [Google Scholar] [CrossRef]
- Rao, G.G.; Konicki, R.; Cattaneo, D.; Alffenaar, J.-W.; Marriott, D.J.E.; Neely, M.; On behalf of the IATDMCT Antimicrobial Scientific Committee. Therapeutic Drug Monitoring Can Improve Linezolid Dosing Regimens in Current Clinical Practice: A Review of Linezolid Pharmacokinetics and Pharmacodynamics. Ther. Drug Monit. 2020, 42, 83–92. [Google Scholar] [CrossRef]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2013, 40, 380–387. [Google Scholar] [CrossRef]
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem use and therapeutic drug monitoring in clinical practice: A literature review. J. Clin. Pharm. Ther. 2021, 46, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Wicha, S.G.; Märtson, A.; Nielsen, E.I.; Koch, B.C.; Friberg, L.E.; Alffenaar, J.; Minichmayr, I.K. The International Society of Anti-Infective Pharmacology (ISAP), the PK/PD study group of the European Society of Clinical Microbiology, Infectious Diseases (EPASG) From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef]
- Osorio, C.; Garzón, L.; Jaimes, D.; Silva, E.; Bustos, R.-H. Impact on Antibiotic Resistance, Therapeutic Success, and Control of Side Effects in Therapeutic Drug Monitoring (TDM) of Daptomycin: A Scoping Review. Antibiotics 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Russo, A.; Cassetta, M.I.; Lappa, A.; Tritapepe, L.; D'Ettorre, G.; Fallani, S.; Novelli, A.; Venditti, M. Variability of pharmacokinetic parameters in patients receiving different dosages of daptomycin: Is therapeutic drug monitoring necessary? J. Infect. Chemother. 2013, 19, 732–739. [Google Scholar] [CrossRef]
- Galar, A.; Valerio, M.; Muñoz, P.; Alcalá, L.; García-González, X.; Burillo, A.; Sanjurjo, M.; Grau, S.; Bouza, E. Systematic Therapeutic Drug Monitoring for Linezolid: Variability and Clinical Impact. Antimicrob. Agents Chemother. 2017, 61, e00687-17. [Google Scholar] [CrossRef] [PubMed]
- Galar, A.; Muñoz, P.; Valerio, M.; Cercenado, E.; García-González, X.; Burillo, A.; Sánchez-Somolinos, M.; Juárez, M.; Verde, E.; Bouza, E. Current use of daptomycin and systematic therapeutic drug monitoring: Clinical experience in a tertiary care institution. Int. J. Antimicrob. Agents 2018, 53, 40–48. [Google Scholar] [CrossRef]
- Takahashi, N.; Kondo, Y.; Kubo, K.; Egi, M.; Kano, K.-I.; Ohshima, Y.; Nakada, T.-A. Efficacy of therapeutic drug monitoring-based antibiotic regimen in critically ill patients: A systematic review and meta-analysis of randomized controlled trials. J. Intensive Care 2023, 11, 48. [Google Scholar] [CrossRef]
- Mangalore, R.P.; Ashok, A.; Lee, S.J.; Romero, L.; Peel, T.N.; A Udy, A.; Peleg, A.Y. Beta-Lactam Antibiotic Therapeutic Drug Monitoring in Critically Ill Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 1848–1860. [Google Scholar] [CrossRef]
- Al-Shaer, M.H.; Rubido, E.; Cherabuddi, K.; Venugopalan, V.; Klinker, K.; Peloquin, C. Early therapeutic monitoring of β-lactams and associated therapy outcomes in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 3644–3651. [Google Scholar] [CrossRef]
- Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Muller, A.E.; de Winter, B.C.M.; Hunfeld, N.G.M.; Purmer, I.M.; van Vliet, P.; Wils, E.-J.; Haringman, J.; et al. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: A multicentre randomised clinical trial. Intensive Care Med. 2022, 48, 1760–1771. [Google Scholar] [CrossRef]
- on behalf of the TARGET Study Group; Hagel, S.; Fiedler, S.; Hohn, A.; Brinkmann, A.; Frey, O.R.; Hoyer, H.; Schlattmann, P.; Kiehntopf, M.; Roberts, J.A.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin/tazobactam to improve outcome in patients with sepsis (TARGET): A prospective, multi-centre, randomised controlled trial. Trials 2019, 20, 330. [Google Scholar] [CrossRef]
- Hansel, J.; Mannan, F.; Robey, R.; Kumarendran, M.; Bladon, S.; Mathioudakis, A.G.; Ogungbenro, K.; Dark, P.; Felton, T.W. Covariates in population pharmacokinetic studies of critically ill adults receiving β-lactam antimicrobials: A systematic review and narrative synthesis. JAC-Antimicrobial Resist. 2023, 6, dlae030. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Pea, F. Impact of attaining aggressive vs. conservative PK/PD target on the clinical efficacy of beta-lactams for the treatment of Gram-negative infections in the critically ill patients: A systematic review and meta-analysis. Crit. Care 2024, 28, 123. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.M.; Roberts, J.A.; Lipman, J. Antimicrobial Pharmacokinetic and Pharmacodynamic Issues in the Critically Ill with Severe Sepsis and Septic Shock. Crit. Care Clin. 2011, 27, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hites, M.; Taccone, F.S. Optimization of antibiotic therapy in the obese, critically ill patient. Reanim. 2015, 24, 278–294. [Google Scholar] [CrossRef]
- Pea, F.; Furlanut, M.; Negri, C.; Pavan, F.; Crapis, M.; Cristini, F.; Viale, P. Prospectively Validated Dosing Nomograms for Maximizing the Pharmacodynamics of Vancomycin Administered by Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2009, 53, 1863–1867. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kanji, S.; Hayes, M.; Ling, A.; Shamseer, L.; Chant, C.; Edwards, D.J.; Edwards, S.; Ensom, M.H.H.; Foster, D.R.; Hardy, B.; et al. Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clin. Pharmacokinet. 2015, 54, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, T.; Cepeda, J.A.; Shulman, R.; Aarons, L.; Nalda-Molina, R.; Tobin, C.; MacGowan, A.; Shaw, S.; Kibbler, C.; Singer, M.; et al. Pharmacokinetic studies of linezolid and teicoplanin in the critically ill. J. Antimicrob. Chemother. 2005, 55, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, O.; Kumar, V.; Katterwe, D.; Majcher-Peszynska, J.; Drewelow, B.; Derendorf, H.; Welte, T. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: Pharmacokinetics with special consideration of free-drug concentration. J. Antimicrob. Chemother. 2006, 59, 277–284. [Google Scholar] [CrossRef]
- Roos, J.F.; Lipman, J.; Kirkpatrick, C.M.J. Population pharmacokinetics and pharmacodynamics of cefpirome in critically ill patients against Gram-negative bacteria. Intensive Care Med. 2007, 33, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Field, J.; Visser, A.; Whitbread, R.; Tallot, M.; Lipman, J.; Kirkpatrick, C.M.J. Using Population Pharmacokinetics To Determine Gentamicin Dosing during Extended Daily Diafiltration in Critically Ill Patients with Acute Kidney Injury. Antimicrob. Agents Chemother. 2010, 54, 3635–3640. [Google Scholar] [CrossRef]
- Asin-Prieto, E.; Rodriguez-Gascon, A.; Troconiz, I.F.; Soraluce, A.; Maynar, J.; Sanchez-Izquierdo, J.A.; Isla, A. Population pharmacokinetics of piperacillin and tazobactam in critically ill patients undergoing continuous renal replacement therapy: Application to pharmacokinetic/pharmacodynamic analysis. J. Antimicrob. Chemother. 2013, 69, 180–189. [Google Scholar] [CrossRef]
- Escobar, L.; Andresen, M.; Downey, P.; Gai, M.N.; Regueira, T.; Bórquez, T.; Lipman, J.; Roberts, J.A. Population pharmacokinetics and dose simulation of vancomycin in critically ill patients during high-volume haemofiltration. Int. J. Antimicrob. Agents 2014, 44, 163–167. [Google Scholar] [CrossRef]
- Couffignal, C.; Pajot, O.; Laouénan, C.; Burdet, C.; Foucrier, A.; Wolff, M.; Armand-Lefevre, L.; Mentré, F.; Massias, L. Population pharmacokinetics of imipenem in critically ill patients with suspected ventilator-associated pneumonia and evaluation of dosage regimens. Br. J. Clin. Pharmacol. 2014, 78, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Noe, M.; Roberts, J.A.; Stove, V.; Verstraete, A.G.; Lipman, J.; De Waele, J.J. Population pharmacokinetics and dosing simulations of cefuroxime in critically ill patients: Non-standard dosing approaches are required to achieve therapeutic exposures. J. Antimicrob. Chemother. 2014, 69, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Zoller, M.; Maier, B.; Hornuss, C.; Neugebauer, C.; Döbbeler, G.; Nagel, D.; Holdt, L.M.; Bruegel, M.; Weig, T.; Grabein, B.; et al. Variability of linezolid concentrations after standard dosing in critically ill patients: A prospective observational study. Crit. Care 2014, 18, R148. [Google Scholar] [CrossRef]
- Luque, S.; Grau, S.; Alvarez-Lerma, F.; Ferrández, O.; Campillo, N.; Horcajada, J.; Basas, M.; Lipman, J.; Roberts, J. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int. J. Antimicrob. Agents 2014, 44, 409–415. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Soy, D.; Llaurado-Serra, M.; Vaquer, S.; Castro, P.; Rodríguez, A.H.; Pontes, C.; Calvo, G.; Torres, A.; Martín-Loeches, I. Meropenem Population Pharmacokinetics in Critically Ill Patients with Septic Shock and Continuous Renal Replacement Therapy: Influence of Residual Diuresis on Dose Requirements. Antimicrob. Agents Chemother. 2015, 59, 5520–5528. [Google Scholar] [CrossRef]
- Kees, M.G.; Minichmayr, I.K.; Moritz, S.; Beck, S.; Wicha, S.G.; Kees, F.; Kloft, C.; Steinke, T. Population pharmacokinetics of meropenem during continuous infusion in surgical ICU patients. J. Clin. Pharmacol. 2015, 56, 307–315. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Rahman, A.N.A.; Mat-Nor, M.-B.; Sulaiman, H.; Wallis, S.C.; Lipman, J.; Roberts, J.A.; Staatz, C.E. Population Pharmacokinetics of Doripenem in Critically Ill Patients with Sepsis in a Malaysian Intensive Care Unit. Antimicrob. Agents Chemother. 2016, 60, 206–214. [Google Scholar] [CrossRef][Green Version]
- Roger, C.; Muller, L.; Wallis, S.C.; Louart, B.; Saissi, G.; Lipman, J.; Lefrant, J.Y.; Roberts, J.A. Population pharmacokinetics of linezolid in critically ill patients on renal replacement therapy: Comparison of equal doses in continuous venovenous haemofiltration and continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 2015, 71, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Cotta, M.O.; Cojutti, P.; Lugano, M.; Della Rocca, G.; Pea, F. Does Critical Illness Change Levofloxacin Pharmacokinetics? Antimicrob. Agents Chemother. 2016, 60, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Martín-Loeches, I.; Llauradó-Serra, M.; Fernández, J.; Vaquer, S.; Rodríguez, A.; Pontes, C.; Calvo, G.; Torres, A.; Soy, D. Piperacillin population pharmacokinetics in critically ill patients with multiple organ dysfunction syndrome receiving continuous venovenous haemodiafiltration: Effect of type of dialysis membrane on dosing requirements. J. Antimicrob. Chemother. 2016, 71, 1651–1659. [Google Scholar] [CrossRef]
- Roger, C.; Wallis, S.C.; Louart, B.; Lefrant, J.-Y.; Lipman, J.; Muller, L.; Roberts, J.A. Comparison of equal doses of continuous venovenous haemofiltration and haemodiafiltration on ciprofloxacin population pharmacokinetics in critically ill patients. J. Antimicrob. Chemother. 2016, 71, 1643–1650. [Google Scholar] [CrossRef]
- Alobaid, A.S.; Wallis, S.C.; Jarrett, P.; Starr, T.; Stuart, J.; Lassig-Smith, M.; Mejia, J.L.O.; Roberts, M.S.; Lipman, J.; Roberts, J.A. Effect of Obesity on the Population Pharmacokinetics of Meropenem in Critically Ill Patients. Antimicrob. Agents Chemother. 2016, 60, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Zoller, M.; Maier, B.; Frechen, S.; Scharf, C.; Holdt, L.-M.; Frey, L.; Vogeser, M.; Fuhr, U.; Zander, J. Predictors of Inadequate Linezolid Concentrations after Standard Dosing in Critically Ill Patients. Antimicrob. Agents Chemother. 2016, 60, 5254–5261. [Google Scholar] [CrossRef]
- Tsai, D.; Stewart, P.; Goud, R.; Gourley, S.; Hewagama, S.; Krishnaswamy, S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Optimising meropenem dosing in critically ill Australian Indigenous patients with severe sepsis. Int. J. Antimicrob. Agents 2016, 48, 542–546. [Google Scholar] [CrossRef]
- Blassmann, U.; Roehr, A.C.; Frey, O.R.; Vetter-Kerkhoff, C.; Thon, N.; Hope, W.; Briegel, J.; Huge, V. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: A prospective observational study. Crit. Care 2016, 20, 343. [Google Scholar] [CrossRef]
- Rahbar, A.J.; Lodise, T.P.; Abraham, P.; Lockwood, A.; Pai, M.P.; Patka, J.; Rabinovich, M.; Curzio, K.; Chester, K.; Williams, B.; et al. Pharmacokinetic and Pharmacodynamic Evaluation of Doripenem in Critically Ill Trauma Patients with Sepsis. Surg. Infect. 2016, 17, 675–682. [Google Scholar] [CrossRef]
- Naik, B.I.; Roger, C.; Ikeda, K.; Todorovic, M.S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Comparative total and unbound pharmacokinetics of cefazolin administered by bolus versus continuous infusion in patients undergoing major surgery: A randomized controlled trial. Br. J. Anaesth. 2017, 118, 876–882. [Google Scholar] [CrossRef]
- Xie, J.; Roberts, J.A.; Alobaid, A.S.; Roger, C.; Wang, Y.; Yang, Q.; Sun, J.; Dong, H.; Wang, X.; Xing, J.; et al. Population Pharmacokinetics of Tigecycline in Critically Ill Patients with Severe Infections. Antimicrob. Agents Chemother. 2017, 61, e00345-17. [Google Scholar] [CrossRef] [PubMed]
- Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Alraish, R.; Wuensch, T.; Kaffarnik, M. Linezolid in liver failure: Exploring the value of the maximal liver function capacity (LiMAx) test in a pharmacokinetic pilot study. Int. J. Antimicrob. Agents 2017, 50, 557–563. [Google Scholar] [CrossRef]
- Sime, F.B.; Hahn, U.; Warner, M.S.; Tiong, I.S.; Roberts, M.S.; Lipman, J.; Peake, S.L.; Roberts, J.A. Using Population Pharmacokinetic Modeling and Monte Carlo Simulations To Determine whether Standard Doses of Piperacillin in Piperacillin-Tazobactam Regimens Are Adequate for the Management of Febrile Neutropenia. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Sjövall, F.; Alobaid, A.S.; Wallis, S.C.; Perner, A.; Lipman, J.; A Roberts, J. Maximally effective dosing regimens of meropenem in patients with septic shock. J. Antimicrob. Chemother. 2017, 73, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.B.-D.; Rypulak, E.; Potręć, B.; Piwowarczyk, P.; Borys, M.; Sysiak, J.; Onichimowski, D.; Raszewski, G.; Czuczwar, M.; Wiczling, P. Population Pharmacokinetics of High-Dose Tigecycline in Patients with Sepsis or Septic Shock. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Braune, S.; König, C.; Roberts, J.A.; Nierhaus, A.; Steinmetz, O.; Baehr, M.; Kluge, S.; Langebrake, C. Pharmacokinetics of meropenem in septic patients on sustained low-efficiency dialysis: A population pharmacokinetic study. Crit. Care 2018, 22, 25. [Google Scholar] [CrossRef]
- Bos, J.C.; Prins, J.M.; Mistício, M.C.; Nunguiane, G.; Lang, C.N.; Beirão, J.C.; A A Mathôt, R.; van Hest, R.M. Pharmacokinetics and pharmacodynamic target attainment of ceftriaxone in adult severely ill sub-Saharan African patients: A population pharmacokinetic modelling study. J. Antimicrob. Chemother. 2018, 73, 1620–1629. [Google Scholar] [CrossRef]
- Hanberg, P.; Öbrink-Hansen, K.; Thorsted, A.; Bue, M.; Tøttrup, M.; Friberg, L.E.; Hardlei, T.F.; Søballe, K.; Gjedsted, J. Population Pharmacokinetics of Meropenem in Plasma and Subcutis from Patients on Extracorporeal Membrane Oxygenation Treatment. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Soraluce, A.; Asín-Prieto, E.; Rodríguez-Gascón, A.; Barrasa, H.; Maynar, J.; Carcelero, E.; Soy, D.; Isla, A. Population pharmacokinetics of daptomycin in critically ill patients. Int. J. Antimicrob. Agents 2018, 52, 158–165. [Google Scholar] [CrossRef]
- Kanji, S.; Roberts, J.A.; Xie, J.; Alobaid, A.; Zelenitsky, S.; Hiremath, S.; Zhang, G.; Watpool, I.; Porteous, R.; Patel, R. Piperacillin Population Pharmacokinetics in Critically Ill Adults During Sustained Low-Efficiency Dialysis. Ann. Pharmacother. 2018, 52, 965–973. [Google Scholar] [CrossRef]
- Fournier, A.; Goutelle, S.; Que, Y.-A.; Eggimann, P.; Pantet, O.; Sadeghipour, F.; Voirol, P.; Csajka, C. Population Pharmacokinetic Study of Amoxicillin-Treated Burn Patients Hospitalized at a Swiss Tertiary-Care Center. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Tsai, D.; Stewart, P.C.; Hewagama, S.; Krishnaswamy, S.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Optimised dosing of vancomycin in critically ill Indigenous Australian patients with severe sepsis. Anaesth. Intensive Care 2018, 46, 374–380. [Google Scholar] [CrossRef]
- Turner, R.B.; Kojiro, K.; Won, R.; Chang, E.; Chan, D.; Elbarbry, F. Prospective evaluation of vancomycin pharmacokinetics in a heterogeneous critically ill population. Diagn. Microbiol. Infect. Dis. 2018, 92, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.E.; Smith, C.L.; Scharmen, A.; Kidd, J.M.; Cooper, C.; Kuti, J.; Mitra, S.; Nicolau, D.P.; Havlichek, D.H. Pharmacokinetic and Pharmacodynamic Analysis of Ceftazidime/Avibactam in Critically Ill Patients. Surg. Infect. 2019, 20, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Leuppi-Taegtmeyer, A.B.; Decosterd, L.; Osthoff, M.; Mueller, N.J.; Buclin, T.; Corti, N. Multicenter Population Pharmacokinetic Study of Colistimethate Sodium and Colistin Dosed as in Normal Renal Function in Patients on Continuous Renal Replacement Therapy. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Sukarnjanaset, W.; Jaruratanasirikul, S.; Wattanavijitkul, T. Population pharmacokinetics and pharmacodynamics of piperacillin in critically ill patients during the early phase of sepsis. J. Pharmacokinet. Pharmacodyn. 2019, 46, 251–261. [Google Scholar] [CrossRef]
- Zamora, A.P.; Roig, R.J.; Badosa, E.L.; Riera, J.S.; Fernández, X.L.P.; Campos, P.C.; Bonin, R.R.; Ramos, P.A.; Quintano, F.T.; Martinez, E.S.; et al. Optimized meropenem dosage regimens using a pharmacokinetic/pharmacodynamic population approach in patients undergoing continuous venovenous haemodiafiltration with high-adsorbent membrane. J. Antimicrob. Chemother. 2019, 74, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Population Pharmacokinetics of Unbound Ceftolozane and Tazobactam in Critically Ill Patients without Renal Dysfunction. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Kanji, S.; Roberts, J.A.; Xie, J.; Zelenitsky, S.; Hiremath, S.; Zhang, G.; Watpool, I.; Porteous, R.; Patel, R. Vancomycin Population Pharmacokinetics in Critically Ill Adults During Sustained Low-Efficiency Dialysis. Clin. Pharmacokinet. 2020, 59, 327–334. [Google Scholar] [CrossRef]
- Sime, F.B.; Lassig-Smith, M.; Starr, T.; Stuart, J.; Pandey, S.; Parker, S.L.; Wallis, S.C.; Lipman, J.; Roberts, J.A. A Population Pharmacokinetic Model-Guided Evaluation of Ceftolozane-Tazobactam Dosing in Critically Ill Patients Undergoing Continuous Venovenous Hemodiafiltration. Antimicrob. Agents Chemother. 2019, 64. [Google Scholar] [CrossRef]
- Kovacevic, T.; Miljkovic, B.; Kovacevic, P.; Dragic, S.; Momcicevic, D.; Avram, S.; Jovanovic, M.; Vucicevic, K. Population pharmacokinetic model of Vancomycin based on therapeutic drug monitoring data in critically ill septic patients. J. Crit. Care 2019, 55, 116–121. [Google Scholar] [CrossRef]
- Kalaria, S.N.; Gopalakrishnan, M.; Heil, E.L. A Population Pharmacokinetics and Pharmacodynamic Approach To Optimize Tazobactam Activity in Critically Ill Patients. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Masich, A.M.; Kalaria, S.N.; Gonzales, J.P.; Heil, E.L.; Tata, A.L.; Claeys, K.C.; Patel, D.; Gopalakrishnan, M. Vancomycin Pharmacokinetics in Obese Patients with Sepsis or Septic Shock. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 211–220. [Google Scholar] [CrossRef]
- Bue, M.; Sou, T.; Okkels, A.S.L.; Hanberg, P.; Thorsted, A.; Friberg, L.E.; Andersson, T.L.; Öbrink-Hansen, K.; Christensen, S. Population pharmacokinetics of piperacillin in plasma and subcutaneous tissue in patients on continuous renal replacement therapy. Int. J. Infect. Dis. 2020, 92, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Niibe, Y.B.; Suzuki, T.B.; Yamazaki, S.M.; Suzuki, T.; Takahashi, N.; Hattori, N.; Nakada, T.-A.; Oda, S.; Ishii, I. Population Pharmacokinetic Analysis of Meropenem in Critically Ill Patients With Acute Kidney Injury Treated With Continuous Hemodiafiltration. Ther. Drug Monit. 2020, 42, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Onichimowski, D.; Będźkowska, A.; Ziółkowski, H.; Jaroszewski, J.; Borys, M.; Czuczwar, M.; Wiczling, P. Population pharmacokinetics of standard-dose meropenem in critically ill patients on continuous renal replacement therapy: A prospective observational trial. Pharmacol. Rep. 2020, 72, 719–729. [Google Scholar] [CrossRef]
- Smit, C.; van Schip, A.M.; A van Dongen, E.P.; Brüggemann, R.J.M.; Becker, M.L.; Knibbe, C.A.J. Dose recommendations for gentamicin in the real-world obese population with varying body weight and renal (dys)function. J. Antimicrob. Chemother. 2020, 75, 3286–3292. [Google Scholar] [CrossRef]
- Blackman, A.L.; Jarugula, P.; Nicolau, D.P.; Chui, S.H.; Joshi, M.; Heil, E.L.; Gopalakrishnan, M. Evaluation of Linezolid Pharmacokinetics in Critically Ill Obese Patients with Severe Skin and Soft Tissue Infections. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Bastida, C.; Llauradó-Serra, M.; Csajka, C.; Rodríguez, A.; Badia, J.R.; Martín-Loeches, I.; Soy, D. Once-daily 1 g ceftriaxone optimizes exposure in patients with septic shock and hypoalbuminemia receiving continuous veno-venous hemodiafiltration. Eur. J. Clin. Pharmacol. 2021, 77, 1169–1180. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yao, F.; Chen, S.; Hou, Y.; Zheng, Z.; Luo, J.; Qiu, B.; Li, Z.; Wang, Y.; et al. Pharmacokinetics of Linezolid Dose Adjustment for Creatinine Clearance in Critically Ill Patients: A Multicenter, Prospective, Open-Label, Observational Study. Drug Des. Dev. Ther. 2021, ume 15, 2129–2141. [Google Scholar] [CrossRef]
- De Winter, S.; van Hest, R.; Dreesen, E.; Annaert, P.; Wauters, J.; Meersseman, W.; Eede, N.V.D.; Desmet, S.; Verelst, S.; Vanbrabant, P.; et al. Quantification and Explanation of the Variability of First-Dose Amikacin Concentrations in Critically Ill Patients Admitted to the Emergency Department: A Population Pharmacokinetic Analysis. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 653–663. [Google Scholar] [CrossRef]
- Cheng, V.; Abdul-Aziz, M.H.; Burrows, F.; Buscher, H.; Corley, A.; Diehl, A.; Jakob, S.M.; Levkovich, B.J.; Pellegrino, V.; Que, Y.-A.; et al. Population pharmacokinetics of cefepime in critically ill patients receiving extracorporeal membrane oxygenation (an ASAP ECMO study). Int. J. Antimicrob. Agents 2021, 58, 106466. [Google Scholar] [CrossRef]
- Lan, J.; Wu, Z.; Wang, X.; Wang, Y.; Yao, F.; Zhao, B.-X.; Wang, Y.; Chen, J.; Chen, C. Population Pharmacokinetics Analysis and Dosing Simulations Of Meropenem in Critically Ill Patients with Pulmonary Infection. J. Pharm. Sci. 2022, 111, 1833–1842. [Google Scholar] [CrossRef]
- Dreesen, E.; Gijsen, M.; Elkayal, O.; Annaert, P.; Debaveye, Y.; Wauters, J.; O Karlsson, M.; Spriet, I. Ceftriaxone dosing based on the predicted probability of augmented renal clearance in critically ill patients with pneumonia. J. Antimicrob. Chemother. 2022, 77, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Meenks, S.D.; le Noble, J.L.; Foudraine, N.A.; de Vries, F.; Neef, K.N.; Janssen, P.K. Population pharmacokinetics of unbound ceftriaxone in a critically ill population. Int. J. Clin. Pharmacol. Ther. 2022, 60, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, M.H.; Barlow, B.; Maranchick, N.; Moser, M.; Gramss, L.; Burgmann, H.; Al Jalali, V.; Wölfl-Duchek, M.; Jäger, W.; Poschner, S.; et al. Meropenem Population Pharmacokinetics and Simulations in Plasma, Cerebrospinal Fluid, and Brain Tissue. Antimicrob. Agents Chemother. 2022, 66, e0043822. [Google Scholar] [CrossRef] [PubMed]
- Kumta, N.; Heffernan, A.J.; Cotta, M.O.; Wallis, S.C.; Livermore, A.; Starr, T.; Wong, W.T.; Joynt, G.M.; Lipman, J.; Roberts, J.A. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Meropenem in Neurocritical Care Patients: A Prospective Two-Center Study. Antimicrob. Agents Chemother. 2022, 66, e0014222. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.B.; Ohbayashi, M.; Okada, A.; Kohyama, N.; Tamatsukuri, T.; Inoue, H.; Kato, A.; Kotani, T.; Sagara, H.; Dohi, K.; et al. Population Pharmacokinetic Model and Dosing Simulation of Meropenem Using Measured Creatinine Clearance for Patients with Sepsis. Ther. Drug Monit. 2022, 45, 392–399. [Google Scholar] [CrossRef]
- Meenks, S.D.; Punt, N.; le Noble, J.L.M.L.; Foudraine, N.A.; Neef, K.; Janssen, P.K.C. Target attainment and population pharmacokinetics of flucloxacillin in critically ill patients: A multicenter study. Crit. Care 2023, 27, 82. [Google Scholar] [CrossRef]
- Tang, T.; Li, Y.; Xu, P.; Zhong, Y.; Yang, M.; Ma, W.; Xiang, D.; Zhang, B.; Zhou, Y. Optimization of polymyxin B regimens for the treatment of carbapenem-resistant organism nosocomial pneumonia: A real-world prospective study. Crit. Care 2023, 27, 164. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, F.; Chen, S.; Ouyang, X.; Lan, J.; Wu, Z.; Wang, Y.; Chen, J.; Wang, X.; Chen, C. Optimal Teicoplanin Dosage Regimens in Critically Ill Patients: Population Pharmacokinetics and Dosing Simulations Based on Renal Function and Infection Type. Drug Des. Dev. Ther. 2023, ume 17, 2259–2271. [Google Scholar] [CrossRef]
- Barreto, E.F.; Chang, J.; Rule, A.D.; Mara, K.C.; Meade, L.A.; Paul, J.; Jannetto, P.J.; Athreya, A.P.; Scheetz, M.H.; for the BLOOM Study Group. Population pharmacokinetic model of cefepime for critically ill adults: A comparative assessment of eGFR equations. Antimicrob. Agents Chemother. 2023, 67, e0081023. [Google Scholar] [CrossRef] [PubMed]
- Facca, B.; Frame, B.; Triesenberg, S. Population Pharmacokinetics of Ceftizoxime Administered by Continuous Infusion in Clinically Ill Adult Patients. Antimicrob. Agents Chemother. 1998, 42, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Frame, B.C.; Facca, B.F.; Nicolau, D.P.; Triesenberg, S.N. Population Pharmacokinetics of Continuous Infusion Ceftazidime. Clin. Pharmacokinet. 1999, 37, 343–350. [Google Scholar] [CrossRef]
- Dailly, E.; Arnould, J.; Fraissinet, F.; Naux, E.; de la Bouralière, M.L.; Bouquié, R.; Deslandes, G.; Jolliet, P.; Le Floch, R. Pharmacokinetics of ertapenem in burns patients. Int. J. Antimicrob. Agents 2013, 42, 48–52. [Google Scholar] [CrossRef]
- Beumier, M.; Roberts, J.A.; Kabtouri, H.; Hites, M.; Cotton, F.; Wolff, F.; Lipman, J.; Jacobs, F.; Vincent, J.-L.; Taccone, F.S. A new regimen for continuous infusion of vancomycin during continuous renal replacement therapy. J. Antimicrob. Chemother. 2013, 68, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Öbrink-Hansen, K.; Juul, R.V.; Storgaard, M.; Thomsen, M.K.; Hardlei, T.F.; Brock, B.; Kreilgaard, M.; Gjedsted, J. Population Pharmacokinetics of Piperacillin in the Early Phase of Septic Shock: Does Standard Dosing Result in Therapeutic Plasma Concentrations? Antimicrob. Agents Chemother. 2015, 59, 7018–7026. [Google Scholar] [CrossRef]
- Lin, W.-W.; Wu, W.; Jiao, Z.; Lin, R.-F.; Jiang, C.-Z.; Huang, P.-F.; Liu, Y.-W.; Wang, C.-L. Population pharmacokinetics of vancomycin in adult Chinese patients with post-craniotomy meningitis and its application in individualised dosage regimens. Eur. J. Clin. Pharmacol. 2015, 72, 29–37. [Google Scholar] [CrossRef]
- Ide, T.; Takesue, Y.; Ikawa, K.; Morikawa, N.; Ueda, T.; Takahashi, Y.; Nakajima, K.; Takeda, K.; Nishi, S. Population pharmacokinetics/pharmacodynamics of linezolid in sepsis patients with and without continuous renal replacement therapy. Int. J. Antimicrob. Agents 2018, 51, 745–751. [Google Scholar] [CrossRef]
- Kang, S.; Jang, J.Y.; Hahn, J.; Kim, D.; Lee, J.Y.; Min, K.L.; Yang, S.; Wi, J.; Chang, M.J. Dose Optimization of Cefpirome Based on Population Pharmacokinetics and Target Attainment during Extracorporeal Membrane Oxygenation. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Grensemann, J.; Busse, D.; König, C.; Roedl, K.; Jäger, W.; Jarczak, D.; Iwersen-Bergmann, S.; Manthey, C.; Kluge, S.; Kloft, C.; et al. Acute-on-chronic liver failure alters meropenem pharmacokinetics in critically ill patients with continuous hemodialysis: An observational study. Ann. Intensive Care 2020, 10, 48. [Google Scholar] [CrossRef]
- Liu, D.; Chen, W.; Wang, Q.; Li, M.; Zhang, Z.; Cui, G.; Li, P.; Zhang, X.; Ma, Y.; Zhan, Q.; et al. Influence of venovenous extracorporeal membrane oxygenation on pharmacokinetics of vancomycin in lung transplant recipients. J. Clin. Pharm. Ther. 2020, 45, 1066–1075. [Google Scholar] [CrossRef]
- Ruiz, J.; Ramirez, P.; Villarreal, E.; Gordon, M.; Sánchez, M.; Martín, M.; Castellanos-Ortega. Effect of pharmacokinetic/pharmacodynamic ratio on tigecycline clinical response and toxicity in critically ill patients with multidrug-resistant Gram-negative infections. SAGE Open Med. 2020, 8. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, D.-Y.; Zhu, Y.-W.; Jiang, Z.-L.; Cui, K.; Zhang, S.; Chen, L.-H. Population pharmacokinetic modeling and clinical application of vancomycin in Chinese patients hospitalized in intensive care units. Sci. Rep. 2021, 11, 2670. [Google Scholar] [CrossRef]
- Šíma, M.; Michaličková, D.; Ryšánek, P.; Cihlářová, P.; Kuchař, M.; Lžičařová, D.; Beroušek, J.; Hartinger, J.M.; Vymazal, T.; Slanař, O. No Time Dependence of Ciprofloxacin Pharmacokinetics in Critically Ill Adults: Comparison of Individual and Population Analyses. Pharmaceutics 2021, 13, 1156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-C.; Zou, Y.; Xiao, Y.-W.; Wang, F.; Zhang, B.-K.; Xiang, D.-X.; Yu, F.; Luo, H.; Sandaradura, I.; Yan, M. Does Prolonged Infusion Time Really Improve the Efficacy of Meropenem Therapy? A Prospective Study in Critically Ill Patients. Infect. Dis. Ther. 2021, 11, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Alsultan, A.; Dasuqi, S.A.; Aljamaan, F.; Omran, R.A.; Syed, S.A.; AlJaloud, T.; AlAhmadi, A.; Alqahtani, S.; Hamad, M.A. Pharmacokinetics of meropenem in critically ill patients in Saudi Arabia. Saudi Pharm. J. 2021, 29, 1272–1277. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, X.; Zhao, S.; He, N.; Zhai, S.; Ge, Q. Dose Optimization of Vancomycin for Critically Ill Patients Undergoing CVVH: A Prospective Population PK/PD Analysis. Antibiotics 2021, 10, 1392. [Google Scholar] [CrossRef]
- Busse, D.; Simon, P.; Schmitt, L.; Petroff, D.; Dorn, C.; Dietrich, A.; Zeitlinger, M.; Huisinga, W.; Michelet, R.; Wrigge, H.; et al. Comparative Plasma and Interstitial Tissue Fluid Pharmacokinetics of Meropenem Demonstrate the Need for Increasing Dose and Infusion Duration in Obese and Non-obese Patients. Clin. Pharmacokinet. 2021, 61, 655–672. [Google Scholar] [CrossRef]
- Farkas, A.; Oikonomou, K.; Ghanbar, M.; Villasurda, P.; Varghese, J.; Lipman, J.; Sassine, J.; Ranganathan, D.; Roberts, J.A. Population Pharmacokinetics of Intraperitoneal Gentamicin and the Impact of Varying Dwell Times on Pharmacodynamic Target Attainment in Patients with Acute Peritonitis Undergoing Peritoneal Dialysis. Antimicrob. Agents Chemother. 2022, 66, e0167921. [Google Scholar] [CrossRef]
- Hahn, J.; Min, K.L.; Kang, S.; Yang, S.; Park, M.S.; Wi, J.; Chang, M.J. Population Pharmacokinetics and Dosing Optimization of Piperacillin-Tazobactam in Critically Ill Patients on Extracorporeal Membrane Oxygenation and the Influence of Concomitant Renal Replacement Therapy. Microbiol. Spectr. 2021, 9, e0063321. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, M.; Elkayal, O.; Annaert, P.; Van Daele, R.; Meersseman, P.; Debaveye, Y.; Wauters, J.; Dreesen, E.; Spriet, I. Meropenem Target Attainment and Population Pharmacokinetics in Critically Ill Septic Patients with Preserved or Increased Renal Function. Infect. Drug Resist. 2022, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pressiat, C.; Kudela, A.; De Roux, Q.; Khoudour, N.; Alessandri, C.; Haouache, H.; Vodovar, D.; Woerther, P.-L.; Hutin, A.; Ghaleh, B.; et al. Population Pharmacokinetics of Amikacin in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation. Pharmaceutics 2022, 14, 289. [Google Scholar] [CrossRef]
- Alihodzic, D.; Wicha, S.G.; Frey, O.R.; König, C.; Baehr, M.; Jarczak, D.; Kluge, S.; Langebrake, C. Ciprofloxacin in Patients Undergoing Extracorporeal Membrane Oxygenation (ECMO): A Population Pharmacokinetic Study. Pharmaceutics 2022, 14, 965. [Google Scholar] [CrossRef]
- Šíma, M.; Bobek, D.; Cihlářová, P.; Ryšánek, P.; Roušarová, J.; Beroušek, J.; Kuchař, M.; Vymazal, T.; Slanař, O. Factors Affecting the Metabolic Conversion of Ciprofloxacin and Exposure to Its Main Active Metabolites in Critically Ill Patients: Population Pharmacokinetic Analysis of Desethylene Ciprofloxacin. Pharmaceutics 2022, 14, 1627. [Google Scholar] [CrossRef]
- Kang, S.; Yang, S.; Hahn, J.; Jang, J.Y.; Min, K.L.; Wi, J.; Chang, M.J. Dose Optimization of Meropenem in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation in Critically Ill Cardiac Patients: Pharmacokinetic/Pharmacodynamic Modeling. J. Clin. Med. 2022, 11, 6621. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Creech, C.B.; Wu, N.; Nation, R.L.; Gu, K.; Nalbant, D.; Jimenez-Truque, N.; Fissell, W.; Rolsma, S.; Patel, P.C.; et al. Evaluation of Empirical Dosing Regimens for Meropenem in Intensive Care Unit Patients Using Population Pharmacokinetic Modeling and Target Attainment Analysis. Antimicrob. Agents Chemother. 2023, 67, e0131222. [Google Scholar] [CrossRef]
- Bai, J.; Wen, A.; Li, Z.; Li, X.; Duan, M. Population pharmacokinetics and dosing optimisation of imipenem in critically ill patients. Eur. J. Hosp. Pharm. 2023. [Google Scholar] [CrossRef]
- Martínez-Casanova, J.; Esteve-Pitarch, E.; Colom-Codina, H.; Gumucio-Sanguino, V.D.; Cobo-Sacristán, S.; Shaw, E.; Maisterra-Santos, K.; Sabater-Riera, J.; Pérez-Fernandez, X.L.; Rigo-Bonnin, R.; et al. Predictive Factors of Piperacillin Exposure and the Impact on Target Attainment after Continuous Infusion Administration to Critically Ill Patients. Antibiotics 2023, 12, 531. [Google Scholar] [CrossRef]
- An, G.; Creech, C.B.; Wu, N.; Nation, R.L.; Gu, K.; Nalbant, D.; Jimenez-Truque, N.; Fissell, W.; Patel, P.C.; Fishbane, N.; et al. Population pharmacokinetics and target attainment analyses to identify a rational empirical dosing strategy for cefepime in critically ill patients. J. Antimicrob. Chemother. 2023, 78, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zoller, M.; Fuhr, U.; Jaehde, U.; Ullah, S.; Liebchen, U.; Büsker, S.; Zander, J.; Flury, B.B.; Taubert, M. Cefepime Population Pharmacokinetics, Antibacterial Target Attainment, and Estimated Probability of Neurotoxicity in Critically Ill Patients. Antimicrob. Agents Chemother. 2023, 67, e0030923. [Google Scholar] [CrossRef]
- Ehmann, L.; Zoller, M.; Minichmayr, I.K.; Scharf, C.; Huisinga, W.; Zander, J.; Kloft, C. Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis. Int. J. Antimicrob. Agents 2019, 54, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Soraluce, A.; Barrasa, H.; Asín-Prieto, E.; Sánchez-Izquierdo, J.; Maynar, J.; Isla, A.; Rodríguez-Gascón, A. Novel Population Pharmacokinetic Model for Linezolid in Critically Ill Patients and Evaluation of the Adequacy of the Current Dosing Recommendation. Pharmaceutics 2020, 12, 54. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.-H.; Kim, J.S.; Jung, W.-B.; Heo, W.; Kim, Y.K.; Kim, S.H.; No, T.-H.; Jo, K.M.; Ko, J.; et al. Pharmacokinetics and Monte Carlo Simulation of Meropenem in Critically Ill Adult Patients Receiving Extracorporeal Membrane Oxygenation. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Dedkaew, T.; Cressey, T.R.; Punyawudho, B.; Lucksiri, A. Pharmacokinetics of vancomycin in critically ill patients in Thailand. Int. J. Pharm. Pharm. Sci. 2015, 7, 232–237. [Google Scholar]
- Seo, H.; Kim, Y.K.; Park, S.; Kim, H.-I.; Lee, D.-H. Population Pharmacokinetics and Monte Carlo Simulation of Cefepime in Critically Ill Patients with Hospital-Acquired/Ventilator-Associated Pneumonia. Infect. Chemother. 2023, 55, 29–41. [Google Scholar] [CrossRef]
- Mattioli, F.; Fucile, C.; Del Bono, V.; Marini, V.; Parisini, A.; Molin, A.; Zuccoli, M.L.; Milano, G.; Danesi, R.; Marchese, A.; et al. Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients. Eur. J. Clin. Pharmacol. 2016, 72, 839–848. [Google Scholar] [CrossRef]
- Abdulla, A.; Rogouti, O.; Hunfeld, N.G.M.; Endeman, H.; Dijkstra, A.; van Gelder, T.; Muller, A.E.; de Winter, B.C.M.; Koch, B.C.P. Population pharmacokinetics and target attainment of ciprofloxacin in critically ill patients. Eur. J. Clin. Pharmacol. 2020, 76, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Abdul-Aziz, M.H.; Burrows, F.; Buscher, H.; Corley, A.; Diehl, A.; Levkovich, B.J.; Pellegrino, V.; Reynolds, C.; Rudham, S.; et al. Population pharmacokinetics of ciprofloxacin in critically ill patients receiving extracorporeal membrane oxygenation (an ASAP ECMO study). Anaesth. Crit. Care Pain Med. 2022, 41, 101080. [Google Scholar] [CrossRef]
- Isla, A.; Rodríguez-Gascón, A.; Trocóniz, I.F.; Bueno, L.; Solinís, M.; Maynar, J.; Sánchez-Izquierdo, J.; Pedraz, J.L. Population Pharmacokinetics of Meropenem in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Clin. Pharmacokinet. 2008, 47, 173–180. [Google Scholar] [CrossRef]
- Alobaid, A.S.; Wallis, S.C.; Jarrett, P.; Starr, T.; Stuart, J.; Lassig-Smith, M.; Mejia, J.L.O.; Roberts, M.S.; Roger, C.; Udy, A.A.; et al. Population Pharmacokinetics of Piperacillin in Nonobese, Obese, and Morbidly Obese Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e01276-16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, H.S.; Park, S.; Kim, H.-I.; Lee, S.H.; Lee, D.-H. Population pharmacokinetics of piperacillin/tazobactam in critically ill Korean patients and the effects of extracorporeal membrane oxygenation. J. Antimicrob. Chemother. 2022, 77, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Ernest, D.; Cutler, D.J. Gentamicin clearance during continuous arteriovenous hemodiafiltration. Crit. Care Med. 1992, 20, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Shikuma, L.R.; Ackerman, B.H.; Weaver, R.H.; Solem, L.D.; Strate, R.G.; Cerra, F.B.; Zaske, D.E. Effects of treatment and the metabolic response to injury on drug clearance: A prospective study with piperacillin. Crit Care Med. 1990, 18, 37–41. [Google Scholar] [CrossRef]
- Cornwell, E.E.; Belzberg, H.; Berne, T.V.; Gill, M.A.; Theodorou, D.; Kern, J.W.; Yu, W.; Asensio, J.; Demetriades, D. Pharmacokinetics of aztreonam in critically ill surgical patients. Am. J. Heal. Pharm. 1997, 54, 537–540. [Google Scholar] [CrossRef]
- Giles, L.J.; Jennings, A.C.; Thomson, A.H.; Creed, G.; Beale, R.J.; McLuckie, A. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit. Care Med. 2000, 28, 632–637. [Google Scholar] [CrossRef]
- Barletta, J.F.; Johnson, S.B.; Nix, D.E.; Nix, L.C.; Erstad, B.L. Population Pharmacokinetics of Aminoglycosides in Critically Ill Trauma Patients on Once-Daily Regimens. J. Trauma: Inj. Infect. Crit. Care 2000, 49, 869–872. [Google Scholar] [CrossRef]
- Malone, R.S.; Fish, D.N.; Abraham, E.; Teitelbaum, I. Pharmacokinetics of Cefepime during Continuous Renal Replacement Therapy in Critically Ill Patients. Antimicrob. Agents Chemother. 2001, 45, 3148–3155. [Google Scholar] [CrossRef]
- Traunmüller, F.; Schenk, P.; Mittermeyer, C.; Thalhammer-Scherrer, R.; Ratheiser, K.; Thalhammer, F. Clearance of ceftazidime during continuous venovenous haemofiltration in critically ill patients. J. Antimicrob. Chemother. 2002, 49, 129–134. [Google Scholar] [CrossRef][Green Version]
- Uchino, S.; Cole, L.; Morimatsu, H.; Goldsmith, D.; Bellomo, R. Clearance of vancomycin during high-volume haemofiltration: Impact of pre-dilution. Intensive Care Med. 2002, 28, 1664–1667. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Maggiore, U.; Rotelli, C.; Giacosa, R.; Parenti, E.; Picetti, E.; Sagripanti, S.; Manini, P.; Andreoli, R.; Cabassi, A. Removal of linezolid by conventional intermittent hemodialysis, sustained low-efficiency dialysis, or continuous venovenous hemofiltration in patients with acute renal failure. Crit. Care Med. 2004, 32, 2437–2442. [Google Scholar] [CrossRef]
- Meyer, B.; Kornek, G.V.; Nikfardjam, M.; Karth, G.D.; Heinz, G.; Locker, G.J.; Jaeger, W.; Thalhammer, F. Multiple-dose pharmacokinetics of linezolid during continuous venovenous haemofiltration. J. Antimicrob. Chemother. 2005, 56, 172–179. [Google Scholar] [CrossRef]
- Fish, D.N.; Teitelbaum, I.; Abraham, E. Pharmacokinetics and Pharmacodynamics of Imipenem during Continuous Renal Replacement Therapy in Critically Ill Patients. Antimicrob. Agents Chemother. 2005, 49, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Czock, D.; Schöpke, T.; Hafer, C.; Bode-Böger, S.M.; Kuse, E.; Keller, F.; Fliser, D. Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis*. Crit. Care Med. 2006, 34, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Klansuwan, N.; Ratanajamit, C.; Kasiwong, S.; Wangsiripaisan, A. Clearance of vancomycin during high-efficiency hemodialysis. J. Med. Assoc. Thail. 2006, 89, 986–991. [Google Scholar] [PubMed]
- Bracco, D.; Landry, C.; Dubois, M.-J.; Eggimann, P. Pharmacokinetic variability of extended interval tobramycin in burn patients. Burns 2008, 34, 791–796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burkhardt, O.; Hafer, C.; Langhoff, A.; Kaever, V.; Kumar, V.; Welte, T.; Haller, H.; Fliser, D.; Kielstein, J.T. Pharmacokinetics of ertapenem in critically ill patients with acute renal failure undergoing extended daily dialysis. Nephrol. Dial. Transplant. 2008, 24, 267–271. [Google Scholar] [CrossRef][Green Version]
- Golestaneh, L.; Gofran, A.; Mokrzycki, M.; Chen, J. Removal of vancomycin in sustained low-efficiency dialysis (SLED): A need for better surveillance and dosing. Clin. Nephrol. 2009, 72, 286–291. [Google Scholar] [CrossRef]
- Deshpande, P.; Chen, J.; Gofran, A.; Murea, M.; Golestaneh, L. Meropenem removal in critically ill patients undergoing sustained low-efficiency dialysis (SLED). Nephrol. Dial. Transplant. 2010, 25, 2632–2636. [Google Scholar] [CrossRef][Green Version]
- Bilgrami, I.; Roberts, J.A.; Wallis, S.C.; Thomas, J.; Davis, J.; Fowler, S.; Goldrick, P.B.; Lipman, J. Meropenem Dosing in Critically Ill Patients with Sepsis Receiving High-Volume Continuous Venovenous Hemofiltration. Antimicrob. Agents Chemother. 2010, 54, 2974–2978. [Google Scholar] [CrossRef]
- Seyler, L.; Cotton, F.; Taccone, F.S.; De Backer, D.; Macours, P.; Vincent, J.-L.; Jacobs, F. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care 2011, 15, R137. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Sousa, E.; Martins, P.J.; Pimentel, J.M. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int. J. Antimicrob. Agents 2012, 39, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Roberts, J.A.; Roberts, M.S.; Liu, X.; Nair, P.; Cole, L.; Lipman, J.; Bellomo, R. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy. Crit. Care Med. 2012, 40, 1523–1528. [Google Scholar] [CrossRef]
- Petejova, N.; Martinek, A.; Zahalkova, J.; Duricova, J.; Brozmannova, H.; Urbanek, K.; Grundmann, M.; Plasek, J.; Kacirova, I. Vancomycin pharmacokinetics during high-volume continuous venovenous hemofiltration in critically ill septic patients. Biomed. Pap. 2014, 158, 065–072. [Google Scholar] [CrossRef]
- D’arcy, D.M.; Casey, E.; Gowing, C.M.; Donnelly, M.B.; I Corrigan, O. An open prospective study of amikacin pharmacokinetics in critically ill patients during treatment with continuous venovenous haemodiafiltration. BMC Pharmacol. Toxicol. 2012, 13, 14. [Google Scholar] [CrossRef]
- Binder, L.; Schwörer, H.; Hoppe, S.; Streit, F.; Neumann, S.; Beckmann, A.; Wachter, R.; Oellerich, M.; Walson, P.D. Pharmacokinetics of Meropenem in Critically Ill Patients With Severe Infections. Ther. Drug Monit. 2013, 35, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.; Li, J.X.; Wallis, S.C.; Rudd, M.; Jarrett, P.; Paterson, D.L.; Lipman, J.; Udy, A.A.; Roberts, J.A. Pharmacokinetics of meropenem and piperacillin in critically ill patients with indwelling surgical drains. Int. J. Antimicrob. Agents 2013, 42, 90–93. [Google Scholar] [CrossRef]
- Carlier, M.; Carrette, S.; Roberts, J.A.; Stove, V.; Verstraete, A.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; Lipman, J.; Wallis, S.C.; et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: Does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit. Care 2013, 17, R84. [Google Scholar] [CrossRef]
- Sturm, A.W.; Allen, N.; Rafferty, K.D.; Fish, D.N.; Toschlog, E.; Newell, M.; Waibel, B. Pharmacokinetic Analysis of Piperacillin Administered with Tazobactam in Critically Ill, Morbidly Obese Surgical Patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 34, 28–35. [Google Scholar] [CrossRef]
- Carlier, M.; Carrette, S.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int. J. Antimicrob. Agents 2014, 43, 470–473. [Google Scholar] [CrossRef]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Roberts, M.S.; Tiong, I.S.; Gardner, J.H.; Lehman, S.; Peake, S.L.; Hahn, U.; Warner, M.S.; Roberts, J.A. Can therapeutic drug monitoring optimize exposure to piperacillin in febrile neutropenic patients with haematological malignancies? A randomized controlled trial. J. Antimicrob. Chemother. 2015, 70, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Awissi, D.; Beauchamp, A.; Hébert, E.; Lavigne, V.; Munoz, D.L.; Lebrun, G.; Savoie, M.; Fagnan, M.; Amyot, J.; Tétreault, N.; et al. Pharmacokinetics of an Extended 4-hour Infusion of Piperacillin-Tazobactam in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 600–607. [Google Scholar] [CrossRef]
- Villa, G.; Cassetta, M.I.; Tofani, L.; Valente, S.; Chelazzi, C.; Falsini, S.; De Gaudio, A.R.; Novelli, A.; Ronco, C.; Adembri, C. Linezolid extracorporeal removal during haemodialysis with high cut-off membrane in critically ill patients. Int. J. Antimicrob. Agents 2015, 46, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.; Li, Z.; Yu, J.; Li, R.; Cheng, S.; Duan, M.; Bai, J. Clinical Validation of Therapeutic Drug Monitoring of Imipenem in Spent Effluent in Critically Ill Patients Receiving Continuous Renal Replacement Therapy: A Pilot Study. PLOS ONE 2016, 11, e0153927. [Google Scholar] [CrossRef]
- Boucher, B.A.; Hudson, J.Q.; Hill, D.M.; Swanson, J.M.; Wood, G.C.; Laizure, S.C.; Arnold-Ross, A.; Hu, Z.-Y.; Hickerson, W.L. Pharmacokinetics of Imipenem/Cilastatin Burn Intensive Care Unit Patients Undergoing High-Dose Continuous Venovenous Hemofiltration. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Mahul, M.; Breilh, D.; Legeron, R.; Signe, J.; Jean-Pierre, H.; Uhlemann, A.-C.; Molinari, N.; Jaber, S. Repeated Piperacillin-Tazobactam Plasma Concentration Measurements in Severely Obese Versus Nonobese Critically Ill Septic Patients and the Risk of Under– and Overdosing*. Crit. Care Med. 2017, 45, e470–e478. [Google Scholar] [CrossRef]
- Roger, C.; Cotta, M.O.; Muller, L.; Wallis, S.C.; Lipman, J.; Lefrant, J.-Y.; Roberts, J.A. Impact of renal replacement modalities on the clearance of piperacillin-tazobactam administered via continuous infusion in critically ill patients. Int. J. Antimicrob. Agents 2017, 50, 227–231. [Google Scholar] [CrossRef]
- Carrié, C.; Petit, L.; D'Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: A prospective observational study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef]
- Ruiz-Ramos, J.; Villarreal, E.; Gordon, M.; Martin-Cerezula, M.; Broch, M.J.; Marqués, M.R.; Poveda, J.L.; Castellanos-Ortega; Ramírez, P. Implication of Haemodiafiltration Flow Rate on Amikacin Pharmacokinetic Parameters in Critically Ill Patients. Blood Purif. 2017, 45, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Eggimann, P.; Pantet, O.; Pagani, J.L.; Dupuis-Lozeron, E.; Pannatier, A.; Sadeghipour, F.; Voirol, P.; Que, Y.-A. Impact of Real-Time Therapeutic Drug Monitoring on the Prescription of Antibiotics in Burn Patients Requiring Admission to the Intensive Care Unit. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Sinnollareddy, M.G.; Roberts, M.S.; Lipman, J.; Peake, S.L.; A Roberts, J. Pharmacokinetics of piperacillin in critically ill patients with acute kidney injury receiving sustained low-efficiency diafiltration. J. Antimicrob. Chemother. 2018, 73, 1647–1650. [Google Scholar] [CrossRef]
- Wang, S.; Lin, F.; Ruan, J.; Ye, H.; Wang, L. Pharmacokinetics of multiple doses of teicoplanin in Chinese elderly critical patients. Expert Rev. Clin. Pharmacol. 2018, 11, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Kassel, L.E.; Van Matre, E.T.; Foster, C.J.; Fish, D.N.; Mueller, S.W.; Sherman, D.S.; Wempe, M.F.; MacLaren, R.; Neumann, R.T.; Kiser, T.H. A Randomized Pharmacokinetic and Pharmacodynamic Evaluation of Every 8-Hour and 12-Hour Dosing Strategies of Vancomycin and Cefepime in Neurocritically ill Patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Olbrisch, K.; Kisch, T.; Thern, J.; Kramme, E.; Rupp, J.; Graf, T.; Wicha, S.G.; Mailänder, P.; Raasch, W. After standard dosage of piperacillin plasma concentrations of drug are subtherapeutic in burn patients. Naunyn-Schmiedeberg's Arch. Pharmacol. 2018, 392, 229–241. [Google Scholar] [CrossRef]
- Barrasa, H.; Soraluce, A.; Isla, A.; Martín, A.; Maynar, J.; Canut, A.; Sánchez-Izquierdo, J.A.; Rodríguez-Gascón, A. Pharmacokinetics of linezolid in critically ill patients on continuous renal replacement therapy: Influence of residual renal function on PK/PD target attainment. J. Crit. Care 2019, 50, 69–76. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Strunk, A.-K.; David, S.; Bode-Böger, S.M.; Martens-Lobenhoffer, J.; Knitsch, W.; Scherneck, S.; Welte, T.; Kielstein, J.T. Single- and multiple-dose pharmacokinetics and total removal of colistin in critically ill patients with acute kidney injury undergoing prolonged intermittent renal replacement therapy. J. Antimicrob. Chemother. 2019, 74, 997–1002. [Google Scholar] [CrossRef]
- Singhan, W.; Vadcharavivad, S.; Areepium, N.; Wittayalertpanya, S.; Chaijamorn, W.; Srisawat, N. The effect of direct hemoperfusion with polymyxin B immobilized cartridge on meropenem in critically ill patients requiring renal support. J. Crit. Care 2019, 51, 71–76. [Google Scholar] [CrossRef]
- Bouglé, A.; Dujardin, O.; Lepère, V.; Hamou, N.A.; Vidal, C.; Lebreton, G.; Salem, J.-E.; El-Helali, N.; Petijean, G.; Amour, J. PHARMECMO: Therapeutic drug monitoring and adequacy of current dosing regimens of antibiotics in patients on Extracorporeal Life Support. Anaesth. Crit. Care Pain Med. 2019, 38, 493–497. [Google Scholar] [CrossRef]
- Dhaese, S.A.; Thooft, A.D.; Farkas, A.; Lipman, J.; Verstraete, A.G.; Stove, V.; Roberts, J.A.; De Waele, J.J. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: A prospective observational study. J. Crit. Care 2019, 52, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.; Guerci, P.; Pape, E.; Thilly, N.; Luc, A.; Germain, A.; Butin-Druoton, A.-L.; Losser, M.-R.; Birckener, J.; Scala-Bertola, J.; et al. Serum and peritoneal exudate concentrations after high doses of β-lactams in critically ill patients with severe intra-abdominal infections: An observational prospective study. J. Antimicrob. Chemother. 2019, 75, 156–161. [Google Scholar] [CrossRef]
- A Roberts, J.; Joynt, G.M.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S.; et al. The Effect of Renal Replacement Therapy and Antibiotic Dose on Antibiotic Concentrations in Critically Ill Patients: Data From the Multinational Sampling Antibiotics in Renal Replacement Therapy Study. Clin. Infect. Dis. 2020, 72, 1369–1378. [Google Scholar] [CrossRef]
- Gieling, E.M.; Wallenburg, E.; Frenzel, T.; de Lange, D.W.; Schouten, J.A.; Oever, J.T.; Kolwijck, E.; Burger, D.M.; Pickkers, P.; ter Heine, R.; et al. Higher Dosage of Ciprofloxacin Necessary in Critically Ill Patients: A New Dosing Algorithm Based on Renal Function and Pathogen Susceptibility. Clin. Pharmacol. Ther. 2020, 108, 770–774. [Google Scholar] [CrossRef]
- Moni, M.; Sudhir, A.S.; Dipu, T.S.; Mohamed, Z.; Prabhu, B.P.; Edathadathil, F.; Balachandran, S.; Singh, S.K.; Prasanna, P.; Menon, V.P.; et al. Clinical efficacy and pharmacokinetics of colistimethate sodium and colistin in critically ill patients in an Indian hospital with high endemic rates of multidrug-resistant Gram-negative bacterial infections: A prospective observational study. Int. J. Infect. Dis. 2020, 100, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Shen, L.-J.; Wu, V.-C.; Ko, W.-J.; Wu, C.-C.; Wu, F.-L.L. Pharmacokinetics and dosing of vancomycin in patients undergoing sustained low efficiency daily diafiltration (SLEDD-f): A prospective study. J. Formos. Med Assoc. 2021, 120, 737–743. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, F.M.; Zamoner, W.; dos Reis, P.F.; Balbi, A.L.; Ponce, D. Vancomycin for Dialytic Therapy in Critically Ill Patients: Analysis of Its Reduction and the Factors Associated with Subtherapeutic Concentrations. Int. J. Environ. Res. Public Heal. 2020, 17, 6861. [Google Scholar] [CrossRef]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M.; et al. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 664. [Google Scholar] [CrossRef]
- Corcione, S.; De Nicolò, A.; Lupia, T.; Segala, F.V.; Pensa, A.; Loia, R.C.; Romeo, M.R.; Di Perri, G.; Stella, M.; D’avolio, A.; et al. Observed concentrations of amikacin and gentamycin in the setting of burn patients with gram-negative bacterial infections: Preliminary data from a prospective study. Therapies 2020, 76, 409–414. [Google Scholar] [CrossRef]
- Petersson, J.; Giske, C.G.; Eliasson, E. Poor Correlation between Meropenem and Piperacillin Plasma Concentrations and Delivered Dose of Continuous Renal Replacement Therapy. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- Nicolau, D.P.; De Waele, J.; Kuti, J.L.; Caro, L.; Larson, K.B.; Yu, B.; Gadzicki, E.; Zeng, Z.; Rhee, E.G.; Rizk, M.L. Pharmacokinetics and Pharmacodynamics of Ceftolozane/Tazobactam in Critically Ill Patients With Augmented Renal Clearance. Int. J. Antimicrob. Agents 2021, 57, 106299. [Google Scholar] [CrossRef] [PubMed]
- Fillâtre, P.; Lemaitre, F.; Nesseler, N.; Schmidt, M.; Besset, S.; Launey, Y.; Maamar, A.; Daufresne, P.; Flecher, E.; Le Tulzo, Y.; et al. Impact of extracorporeal membrane oxygenation (ECMO) support on piperacillin exposure in septic patients: A case–control study. J. Antimicrob. Chemother. 2021, 76, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Rehm, S.; Guertler, N.; Hinic, V.; Dräger, S.; Bassetti, S.; Rentsch, K.M.; Sendi, P.; Osthoff, M. Probability of pharmacological target attainment with flucloxacillin in Staphylococcus aureus bloodstream infection: A prospective cohort study of unbound plasma and individual MICs. J. Antimicrob. Chemother. 2021, 76, 1845–1854. [Google Scholar] [CrossRef]
- Esteve-Pitarch, E.; Gumucio-Sanguino, V.D.; Cobo-Sacristán, S.; Shaw, E.; Maisterra-Santos, K.; Sabater-Riera, J.; Pérez-Fernandez, X.L.; Rigo-Bonnin, R.; Tubau-Quintano, F.; Carratalà, J.; et al. Continuous Infusion of Piperacillin/Tazobactam and Meropenem in ICU Patients Without Renal Dysfunction: Are Patients at Risk of Underexposure? Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Pařízková, R.; Martínková, J.; Havel, E.; Šafránek, P.; Kaška, M.; Astapenko, D.; Bezouška, J.; Chládek, J.; Černý, V. Additional File 1 of Impact of Cumulative Fluid Balance on the Pharmacokinetics of Extended Infusion Meropenem in Critically Ill Patients with Sepsis. Available online: https://pubmed.ncbi.nlm.nih.gov/34274013/ (accessed on 18 July 2024). [CrossRef]
- Liebchen, U.; Paal, M.; Bucher, V.; Vogeser, M.; Irlbeck, M.; Schroeder, I.; Zoller, M.; Scharf, C. Trough concentrations of meropenem and piperacillin during slow extended dialysis in critically ill patients with intermittent and continuous infusion: A prospective observational study. J. Crit. Care 2021, 67, 26–32. [Google Scholar] [CrossRef]
- Messiano, C.G.; Junior, R.M.; Pereira, G.O.; Junior, E.M.d.S.; Gomez, D.D.S.; Santos, S.R.C.J. Therapeutic Target Attainment of 3-Hour Extended Infusion of Meropenem in Patients With Septic Burns. Clin. Ther. 2022. [Google Scholar] [CrossRef]
- Zoller, M.; Paal, M.; Greimel, A.; Kallee, S.; Vogeser, M.; Irlbeck, M.; Schroeder, I.; Liebchen, U.; Scharf, C. Serum linezolid concentrations are reduced in critically ill patients with pulmonary infections: A prospective observational study. J. Crit. Care 2022, 71, 154100. [Google Scholar] [CrossRef]
- Shekar, K.; Abdul-Aziz, M.H.; Cheng, V.; Burrows, F.; Buscher, H.; Cho, Y.-J.; Corley, A.; Diehl, A.; Gilder, E.; Jakob, S.M.; et al. Antimicrobial Exposures in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2023, 207, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Smeets, T.J.; de Geus, H.R.; Rietveld, A.; Rietdijk, W.J.; Koch, B.C.; Endeman, H.; Hunfeld, N.G. Pursuing the Real Vancomycin Clearance during Continuous Renal Replacement Therapy in Intensive Care Unit Patients: Is There Adequate Target Attainment? Blood Purif. 2023, 52, 652–659. [Google Scholar] [CrossRef]
- Correia, P.; Launay, M.; Balluet, R.; Gergele, L.; Gauthier, V.; Morel, J.; Beuret, P.; Mariat, C.; Thiery, G.; Ragey, S.P. Towards optimization of ceftazidime dosing in obese ICU patients: The end of the ‘one-size-fits-all’ approach? J. Antimicrob. Chemother. 2023, 78, 2968–2975. [Google Scholar] [CrossRef]
- Martin, C.; Lambert, D.; Bruguerolle, B.; Saux, P.; Freney, J.; Fleurette, J.; Meugnier, H.; Gouin, F. Ofloxacin pharmacokinetics in mechanically ventilated patients. Antimicrob. Agents Chemother. 1991, 35, 1582–1585. [Google Scholar] [CrossRef][Green Version]
- Akers, K.S.; Niece, K.L.; Chung, K.K.; Cannon, J.W.; Cota, J.M.; Murray, C.K. Modified Augmented Renal Clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J. Trauma: Inj. Infect. Crit. Care 2014, 77, S163–S170. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.S.; Sanches-Giraud, C.; Silva, C.V.; Oliveira, A.M.R.R.; da Silva, J.M.; Gemperli, R.; Santos, S.R. Imipenem in burn patients: Pharmacokinetic profile and PK/PD target attainment. J. Antibiot. 2014, 68, 143–147. [Google Scholar] [CrossRef]
- Ko, A.; Harada, M.Y.; Barmparas, G.; Jay, J.; Sun, B.J.; Chen, E.; Mehrzadi, D.; Patel, B.; Mason, R.; Ley, E.J. Reducing acute kidney injury due to vancomycin in trauma patients. J. Trauma: Inj. Infect. Crit. Care 2016, 81, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Mokline, A.; Gharsallah, L.; Rahmani, I.; Gaies, E.; Tabelsi, S.; A Messadi, A. Pharmacokinetics and pharmacodynamics of Linezolid in burn patients. Ann. Burn. Fire Disasters 2018, 31, 118–121. [Google Scholar]
- Lim, S.K.; Lee, S.A.; Kim, C.; Kang, E.; Choi, Y.H.; Park, I. High variability of teicoplanin concentration in patients with continuous venovenous hemodiafiltration. Hemodial. Int. 2019, 23, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, T.; Miljkovic, B.; Mikov, M.; Satara, S.S.; Dragic, S.; Momcicevic, D.; Kovacevic, P. The Effect of Hypoalbuminemia on the Therapeutic Concentration and Dosage of Vancomycin in Critically Ill Septic Patients in Low-Resource Countries. Dose-Response 2019, 17. [Google Scholar] [CrossRef]
- Wu, C.-C.; Tai, C.H.; Liao, W.-Y.; Wang, C.-C.; Kuo, C.-H.; Lin, S.-W.; Ku, S.-C. Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: A prospective observational study. Infect. Drug Resist. 2019, ume 12, 2531–2541. [Google Scholar] [CrossRef]
- Morbitzer, K.A.; Rhoney, D.H.; Dehne, K.A.; Jordan, J.D. Enhanced renal clearance and impact on vancomycin pharmacokinetic parameters in patients with hemorrhagic stroke. J. Intensive Care 2019, 7, 51. [Google Scholar] [CrossRef]
- Breilh, D.; Honore, P.M.; De Bels, D.; Roberts, J.A.; Gordien, J.B.; Fleureau, C.; Dewitte, A.; Coquin, J.; Rozé, H.; Perez, P.; et al. Pharmacokinetics and pharmacodynamics of anti-infective agents during continuous veno-venous hemofiltration in critically ill patients: Lessons learned from an ancillary study of the IVOIRE trial. J. Transl. Intern. Med. 2019, 7, 155–169. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Machado, A.S.; Mendes, E.T.; Chaves, L.; Neto, L.V.P.; da Silva, C.V.; Santos, S.R.C.J.; Sanches, C.; Macedo, E.; Levin, A.S. Pharmacokinetic and Pharmacodynamic Characteristics of Vancomycin and Meropenem in Critically Ill Patients Receiving Sustained Low-efficiency Dialysis. Clin. Ther. 2020, 42, 625–633. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Avedissian, S.N.; Al-Qamari, A.; Bohling, T.; Pham, M.; Scheetz, M.H. Pharmacokinetic Assessment of Pre- and Post-Oxygenator Vancomycin Concentrations in Extracorporeal Membrane Oxygenation: A Prospective Observational Study. Clin. Pharmacokinet. 2020, 59, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Veillette, J.J.; Winans, S.A.; Maskiewicz, V.K.; Truong, J.; Jones, R.N.; Forland, S.C. Pharmacokinetics and Pharmacodynamics of High-Dose Piperacillin–Tazobactam in Obese Patients. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, M.; Dreesen, E.; Van Daele, R.; Annaert, P.; Debaveye, Y.; Wauters, J.; Spriet, I. Pharmacokinetic/Pharmacodynamic Target Attainment Based on Measured versus Predicted Unbound Ceftriaxone Concentrations in Critically Ill Patients with Pneumonia: An Observational Cohort Study. Antibiotics 2021, 10, 557. [Google Scholar] [CrossRef]
- Shi, L.; Zhuang, Z.; Duan, L.; Zhu, C.; Xue, H.; Wang, X.; Xu, X.; Yuan, Y.; Shi, L.; Li, J.; et al. Dose Optimization of Teicoplanin for Critically Ill Patients With Renal Dysfunction and Continuous Renal Replacement Therapy: Experience From a Prospective Interventional Study. Front. Pharmacol. 2022, 13, 817401. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, Y.; Yang, M.; Liang, X.; Wu, J.; Chen, Y.; Guo, B.; Zhang, H.; Wang, R.; Zhang, F.; et al. Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study. Antibiotics 2022, 11, 837. [Google Scholar] [CrossRef]
- Calov, S.; Munzel, F.; Roehr, A.C.; Frey, O.; Higuita, L.M.S.; Wied, P.; Rosenberger, P.; Haeberle, H.A.; Ngamsri, K.-C. Daptomycin Pharmacokinetics in Blood and Wound Fluid in Critical Ill Patients with Left Ventricle Assist Devices. Antibiotics 2023, 12, 904. [Google Scholar] [CrossRef]
- Tikiso, T.; Fuhrmann, V.; König, C.; Jarczak, D.; Iwersen-Bergmann, S.; Kluge, S.; Wicha, S.G.; Grensemann, J. Acute-on-chronic liver failure alters linezolid pharmacokinetics in critically ill patients with continuous hemodialysis: An observational study. Ann. Intensive Care 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.M.; Liu, X.; A Roberts, J.; Nair, P.; Cole, L.; Roberts, M.S.; Lipman, J.; Bellomo, R. Additional File 1 of A multicenter Study on the Effect of Continuous Hemodiafiltration Intensity on Antibiotic Pharmacokinetics. Available online: https://pubmed.ncbi.nlm.nih.gov/25881576/ (accessed on 18 July 2024). [CrossRef]
- Scharf, C.; Weinelt, F.; Schroeder, I.; Paal, M.; Weigand, M.; Zoller, M.; Irlbeck, M.; Kloft, C.; Briegel, J.; Liebchen, U. Does the cytokine adsorber CytoSorb® reduce vancomycin exposure in critically ill patients with sepsis or septic shock? a prospective observational study. Ann. Intensive Care 2022, 12, 44. [Google Scholar] [CrossRef]
- Wulkersdorfer, B.; Bergmann, F.; Amann, L.; Fochtmann-Frana, A.; Al Jalali, V.; Kurdina, E.; Lackner, E.; Wicha, S.G.; Dorn, C.; Schäfer, B.; et al. Effect of albumin substitution on pharmacokinetics of piperacillin/tazobactam in patients with severe burn injury admitted to the ICU. J. Antimicrob. Chemother. 2023, 79, 262–270. [Google Scholar] [CrossRef]
- Shahrami, B.; Najmeddin, F.; Rouini, M.R.; Najafi, A.; Sadeghi, K.; Amini, S.; Khezrnia, S.S.; Sharifnia, H.R.; Mojtahedzadeh, M. Evaluation of Amikacin Pharmacokinetics in Critically Ill Patients with Intra-abdominal Sepsis. Adv. Pharm. Bull. 2019, 10, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Busse, D.; Petroff, D.; Dorn, C.; Ehmann, L.; Hochstädt, S.; Girrbach, F.; Dietrich, A.; Zeitlinger, M.; Kees, F.; et al. Linezolid Concentrations in Plasma and Subcutaneous Tissue are Reduced in Obese Patients, Resulting in a Higher Risk of Underdosing in Critically Ill Patients: A Controlled Clinical Pharmacokinetic Study. J. Clin. Med. 2020, 9, 1067. [Google Scholar] [CrossRef]
- Del Bono, V.; Giacobbe, D.R.; Marchese, A.; Parisini, A.; Fucile, C.; Coppo, E.; Marini, V.; Arena, A.; Molin, A.; Martelli, A.; et al. Meropenem for treating KPC-producing Klebsiella pneumoniae bloodstream infections: Should we get to the PK/PD root of the paradox? Virulence 2016, 8, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Xie, J.; Li, Q.; He, J.; Hu, L.; Wang, H.; Liu, A.; Xu, J.; Yang, C.; et al. Pharmacokinetic/Pharmacodynamic Parameters of Linezolid in the Epithelial Lining Fluid of Patients With Sepsis. J. Clin. Pharmacol. 2022, 62, 891–897. [Google Scholar] [CrossRef]
- Corti, N.; Rudiger, A.; Chiesa, A.; Marti, I.; Jetter, A.; Rentsch, K.; Müller, D.; Béchir, M.; Maggiorini, M. Pharmacokinetics of Daily Daptomycin in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Chemotherapy 2013, 59, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; D’avolio, A.; Loia, R.C.; Pensa, A.; Segala, F.V.; De Nicolò, A.; Fatiguso, G.; Romeo, M.; Di Perri, G.; Stella, M.; et al. Pharmacokinetics of meropenem in burn patients with infections caused by Gram-negative bacteria: Are we getting close to the right treatment? J. Glob. Antimicrob. Resist. 2020, 20, 22–27. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, Y.; Li, X.; Peng, M.; He, X.; Zhang, P.; Dong, S.; Wang, W.; Wang, D. Selection of piperacillin/tazobactam infusion mode guided by SOFA score in cancer patients with hospital-acquired pneumonia: A randomized controlled study. Ther. Clin. Risk Manag. 2018, 14, 31–37. [Google Scholar] [CrossRef]
- van der Starre, P.J.; Kolz, M.; Lemmens, H.J.; Faix, J.D.; Mitchell, S.; Miller, C. Vancomycin plasma concentrations in cardiac surgery with the use of profound hypothermic circulatory arrest. Eur. J. Cardio-Thoracic Surg. 2010, 38, 741–744. [Google Scholar] [CrossRef]
- Triginer, C.; Izquierdo, I.; Fernández, R.; Rello, J.; Torrent, J.; Benito, S.; Net, A. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med. 1990, 16, 303–306. [Google Scholar] [CrossRef]
- Tang, G.J.; Tang, J.J.; Lin, B.S.; Kong, C.W.; Lee, T.Y. Factors affecting gentamicin pharmacokinetics in septic patients. Acta Anaesthesiol. Scand. 1999, 43, 726–730. [Google Scholar] [CrossRef]
- Rebuck, J.A.; Fish, D.N.; Abraham, E. Pharmacokinetics of Intravenous and Oral Levofloxacin in Critically Ill Adults in a Medical Intensive Care Unit. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002, 22, 1216–1225. [Google Scholar] [CrossRef]
- Belzberg, H.; Zhu, J.; Cornwell, E.E.; Murray, J.A.; Sava, J.; Salim, A.; Velmahos, G.C.; Gill, M.A. Imipenem Levels Are Not Predictable in the Critically Ill Patient. J. Trauma: Inj. Infect. Crit. Care 2004, 56, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.S.; Gandarillas, C.-I.C.; Lerma, F.A.; Menacho, Y.A.; Domínguez-Gil, A. Pharmacokinetics and Pharmacodynamics of Levofloxacin in Intensive Care Patients. Clin. Pharmacokinet. 2005, 44, 627–635. [Google Scholar] [CrossRef]
- van Zanten, A.R.; Polderman, K.H.; van Geijlswijk, I.M.; van der Meer, G.Y.; Schouten, M.A.; Girbes, A.R. Ciprofloxacin pharmacokinetics in critically ill patients: A prospective cohort study. J. Crit. Care 2008, 23, 422–430. [Google Scholar] [CrossRef]
- Brink, A.; Richards, G.; Schillack, V.; Kiem, S.; Schentag, J. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int. J. Antimicrob. Agents 2008, 33, 432–436. [Google Scholar] [CrossRef]
- Chapuis, T.M.; Giannoni, E.; A Majcherczyk, P.; Chioléro, R.; Schaller, M.-D.; Berger, M.M.; Bolay, S.; A Décosterd, L.; Bugnon, D.; Moreillon, P. Prospective monitoring of cefepime in intensive care unit adult patients. Crit. Care 2010, 14, R51. [Google Scholar] [CrossRef] [PubMed]
- Aubron, C.; Corallo, C.E.; Nunn, M.O.; Dooley, M.J.; Cheng, A.C. Evaluation of the Accuracy of a Pharmacokinetic Dosing Program in Predicting Serum Vancomycin Concentrations in Critically Ill Patients. Ann. Pharmacother. 2011, 45, 1193–1198. [Google Scholar] [CrossRef]
- Chung, J.; Oh, J.M.; Cho, E.M.; Jang, H.J.; Hong, S.B.; Lim, C.M.; Koh, Y.S. Optimal Dose of Vancomycin for Treating Methicillin-Resistant Staphylococcus Aureus Pneumonia in Critically Ill Patients. Anaesth. Intensive Care 2011, 39, 1030–1037. [Google Scholar] [CrossRef]
- Karnik, N.D.; Sridharan, K.; Jadhav, S.P.; Kadam, P.P.; Naidu, R.K.; Namjoshi, R.D.; Gupta, V.; Gore, M.S.; Surase, P.V.; Mehta, P.R.; et al. Pharmacokinetics of colistin in critically ill patients with multidrug-resistant Gram-negative bacilli infection. Eur. J. Clin. Pharmacol. 2013, 69, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Koegelenberg, C.F.N.; Nortje, A.; Lalla, U.; Enslin, A.; Irusen, E.M.; Rosenkranz, B.; I Seifart, H.; Bolliger, C.T. The pharmacokinetics of enteral antituberculosis drugs in patients requiring intensive care. South Afr. Med. J. 2013, 103, 394–398. [Google Scholar] [CrossRef]
- de Montmollin, E.; Bouadma, L.; Gault, N.; Mourvillier, B.; Mariotte, E.; Chemam, S.; Massias, L.; Papy, E.; Tubach, F.; Wolff, M.; et al. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med. 2014, 40, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Takasu, O.; Sakai, Y.; Sakamoto, T.; Yamashita, N.; Mori, S.; Morita, T.; Nabeta, M.; Hirayu, N.; Yoshiyama, N.; et al. Development of a teicoplanin loading regimen that rapidly achieves target serum concentrations in critically ill patients with severe infections. J. Infect. Chemother. 2015, 21, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Dulhunty, J.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; et al. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother. 2015, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Zander, J.; Döbbeler, G.; Nagel, D.; Maier, B.; Scharf, C.; Huseyn-Zada, M.; Jung, J.; Frey, L.; Vogeser, M.; Zoller, M. Piperacillin concentration in relation to therapeutic range in critically ill patients—A prospective observational study. Crit. Care 2016, 20, 79. [Google Scholar] [CrossRef]
- Allou, N.; Charifou, Y.; Augustin, P.; Galas, T.; Valance, D.; Corradi, L.; Martinet, O.; Vandroux, D.; Allyn, J. A study to evaluate the first dose of gentamicin needed to achieve a peak plasma concentration of 30 mg/l in patients hospitalized for severe sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1187–1193. [Google Scholar] [CrossRef]
- Obara, V.Y.; Zacas, C.P.; Carrilho, C.M.D.d.M.; Delfino, V.D.A. Currently used dosage regimens of vancomycin fail to achieve therapeutic levels in approximately 40% of intensive care unit patients. Rev. Bras. Ter. Intensive 2016, 28, 380–386. [Google Scholar] [CrossRef]
- Bakke, V.; Sporsem, H.; Von der Lippe, E.; Nordøy, I.; Lao, Y.; Nyrerød, H.C.; Sandvik, L.; Hårvig, K.R.; Bugge, J.F.; Helset, E. Vancomycin levels are frequently subtherapeutic in critically ill patients: A prospective observational study. Acta Anaesthesiol. Scand. 2017, 61, 627–635. [Google Scholar] [CrossRef]
- Ruiz, J.; Ramirez, P.; Company, M.J.; Gordon, M.; Villarreal, E.; Concha, P.; Aroca, M.; Frasquet, J.; Remedios-Marqués, M.; Castellanos-Ortega. Impact of amikacin pharmacokinetic/pharmacodynamic index on treatment response in critically ill patients. J. Glob. Antimicrob. Resist. 2018, 12, 90–95. [Google Scholar] [CrossRef]
- Burger, R.; Guidi, M.; Calpini, V.; Lamoth, F.; Decosterd, L.; Robatel, C.; Buclin, T.; Csajka, C.; Marchetti, O. Effect of renal clearance and continuous renal replacement therapy on appropriateness of recommended meropenem dosing regimens in critically ill patients with susceptible life-threatening infections. J. Antimicrob. Chemother. 2018, 73, 3413–3422. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Xie, J.; Liu, L.; Liu, S.; Guo, F.; Qiu, H.; Yang, Y. Association Between Pathophysiology and Volume of Distribution Among Patients With Sepsis or Septic Shock Treated With Imipenem: A Prospective Cohort Study. J. Infect. Dis. 2020, 221, S272–S278. [Google Scholar] [CrossRef]
- Helset, E.; Nordøy, I.; Sporsem, H.; Bakke, V.D.; Bugge, J.F.; Gammelsrud, K.W.; Zucknick, M.; Lippe, E.; von der Lippe, E. Factors increasing the risk of inappropriate vancomycin therapy in ICU patients: A prospective observational study. Acta Anaesthesiol. Scand. 2020, 64, 1295–1304. [Google Scholar] [CrossRef]
- Ram, K.; Sheikh, S.; Bhati, R.K.; Tripathi, C.D.; Suri, J.C.; Meshram, G.G. Steady-state pharmacokinetic and pharmacodynamic profiling of colistin in critically ill patients with multi-drug-resistant gram-negative bacterial infections, along with differences in clinical, microbiological and safety outcome. Basic Clin. Pharmacol. Toxicol. 2021, 128, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hou, Y.; Li, J.; Gao, Y.; Li, R.; Jin, X.; Zhang, J.; Wang, X.; Wang, G. An evaluation on the association of vancomycin trough concentration with mortality in critically ill patients: A multicenter retrospective study. Clin. Transl. Sci. 2021, 14, 1780–1790. [Google Scholar] [CrossRef]
- Niibe, Y.; Suzuki, T.; Yamazaki, S.; Uchida, M.; Suzuki, T.; Takahashi, N.; Hattori, N.; Nakada, T.-A.; Ishii, I. Identification of factors affecting meropenem pharmacokinetics in critically ill patients: Impact of inflammation on clearance. J. Infect. Chemother. 2022, 28, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Mitton, B.; Paruk, F.; Gous, A.; Chausse, J.; Milne, M.; Becker, P.; Said, M. Evaluating the usefulness of the estimated glomerular filtration rate for determination of imipenem dosage in critically ill patients. South Afr. Med J. 2022, 112, 765–768. [Google Scholar] [CrossRef]
- Helset, E.; Cheng, V.; Sporsem, H.; Thorstensen, C.; Nordøy, I.; Gammelsrud, K.W.; Hanssen, G.; Ponzi, E.; Lipman, J.; von der Lippe, E. Meropenem pharmacokinetic/pharmacodynamic target attainment and clinical response in ICU patients: A prospective observational study. Acta Anaesthesiol. Scand. 2024, 68, 502–511. [Google Scholar] [CrossRef]
- Lejbman, I.A.; Torisson, G.; Resman, F.; Sjövall, F. Beta-lactam antibiotic concentrations in critically ill patients with standard and adjusted dosages: A prospective observational study. Acta Anaesthesiol. Scand. 2024, 68, 530–537. [Google Scholar] [CrossRef]
- Beckhouse, M.J.; Whyte, I.M.; Byth, P.L.; Napier, J.C.; Smith, A.J. Altered Aminoglycoside Pharmacokinetics in the Critically Ill. Anaesth. Intensive Care 1988, 16, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Zeitlinger, B.S.; Zeitlinger, M.; Leitner, I.; Müller, M.; Joukhadar, C. Clinical Scoring System for the Prediction of Target Site Penetration of Antimicrobials in Patients with Sepsis. Clin. Pharmacokinet. 2007, 46, 75–83. [Google Scholar] [CrossRef]
- Cristallini, S.; Hites, M.; Kabtouri, H.; Roberts, J.A.; Beumier, M.; Cotton, F.; Lipman, J.; Jacobs, F.; Vincent, J.-L.; Creteur, J.; et al. New Regimen for Continuous Infusion of Vancomycin in Critically Ill Patients. Antimicrob. Agents Chemother. 2016, 60, 4750–4756. [Google Scholar] [CrossRef]
- Ehmann, L.; Zoller, M.; Minichmayr, I.K.; Scharf, C.; Maier, B.; Schmitt, M.V.; Hartung, N.; Huisinga, W.; Vogeser, M.; Frey, L.; et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: A prospective observational study. Crit. Care 2017, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Deslandes, G.; Jalin, L.; Corvec, S.; Caillon, J.; Boutoille, D.; Grégoire, M.; Bretonnière, C. PK/PD targets of amikacin and gentamicin in ICU patients. Med. Et Mal. Infect. 2019, 50, 709–714. [Google Scholar] [CrossRef]
- Logre, E.; Enser, M.; Tanaka, S.; Dubert, M.; Claudinon, A.; Grall, N.; Mentec, H.; Montravers, P.; Pajot, O. Amikacin pharmacokinetic/pharmacodynamic in intensive care unit: A prospective database. Ann. Intensive Care 2020, 10, 75. [Google Scholar] [CrossRef]
- De Corte, T.; Verhaeghe, J.; Dhaese, S.; Van Vooren, S.; Boelens, J.; Verstraete, A.G.; Stove, V.; Ongenae, F.; De Bus, L.; Depuydt, P.; et al. Pathogen-based target attainment of optimized continuous infusion dosing regimens of piperacillin-tazobactam and meropenem in surgical ICU patients: A prospective single center observational study. Ann. Intensive Care 2023, 13, 35. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Chevrier, C.; Setti, J.L.; Jouve, E.; Marsot, A.; Julian, N.; Blin, O.; Simeone, P.; Lagier, D.; Mokart, D.; et al. β-Lactam Pharmacokinetic/Pharmacodynamic Target Attainment in Intensive Care Unit Patients: A Prospective, Observational, Cohort Study. Antibiotics 2023, 12, 1289. [Google Scholar] [CrossRef]
- El-Haffaf, I.; Marsot, A.; Hachemi, D.; Pesout, T.; Williams, V.; Smith, M.-A.; Albert, M.; Williamson, D. Exposure levels and target attainment of piperacillin/tazobactam in adult patients admitted to the intensive care unit: A prospective observational study. Can. J. Anaesth. 2024, 71, 511–522. [Google Scholar] [CrossRef]
- Campassi, M.L.; Gonzalez, M.C.; Masevicius, F.D.; Vazquez, A.R.; Moseinco, M.; Navarro, N.C.; Previgliano, L.; Rubatto, N.P.; Benites, M.H.; Estenssoro, E.; et al. Augmented renal clearance in critically ill patients: Incidence, associated factors and effects on vancomycin treatment. Rev. Bras. Ter. Intensive 2014, 26, 13–20. [Google Scholar] [CrossRef]
- Zeng, J.; Leng, B.; Guan, X.; Jiang, S.; Xie, M.; Zhu, W.; Tang, Y.; Zhang, L.; Sha, J.; Wang, T.; et al. Comparative pharmacokinetics of polymyxin B in critically ill elderly patients with extensively drug-resistant gram-negative bacteria infections. Front. Pharmacol. 2024, 15, 1347130. [Google Scholar] [CrossRef] [PubMed]
- Frazee, E.; Rule, A.D.; Lieske, J.C.; Kashani, K.B.; Barreto, J.N.; Virk, A.; Kuper, P.J.; Dierkhising, R.A.; Leung, N. Cystatin C–Guided Vancomycin Dosing in Critically Ill Patients: A Quality Improvement Project. Am. J. Kidney Dis. 2017, 69, 658–666. [Google Scholar] [CrossRef]
- You, X.; Dai, Q.; Hu, J.; Yu, M.; Wang, X.; Weng, B.; Cheng, L.; Sun, F. Therapeutic drug monitoring of imipenem/cilastatin and meropenem in critically ill adult patients. J. Glob. Antimicrob. Resist. 2024, 36, 252–259. [Google Scholar] [CrossRef]
- Huang, F.; Cao, W.-X.; Yan, Y.-Y.; Mao, T.-T.; Wang, X.-W.; Huang, D.; Qiu, Y.-S.; Lu, W.-J.; Li, D.-J.; Zhuang, Y.-G. Influence of continuous renal replacement therapy on the plasma concentration of tigecycline in patients with septic shock: A prospective observational study. Front. Pharmacol. 2023, 14, 1118788. [Google Scholar] [CrossRef]
- Poli, E.C.; Simoni, C.; André, P.; Buclin, T.; Longchamp, D.; Perez, M.-H.; Ferry, T.; Schneider, A.G. Clindamycin clearance during Cytosorb® hemoadsorption: A case report and pharmacokinetic study. Int. J. Artif. Organs 2019, 42, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Dimski, T.; Brandenburger, T.; MacKenzie, C.; Kindgen-Milles, D. Elimination of glycopeptide antibiotics by cytokine hemoadsorption in patients with septic shock: A study of three cases. Int. J. Artif. Organs 2020, 43, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Schwier, E.; Kirchner, C.; Winde, G.; Henzler, D.; Eickmeyer, C. Hemoadsorption with CytoSorb® and the early course of linezolid plasma concentration during septic shock. J. Artif. Organs 2021, 25, 86–90. [Google Scholar] [CrossRef]
- Liebchen, U.; Scharf, C.; Zoller, M.; Weinelt, F.; Kloft, C.; the CytoMero Collaboration Team; Michelet, R.; Schroeder, I.; Paal, M.; Vogeser, M.; et al. No clinically relevant removal of meropenem by cytokine adsorber CytoSorb® in critically ill patients with sepsis or septic shock. Intensive Care Med. 2021, 47, 1332–1333. [Google Scholar] [CrossRef]
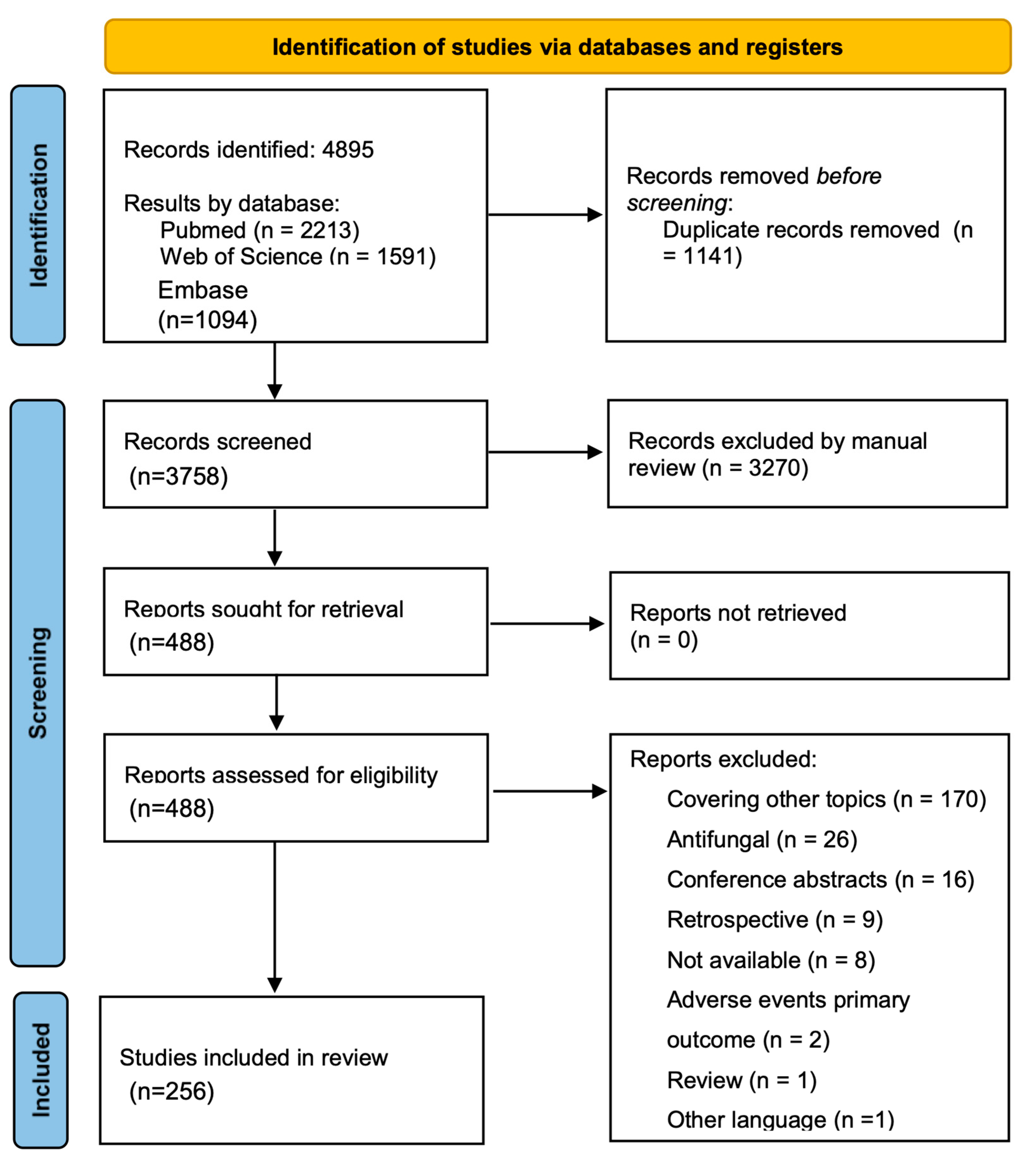
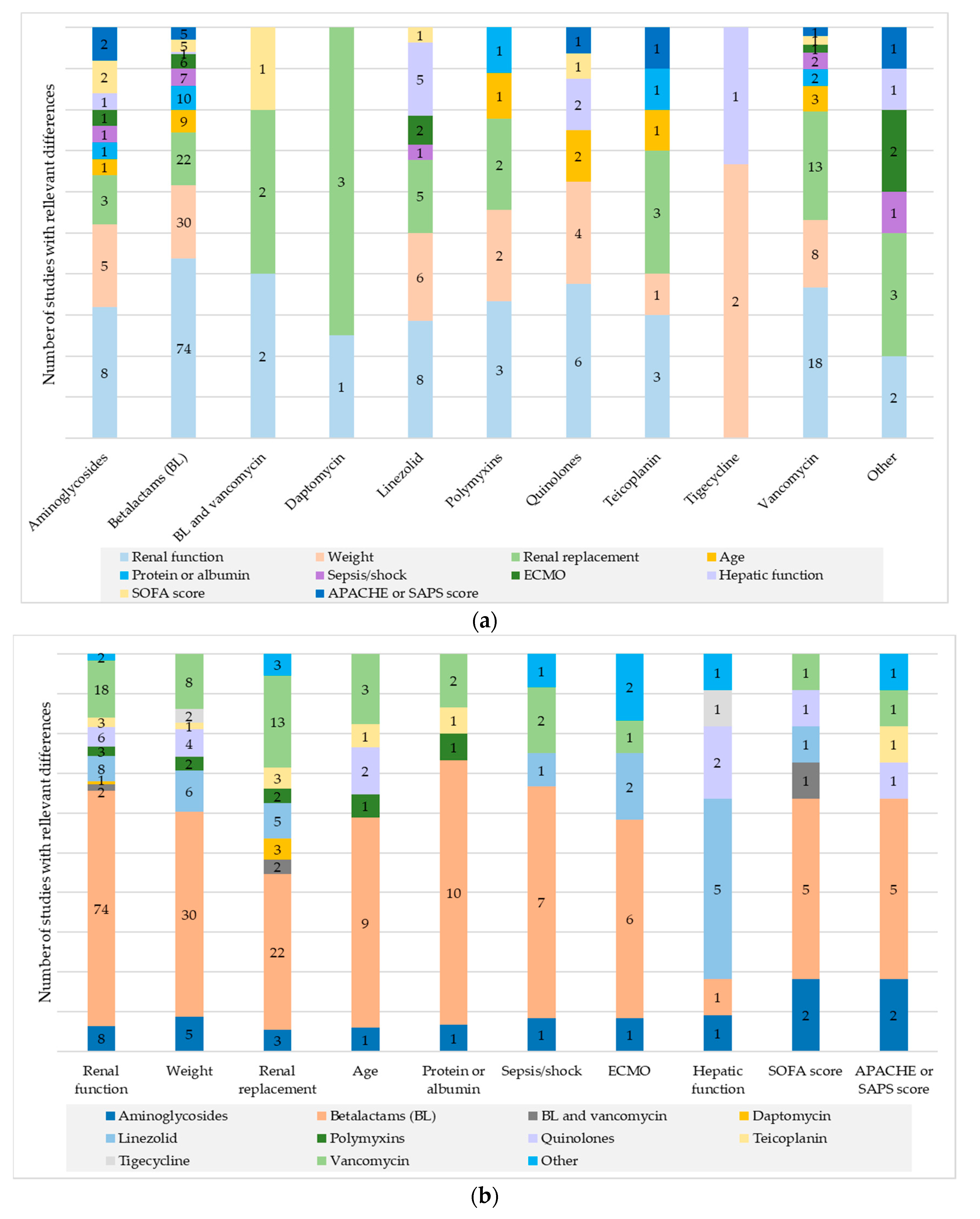

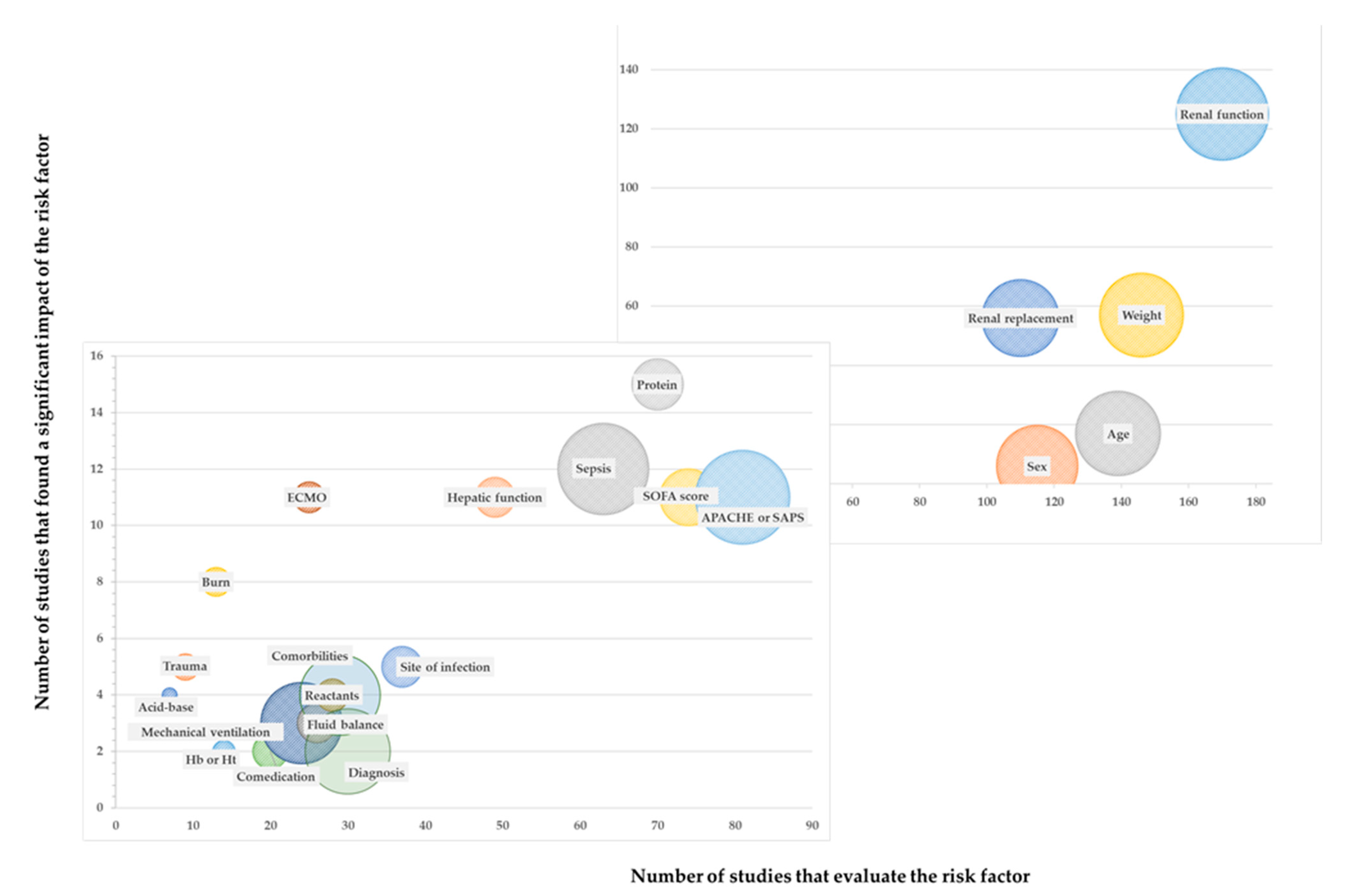
| Number of Studies | Number of Patients | ||
|---|---|---|---|
| Number of Centers | Unicentric | 222 | 11,342 |
| Multicentric | 34 | 10,157 | |
| Study Design | Prospective observational | 246 | 21,089 |
| RCT | 10 | 411 | |
| Outcome type | Exposure | 113 | 15,702 |
| PKPOP | 101 | 4628 | |
| PK | 42 | 1170 | |
| Type of analysis | Subgroup | 102 | 4827 |
| Subgroup and PKPOP | 62 | 2093 | |
| Risk factor, no PKPOP | 53 | 12,045 | |
| Risk factor and PKPOP | 39 | 2535 | |
| Distribution of studies by area | Europe | 125 | 7036 |
| North America | 39 | 9303 | |
| East Asia | 39 | 2707 | |
| Oceania | 21 | 700 | |
| South Asia | 9 | 335 | |
| South America | 8 | 689 | |
| Africa | 5 | 187 | |
| More than one area | 5 | 493 | |
| Middle East | 1 | 43 | |
| Publication year | 1988–1996 (9 years) | 5 | 117 |
| 1997–2005 (9 years) | 16 | 445 | |
| 2006–2014 (9 years) | 43 | 1997 | |
| 2015–2024 (9.2 years) | 192 | 18,941 | |
| Antibiotic group evaluated | More than two antibiotic groups | 6 | 256 |
| Aminoglycosides | 20 | 1767 | |
| Antituberculous | 1 | 10 | |
| Beta lactams | 137 | 7395 | |
| Daptomycin | 4 | 101 | |
| Linezolid | 23 | 643 | |
| Polimyxins | 7 | 207 | |
| Quinolones | 11 | 258 | |
| Teicoplanin | 7 | 416 | |
| Tigecycline | 4 | 89 | |
| Vancomycin | 42 | 10,602 |
| Antibiotic Group | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Factor/Primary Outcome | Amino-Glycosides | B-Lactams 2 | B-Lactams Vancomycin | Daptomycin | Linezolid 2 | Polymyxins | Quinolone | Teicoplanin | Tigecycline | Vancomycin | Other 1 | Number of Studies |
| Age | 1/10 (10.0) | 9/80 (11.3) | 0/1 (0) | 0/2 (0) | 0/11 (0) | 1/4 (25.0) | 2/9 (22.2) | 1/4 (25.0) | 0/3(0) | 3/16 (18.8) | 0 | 17/139 (12.2) |
| Exposure | 1/5 (20.0) | 3/20 (15.0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/3 (33.3) | 1/2 (50.0) | 1/2(50.0) | 0 | 1/6 (16.7) | 0 | 8/41 (19.5) |
| PK/PKPOP | 0/5 (0) | 6/60 (10.0) | 0 | 0/1 (0) | 0/10 (0) | 0/1 (0) | 1/7 (14.3) | 0/2 (0) | 0/3(0) | 2/10 (20.0) | 0 | 9/98 (9.2) |
| Sex | 0/8 (0) | 4/68 (5.9) | 0 | 0/2 (0) | 0/8 (0) | 0/3 (0) | 2/6 (33.3) | 0/3 (0) | 0/3 (0) | 0/15 (0) | 0 | 6/115 (5.2) |
| Exposure | 0/4 (0) | 1/15 (6.7) | 0 | 0/1 (0) | 0 | 0/2 (0) | 2/2 (100) | 0/1 (0) | 0 | 0/6 (0) | 0 | 3/31 (9.7) |
| PK/PKPOP | 0/4 (0) | 3/53 (5.7) | 0 | 0/1 (0) | 0/8 (0) | 0/1 (0) | 0/4 (0) | 0/2 (0) | 0/3 (0) | 0/9 (0) | 0 | 3/84 (3.6) |
| Weight | 5/7 (71.4) | 30/88 (34.1) | 0/1 | 0/2 (0) | 6/12 (50.0) | 2/6 (33.3) | 4/8 (50.0) | 1/3 (33.3) | 2/3 (66.7) | 8/17 (47.1) | 0 | 57/146 (39.0) |
| Exposure | 1/2 (50.0) | 6/22 (27.3) | 0/1 | 0/1 (0) | 1/2 (50.0) | 2/4 (50.0) | 1/2 (50.0) | 0/1 (0) | 0 | 3/5 (60.0) | 0 | 14/40 (35.0) |
| PK/PKPOP | 4/5 (80.0) | 24/66 (36.4) | 0 | 0/1 (0) | 5/10 (50.0) | 0/2 (0) | 3/6 (50.0) | 1/2 (50.0) | 2/3 (66.7) | 5/12 (41.7) | 0 | 43/106 (40.6) |
| Renal function | 8/11 (72.7) | 74/98 (75.5) | 2/2 (100) | 1/2 (50.0) | 8/13 (61.5) | 3/5 (60.0) | 6/8 (75.0) | 3/4 (75.0) | 0/2 (0) | 18/24 (75.0) | 2/2 (100) | 125/170 (73.5) |
| Exposure | 4/5 (80.0) | 24/30 (80.0) | 2/2 (100) | 0/1 (0) | 1/2 (50.0) | 2/3 (66.7) | 2/3 (66.7) | 1/1 (100) | 0 | 10/12 (83.3) | 2/2 (100) | 48/61 (78.7) |
| PK/PKPOP | 4/6 (66.7) | 50/68 (73.5) | 0 | 1/1 (100) | 7/11 (63.6) | 1/2 (50.0) | 4/5 (80.0) | 2/3 (66.7) | 0/2 (0) | 8/12 (66.7) | 0 | 77/109 (70.6) |
| Renal replacement | 3/7 (42.9) | 22/50 (44.0) | 2/3 (66.7) | 3/3 (100) | 5/14 (35.7) | 2/2 (100) | 0/5 (0) | 3/5 (60.0) | 1/3 (33.3) | 13/17 (76.5) | 3/3 (100) | 57/110 (51.8) |
| Exposure | 1/4 (25.0) | 9/21 (42.9) | 1/2 (50.0) | 1/1 (100) | 2/4 (50.0) | 1/1 (100) | 0 | 2/2 (100) | 1/1 (100) | 7/9 (77.8) | 1/1 (100) | 26/45 (57.8) |
| PK/PKPOP | 2/3 (66.7) | 13/29 (44.8) | 1/1 (100) | 2/2 (100) | 3/10 (30.0) | 1/1 (100) | 0/5 (0) | 1/3 (33.3) | 0/2 (0) | 6/8 (75.0) | 2/2 (100) | 31/65 (47.7) |
| Protein or albumin | 1/4 (25.0) | 10/49 (20.4) | 0/1 (0) | 0/1 (0) | 0/2 (0) | 1/4 (25.0) | 0/1 (0) | 1/2 (50.0) | 0/2 (0) | 2/4 (50.0) | 0 | 15/70 (21.4) |
| Exposure | 0/2 (0) | 1/10 (10.0) | 0/1 (0) | 0 | 0 | 1/2 (50.0) | 0 | 1/1 (100) | 0 | 1/1 (100) | 0 | 4/17 (23.5) |
| PK/PKPOP | 1/2 (50.0) | 9/39 (23.1) | 0 | 0/1 (0) | 0/2 (0) | 0/2 (0) | 0/1 (0) | 0/1 (0) | 0/2 (0) | 1/3 (33.3) | 0 | 11/53 (20.8) |
| APACHE or SAPS | 2/6 (33.3) | 5/46 (10.9) | 0/1 (0) | 0/1 (0) | 0/6 (0) | 0/2 (0) | 1/3 (33.3) | 1/2 (50.0) | 0/1 (0) | 1/11 (9.1) | 1/2 (50.0) | 11/81 (13.6) |
| Exposure | 2/4 (50.0) | 3/14 (21.4) | 0/1 (0) | 0 | 0/1 (0) | 0/1 (0) | 0 | 1/1 (100) | 0 | 1/6 (16.7) | 1/2 (50.0) | 8/30 (26.7) |
| PK/PKPOP | 0/2 (0) | 2/32 (6.3) | 0 | 0/1 (0) | 0/5 (0) | 0/1 (0) | 1/3 (33.3) | 0/1 (0) | 0/1 (0) | 0/5 (0) | 0 | 3/51 (5.9) |
| SOFA score | 2/5 (40.0) | 5/46 (10.9) | 1/1 (100) | 0 | 1/5 (20.0) | 0/2 (0) | 1/3 (33.3) | 0 | 0/1 (0) | 1/10 (10.0) | 0/1 (0) | 11/74 (14.9) |
| Exposure | 2/3 (66.7) | 4/13 (30.8) | 1/1 (100) | 0 | 0 | 0/2 (0) | 1/1 (100) | 0 | 0 | 0/4 (0) | 0/1 (0) | 8/25 (32.0) |
| PK/PKPOP | 0/2 (0) | 1/33 (3.0) | 0 | 0 | 1/5 (20.0) | 0 | 0/2 (0) | 0 | 0/1 (0) | 1/6 (16.7) | 0 | 3/49 (6.1) |
| Hepatic function | 1/3 (33.3) | 1/23 (4.3) | 0 | 0/1 (0) | 5/8 (62.5) | 0/3 (0) | 2/6 (33.3) | 0 | 1/1 (100) | 0/3 (0) | 1/1 (100) | 11/49 (22.4) |
| Exposure | 1/2 (50.0) | 1/3 (33.3) | 0 | 0 | 0 | 0/2 (0) | 0/2 (0) | 0 | 0 | 0/1 (0) | 1/1 (100) | 3/11 (27.3) |
| PK/PKPOP | 0/1 (0) | 0/20 (0) | 0 | 0/1 (0) | 5/8 (62.5) | 0/1 (0) | 2/4 (50.0) | 0 | 1/1 (100) | 0/2 (0) | 0 | 8/38 (21.1) |
| Sepsis/shock | 1/6 (16.7) | 7/39 (17.9) | 0 | 0 | 1/2 (50.0) | 0 | 0/3 (0) | 0 | 0 | 2/11 (18.2) | 1/2 (50.0) | 12/63 (19.0) |
| Exposure | 0/3 (0) | 3/13 (23.1) | 0 | 0 | 1/1 (100) | 0 | 0/1 (0) | 0 | 0 | 2/7 (28.6) | 1/2 (50.0) | 7/27 (25.9) |
| PK/PKPOP | 1/3 (33.3) | 4/26 (15.4) | 0 | 0 | 0/1 (0) | 0 | 0/2 (0) | 0 | 0 | 0/4 (0) | 0 | 5/36 (13.9) |
| Admission Diagnosis | 0/1 (0) | 1/17 (5.9) | 0/1 (0) | 0/1 (0) | 1/2 (50.0) | 0 | 0 | 0/1 (0) | 0 | 0/7 (0) | 0 | 2/30 (6.7) |
| Exposure | 0/1 (0) | 1/6 (16.7) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0 | 0 | 0/1 (0) | 0 | 0/4 (0) | 0 | 1/15 (6.7) |
| PK/PKPOP | 0 | 0/11 (0) | 0 | 0 | 1/1 (100) | 0 | 0 | 0 | 0 | 0/3 (0) | 0 | 1/15 (6.7) |
| Trauma | 1/1 (100) | 3/5 (60.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1/3 (33.3) | 0 | 5/9 (55.6) |
| Exposure | 1/1 (100) | 0/2 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1/3 (33.3) | 0 | 2/6 (33.3) |
| PK/PKPOP | 0 | 3/3 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3/3 (100) |
| Burn | 2/2 (100) | 6/9 (66.7) | 0 | 0 | 0/1 (0) | 0 | 0 | 0 | 0 | 0/1 (0) | 0 | 8/13 (61.5) |
| Exposure | 1/1 (100) | 5/6 (83.3) | 0 | 0 | 0/1 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 6/8 (75.0) |
| PK/PKPOP | 1/1 (100) | 1/3 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/1 (0) | 0 | 2/5 (40.0) |
| ECMO | 1/2 (50.0) | 6/14 (42.9) | 0 | 0/1 (0) | 2/2 (100) | 0 | 0/2 (0) | 0 | 0/1 (0) | 1/2 (50.0) | 2/2 (100) | 11/25 (44.0) |
| Exposure | 0/1 (0) | 1/5 (20.0) | 0 | 0/1 (0) | 2/2 (100) | 0 | 0 | 0 | 0 | 1/1 (100) | 2/2 (100) | 5/11 (45.5) |
| PK/PKPOP | 1/1 (100) | 5/9 (55.6) | 0 | 0 | 0 | 0 | 0/2 (0) | 0 | 0/1 (0) | 0/1 (0) | 0 | 6/14 (42.9) |
| Mechanical ventilation | 0/4 (0) | 1/14 (7.1) | 0 | 0 | 0 | 0 | 1/1 (100) | 0 | 0 | 1/5 (20.0) | 0 | 3/24 (12.5) |
| Exposure | 0/3 (0) | 0/4 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1/4 (25.0) | 0 | 1/11 (9.1) |
| PK/PKPOP | 0/1 (0) | 1/10 (10.0) | 0 | 0 | 0 | 0 | 1/1 (100) | 0 | 0 | 0/1 (0) | 0 | 2/13 (15.4) |
| pH parameters | 0 | 1/3 (33.3) | 0 | 0 | 1/1 (100) | 0 | 1/1 (100) | 0/1 (0) | 0 | 0 | 1/1 (100) | 4/7 (57.1) |
| Exposure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/1 (0) | 0 | 0 | 1/1 (100) | 1/2 (50.0) |
| PK/PKPOP | 0 | 1/3 (33.3) | 0 | 0 | 1/1 (100) | 0 | 1/1 (100) | 0 | 0 | 0 | 0 | 3/5 (60.0) |
| Acute reactants | 1/3 (33.3) | 3/19 (15.8) | 0 | 0/1 (0) | 0/1 (0) | 0 | 0 | 0/1 (0) | 0/1 (0) | 0/2 (0) | 0 | 4/28 (14.3) |
| Exposure | 1/2 (50.0) | 1/4 (25.0) | 0 | 0 | 0 | 0 | 0 | 0/1 (0) | 0 | 0/1 (0) | 0 | 2/8 (25.0) |
| PK/PKPOP | 0/1 (0) | 2/15 (13.3) | 0 | 0/1 (0) | 0/1 (0) | 0 | 0 | 0 | 0/1 (0) | 0/1 (0) | 0 | 2/20 (10.0) |
| Hemoglobin/hematocrit | 0/2 (0) | 1/7 (14.3) | 0 | 0/2 (0) | 0/1 (0) | 1/1 (100) | 0 | 0 | 0 | 0/1 (0) | 0 | 2/14 (14.3) |
| Exposure | 0/1 (0) | 0/1 (0) | 0 | 0/1 (0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/3 (0) |
| PK/PKPOP | 0/1 (0) | 1/6 (16.7) | 0 | 0/1 (0) | 0/1 (0) | 1/1 (100) | 0 | 0 | 0 | 0/1 (0) | 0 | 2/11 (18.2) |
| Fluid balance | 1/4 (25.0) | 2/15 (13.3) | 0/1 (0) | 0 | 0 | 0 | 0/2 (0) | 0 | 0/2 (0) | 0/2 (0) | 0 | 3/26 (11.5) |
| Exposure | 0/3 (0) | 1/3 (33.3) | 0/1 (0) | 0 | 0 | 0 | 0/1 (0) | 0 | 0 | 0/1 (0) | 0 | 1/9 (11.1) |
| PK/PKPOP | 1/1 (100) | 1/12 (8.3) | 0 | 0 | 0 | 0 | 0/1 (0) | 0 | 0/2 (0) | 0/1 (0) | 0 | 2/17 (11.8) |
| Comorbidities | 0/4 (0) | 3/14 (21.4) | 0 | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/2 (0) | 0 | 0 | 1/6 (16.7) | 0/1 (0) | 4/29 (13.8) |
| Exposure | 0/4 (0) | 1/6 (16.7) | 0 | 0/1 (0) | 0 | 0/1 (0) | 0 | 0 | 0 | 1/2 (50.0) | 0/1 (0) | 2/15 (13.3) |
| PK/PKPOP | 0 | 2/8 (25.0) | 0 | 0 | 0/1 (0) | 0 | 0/2 (0) | 0 | 0 | 0/4 (0) | 0 | 2/14 (14.3) |
| Comedication | 0/1 (0) | 0/10 (0) | 0 | 1/1 (100) | 0 | 0/2 (0) | 1/2 (50.0) | 0 | 0 | 0/3 (0) | 0/1 (0) | 2/20 (10.0) |
| Exposure | 0/1 (0) | 0/7 (0) | 0 | 1/1 (100) | 0 | 0/1 (0) | 0 | 0 | 0 | 0/1 (0) | 0/1 (0) | 1/12 (8.3) |
| PK/PKPOP | 0 | 0/3 (0) | 0 | 0 | 0 | 0/1 (0) | 1/2 (50.0) | 0 | 0 | 0/2 (0) | 0 | 1/8 (12.5) |
| Site of infection | 1/3 (33.3) | 3/21 (14.3) | 0 | 0/1 (0) | 1/4 (25.0) | 0/2 (0) | 0/2 (0) | 0/1 (0) | 0 | 0/3 (0) | 0 | 5/37 (13.5) |
| Exposure | 0/1 (0) | 3/9 (33.3) | 0 | 0/1 (0) | 1/2 (50.0) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0 | 0/1 (0) | 0 | 4/17 (23.5) |
| PK/PKPOP | 1/2 (50.0) | 0/12 (0) | 0 | 0 | 0/2 (0) | 0/1 (0) | 0/1 (0) | 0 | 0 | 0/2 (0) | 0 | 1/20 (5.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gras-Martín, L.; Plaza-Diaz, A.; Zarate-Tamames, B.; Vera-Artazcoz, P.; Torres, O.H.; Bastida, C.; Soy, D.; Ruiz-Ramos, J. Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review. Antibiotics 2024, 13, 801. https://doi.org/10.3390/antibiotics13090801
Gras-Martín L, Plaza-Diaz A, Zarate-Tamames B, Vera-Artazcoz P, Torres OH, Bastida C, Soy D, Ruiz-Ramos J. Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review. Antibiotics. 2024; 13(9):801. https://doi.org/10.3390/antibiotics13090801
Chicago/Turabian StyleGras-Martín, Laura, Adrián Plaza-Diaz, Borja Zarate-Tamames, Paula Vera-Artazcoz, Olga H. Torres, Carla Bastida, Dolors Soy, and Jesús Ruiz-Ramos. 2024. "Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review" Antibiotics 13, no. 9: 801. https://doi.org/10.3390/antibiotics13090801
APA StyleGras-Martín, L., Plaza-Diaz, A., Zarate-Tamames, B., Vera-Artazcoz, P., Torres, O. H., Bastida, C., Soy, D., & Ruiz-Ramos, J. (2024). Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review. Antibiotics, 13(9), 801. https://doi.org/10.3390/antibiotics13090801







