Abstract
Applying a saturated potassium iodide (KI) solution immediately after silver diamine fluoride (SDF) application may affect the inhibitory effects of SDF on biofilm formation. This study compared the efficacy of 38% SDF with and without KI on preventing mixed-species biofilm formation on human root dentin surfaces and assessed ion incorporation into root dentin. The biofilms, composed of Streptococcus mutans, Lactobacillus rhamnosus, and Actinomyces naeslundii, were grown on specimen surfaces treated with either SDF or SDF + KI. After 24 h, the biofilms were evaluated using scanning electron microscopy, live/dead staining, adenosine triphosphate (ATP) assays, colony-forming unit (CFU) counts, and quantitative polymerase chain reaction. A Mann–Whitney U test was used to compare the results between the groups. Ion incorporation was assessed using an electron probe microanalyzer. The relative ATP content in the SDF + KI group was significantly higher than that in the SDF group (p < 0.05). However, biofilm morphology and the logarithmic reduction in CFUs and bacterial DNA were comparable across the groups. The SDF + KI treatment resulted in less silver and fluoride ion incorporation than that yielded by SDF alone. The inhibitory effects of SDF and SDF + KI on mixed-species biofilm formation were almost equivalent, although KI application affected the ion incorporation.
1. Introduction
Root caries is a remarkable dental problem among older adults, particularly in Japan, where there is an increasing prevalence of this condition [1]. A recent prospective study highlighted that 59.6% of older adults requiring nursing care experience new instances of root caries annually [2]. An important consequence of untreated root caries is tooth loss, which significantly negatively affects oral health-related quality of life. Preventive interventions for root caries are needed for older adults to maintain their dentition and oral function throughout their lives.
Dental caries is a disease that results from the dysbiosis of biofilm adherent to the tooth surface, dominated by acidogenic and aciduric bacteria (e.g., Streptococcus mutans and lactobacilli) [3,4]. Research on the microbial etiology of root caries has identified that Streptococcus and Actinomyces species are predominant in supragingival lesions and that Actinomyces species are the only cariogenic bacteria that remain predominant in root caries that progress below the gingival margin [5]. Reducing biofilm formation or reducing specific cariogenic bacteria levels can decrease the likelihood of root caries. Thus, the inhibition of microbial colonization or modulating biofilm quality by pretreatment of root surfaces with potential antibiofilm agents is an approach for root caries prevention [6].
Silver diamine fluoride (SDF) has been widely used in Japanese dental clinics to manage root caries in older adults. Several randomized controlled clinical trials have demonstrated that SDF is effective in arresting root caries [7,8,9]. Moreover, a meta-analysis indicated that annual applications of 38% SDF are more effective than controls in preventing root caries [10]. The efficacy of SDF may be ascribed to its antibiofilm properties, which involve SDF-derived precipitates forming on root dentin surfaces [11]. Silver from SDF integrates into the crystal structure of hydroxyapatite [12], reducing bacterial accumulation [13]. Moreover, root dentin surfaces with an SDF coating inhibit decalcification induced by S. mutans by releasing silver and fluoride at the bacteria/dentin interface [14].
Although no clinical trials have reported serious adverse effects related to SDF application, its major drawback is the irreversible black staining from silver precipitation, which poses an esthetic concern for many patients [15,16]. Recently, it has been suggested that using a saturated potassium iodide (KI) solution immediately after SDF can reduce noticeable staining without altering the efficacy of SDF in arresting caries [17,18]. However, reversing staining by reducing the number of silver ions remaining on the tooth surface may impact SDF’s antibiofilm effect. While some studies have revealed that the SDF/KI combination does not reduce antibacterial efficacy compared to SDF alone [19], its effectiveness in inhibiting biofilm formation on the root dentin surface remains uncertain. This uncertainty arises because a previous study focused on single-species biofilms of S. mutans [11]. To date, no studies on the SDF combined with KI (SDF + KI) application have tested its effects against mixed-species cariogenic biofilm formation after topical application on root dentin.
Therefore, this study aimed to (i) compare the inhibitory effects of 38% SDF and 38% SDF + KI on mixed-species biofilm formation on human root dentin surfaces using a modified Robbins device (MRD) and (ii) assess silver and fluoride incorporation into the root dentin when treated with SDF and SDF combined with KI. An MRD is an in vitro model system that allows reproducible biofilm formation on tooth substrates under controlled flow conditions. The null hypothesis was that there would be no difference in the growth inhibition of mixed-species biofilms on root dentin surfaces between treatments with SDF and SDF + KI.
2. Results
2.1. Scanning Electron Microscopy (SEM) and Confocal Laser Scanning Microscopy (CLSM) Observations
SEM images revealed a considerably lower number of biofilm clusters on the root dentin surfaces in both the SDF and SDF + KI groups (Figure 1c,d,g,h) than those formed in the untreated control groups (Figure 1a,b,e,f). There was clear evidence of reduced biofilm formation when the dentin surfaces were pretreated with SDF or SDF + KI. However, no remarkable differences were observed between the SEM images of the SDF and SDF + KI groups.
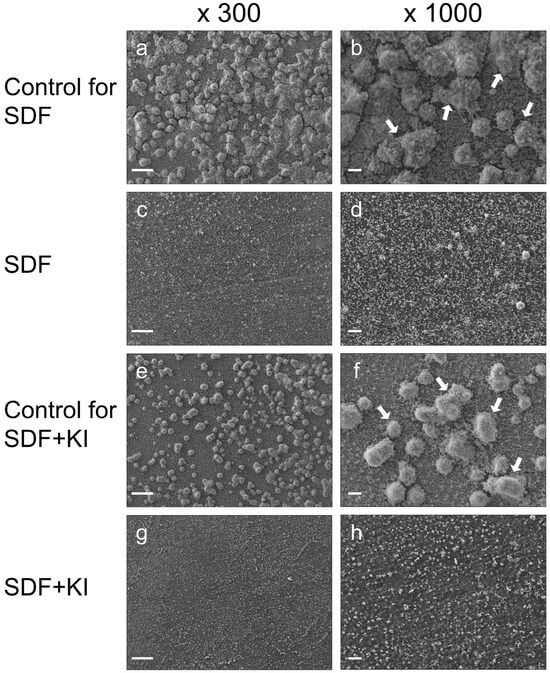
Figure 1.
Representative scanning electron microscopy (SEM) images of cariogenic biofilms formed on root dentin surfaces in the SDF (c,d), SDF + KI (g,h), and corresponding control groups (a,b,e,f) after 24 h of incubation. White arrows indicate the biofilm clusters. SDF: silver diamine fluoride; KI: potassium iodide; scale bars = 100 µm.
Corroborating the SEM findings, three-dimensional reconstructed images from live/dead staining indicate that the biofilms in both the SDF (Figure 2e–h) and SDF + KI groups (Figure 2m–p) were thinner than those in the control groups (Figure 2a–d,i–l). The ratio of dead to live cells in the SDF and SDF + KI groups was notably higher than in the corresponding controls (Figure 2q); however, differences between the SDF and SDF + KI groups were not readily apparent.
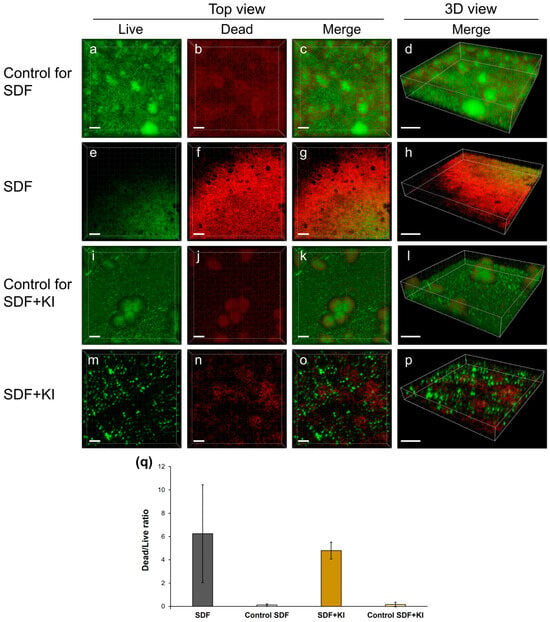
Figure 2.
Confocal laser scanning microscopy (CLSM) analysis of mixed-species biofilms consisting of S. mutans, L. rhamnosus, and A. naeslundii formed on root dentin surfaces. (a–p) Representative three-dimensional reconstructed images corresponding to live/dead staining; scale bars = 20 µm (top view) and 30 µm (3D view). The green signal is due to the SYTO9 dye which indicates live cells, while the red signal is due to propidium iodide which marks the dead cells. (q) Ratio of dead to live cells. SDF: silver diamine fluoride; KI: potassium iodide.
2.2. Relative Adenosine Triphosphate (ATP) Content
Figure 3 shows the percentage of relative ATP content in the biofilms formed on root dentin surfaces in the SDF and SDF + KI groups. The relative ATP content in the SDF group (1.83–4.07) was significantly lower than that in the SDF + KI group (3.65–52.00) (p < 0.05).
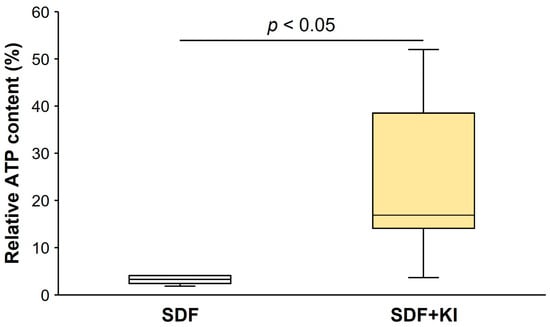
Figure 3.
Relative ATP content of biofilm on root dentin surfaces. Data from the control of each test group were used as the standard for calculating the relative content in comparison with the other groups. ATP: adenosine triphosphate; SDF: silver diamine fluoride; KI: potassium iodide.
2.3. Viable Bacterial Count
The mixed-species biofilm in the control groups constructed with the MRD flow-cell system comprised mainly viable cells of S. mutans and L. rhamnosus. The number of A. naeslundii was lower than that of the other microorganisms (Table 1). Figure 4 shows that the log reduction in viable S. mutans (1.60–3.04) and L. rhamnosus (2.13–4.38) in the SDF group was marginally greater than that in the SDF + KI group (0.97–2.37 and 2.01–3.55, respectively); however, a statistically significant difference was not observed (p = 0.08 and 0.35, respectively). Moreover, there was no significant difference in the log reduction in viable A. naeslundii between the SDF (2.00–2.79) and SDF + KI (2.00–2.70) groups (p = 0.67).

Table 1.
Mean, standard deviation (SD), 95% confidence interval (CI), and median of viable bacterial count (log CFU/mL) in each species for the experimental and corresponding control groups (n = 5).
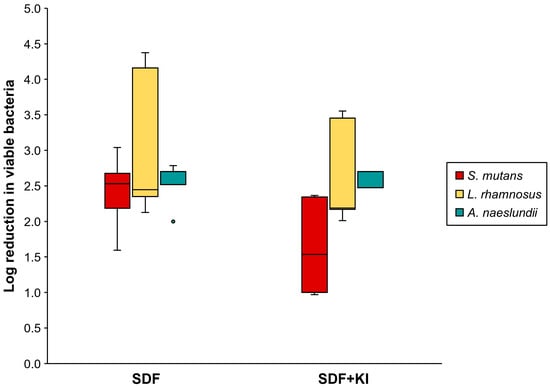
Figure 4.
Log reduction in viable cell number (CFU/mL) of S. mutans, L. rhamnosus, and A. naeslundii in the SDF and SDF + KI groups (n = 5). Medians, quartiles, and extreme values are given. SDF: silver diamine fluoride; KI: potassium iodide.
2.4. Bacterial DNA Quantification
The log reduction in bacterial DNA concentration for each group is shown in Figure 5. The log reduction in L. rhamnosus in the SDF group (0.40–2.24) was significantly greater than that in the SDF + KI group (−0.76–1.00) (p < 0.05), whereas no significant difference was observed for the log reduction in total bacterial 16S rDNA, S. mutans, and A. naeslundii.
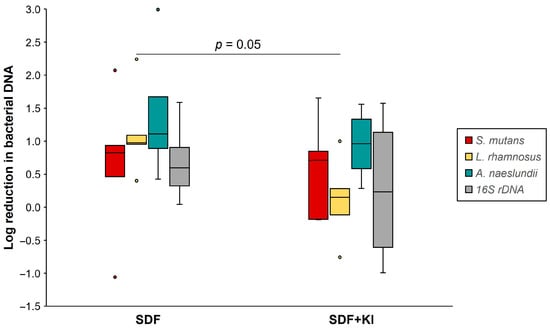
Figure 5.
Log reduction in DNA concentration (copies/mL) of S. mutans, L. rhamnosus, A. naeslundii, and 16S rDNA in the SDF and SDF + KI groups (n = 5). Medians, quartiles, and extreme values are given. SDF: silver diamine fluoride; KI: potassium iodide.
2.5. Ion Incorporation
Figure 6 shows the silver and fluoride incorporation into the root dentin in the SDF and SDF + KI groups compared to that of the untreated control. In the SDF group, there was a concentration gradient of silver and fluoride along the interior (Figure 6b,e), with the highest concentration on the surface (black arrowhead). Moreover, there was a noticeably dense layer of silver deposits on the root dentin superficial surface in the SDF group (Figure 6b), which was absent in the SDF + KI group (Figure 6c). The SDF + KI group showed less ion incorporation into dentin than that of the SDF group (Figure 6c,f). The untreated control did not show silver and fluoride incorporation (Figure 6a,d).
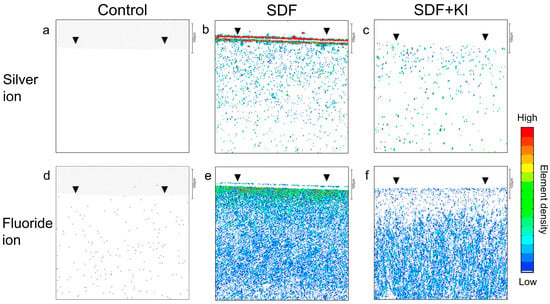
Figure 6.
Silver and fluoride distribution profiles in the longitudinal section of root dentin specimens after incubation for 24 h in the control (a–d), SDF (b–e), and SDF + KI (c–f) groups. Arrowheads indicate the disc surface. SDF: silver diamine fluoride; KI: potassium iodide.
3. Discussion
Applying a saturated KI solution to minimize black staining caused by silver-derived precipitates on dentin surfaces may affect the antibiofilm effect of SDF. This study found no remarkable difference in the efficacy of inhibitory biofilm formation on root dentin when pretreated with SDF alone versus SDF combined with KI, although KI application did affect the amount of silver and fluoride ion incorporation. Thus, the null hypothesis could not be rejected. For patients concerned with esthetics, using an SDF/KI combination as an antibiofilm agent appears to be a promising approach for preventing root caries.
In this study, there was clear evidence of reduced biofilm formation when the dentin surfaces were pretreated with SDF or SDF + KI. The percentage of reduction in viable bacterial cells in both the SDF and SDF + KI groups compared to the controls ranged from 90% to 99.99% (log reduction of 1–4). The effectiveness of these materials is further supported by findings from SEM (Figure 1) and CLSM (Figure 2) imaging. The antibiofilm effects appear to be due to the incorporation and release of silver and fluoride ions from the root dentin. These ions interact with hydroxyapatite in teeth to form calcium fluoride and silver phosphate [20]. Additionally, the high polarizing power of silver ions promotes strong bonding with amino acids in dentin proteins [21]. Evidence suggests that the precipitation of silver particles on tooth surfaces suppresses bacterial adhesion [12,13]. Moreover, a previous in vitro study using inductively coupled plasma mass spectrometry and fluoride ion-specific electrodes detected silver and fluoride ions within S. mutans cells surrounding SDF-coated tooth surfaces [14], indicating that SDF-treated tooth surfaces release these ions at the biofilm/tooth surface interface [14]. Silver ions are bactericidal metal cations that inhibit biofilm formation by inactivating glucosyltransferase enzymes responsible for glucan synthesis [22]. Glucan synthesized from sucrose is essential for mediating the adhesion of bacterial cells to tooth surfaces [23]. Fluoride promotes remineralization and, at high concentrations, impairs the proton-extruding ATPase enzyme related to the carbohydrate metabolism of acidogenic oral bacteria [24].
Although several studies confirm that SDF is effective and safe for patients, the associated tooth staining with SDF creates esthetic concerns, limiting its use in clinical practice [15,16,25]. To address this issue, discoloration by KI immediately after SDF application is recommended [26,27,28,29]. KI reverses tooth staining by preventing the formation of silver phosphate precipitates and creating soluble silver iodide [21]. Previous studies indicate that KI does not modulate the antibacterial efficacy of SDF against plaque biofilms [9,30]. SDF combined with KI shows a stable antibacterial effect against S. mutans at the minimum inhibitory concentration of 20% [31]. The use of SDF combined with KI increases cytocompatibility compared to SDF alone [32,33]. However, research on the inhibitory effects of SDF + KI on biofilm formation is limited [11]. A previous study indicated that the SDF + KI treatment was significantly less effective than 38% SDF alone in reducing S. mutans biofilm formation [11]. The inhibition of bacterial adhesion and co-aggregation on tooth surfaces may relate to the number of ions incorporated and released, as discussed earlier. In this study, SDF alone tended to present higher efficacy than when combined with KI. As shown in Figure 2, the biofilm on the SDF-treated surface consists mostly of dead cells. The relative ATP content from the biofilm in the SDF group was significantly lower than that in the SDF + KI group (Figure 3), while no notable differences were observed between the viable bacterial counts of the SDF and SDF + KI groups (Table 1). Electron probe microanalyzer (EPMA) analysis showed that SDF supplemented with KI reduced silver and fluoride incorporation into the root dentin compared to that with SDF alone (Figure 6). This reduction may have impacted the antibiofilm properties of the SDF/KI-treated surface. However, this study indicates that the remaining SDF-derived precipitates on root dentin after the 38% SDF + KI application, acting as a reservoir of silver and fluoride ions, appeared sufficient to inhibit mixed-species biofilm formation. Further work needs to validate the long-term efficacy of this antibiofilm agent with the varied concentration of SDF.
Herein, viable cells were assessed after 24 h in a biofilm model constructed using an MRD flow-cell system. The biofilm community was dominated by S. mutans, followed by L. rhamnosus and A. naeslundii (Table 1), aligning with previous findings [34]. The low number of viable A. naeslundii may result from shifts in microbial composition related to plaque pH [35]. Previous studies indicate that the microbial composition in the dynamic stability stage of dental biofilms (pH 7.0) is dominated by Actinomyces and non-mutans streptococci, which resemble biofilms on clinically sound enamel surfaces [35,36]. However, an acidic environment from frequent sugar intake decreases the viability of Actinomyces and non-mutans streptococci, shifting the dominance to more acidogenic/aciduric species like S. mutans and lactobacilli. Even when the plaque pH returns to neutral, the recovery of Actinomyces and non-mutans streptococci is slow [35,37]. This slow recovery may explain the reduced growth and low viability of A. naeslundii in our 24 h mixed-species cariogenic biofilm model. However, the microbial composition and characteristics of the biofilm community may differ with an increased incubation time. Further work with longer observation is needed to perform.
Demineralized dentin leads to more silver uptake than sound dentin due to highly porous tissue and exposed collagen [38]. Although this study used sound human root dentin as a substrate for SDF application, silver precipitates acting as an ion reservoir [39,40] were sufficient to provide an antibiofilm effect. Under physiological conditions, negatively charged proteins in the organic components of teeth coagulate by binding to silver ions [21]. Interestingly, a previous study shows that SDF-coated root dentin retains and releases the greatest amount of silver and fluoride ions compared to coronal enamel. Releasing ions at the biofilm/tooth interfaces inhibits the metabolic activity of S. mutans cells [14]. These results support the idea that SDF-derived components are more readily retained on sound root surfaces due to the abundant organic matter in the root dentin. Thus, SDF or SDF + KI could be beneficial as preventive materials on exposed root surfaces, as well as on an arrested carious dentin.
This study compared the effects of 38% SDF with and without KI on mixed-species cariogenic biofilm formation on human root dentin using an MRD flow-cell system, which more closely mimics natural biofilm behavior than mono-species biofilms or static conditions do. Mixed-species biofilm bacteria exhibit distinct growth rates, gene expression patterns, and altered phenotypes reflected in the enhancement of metabolic capacity, stress tolerance, and community-level signaling [41,42,43,44,45]. Previous research indicates that S. mutans within mixed-species biofilms increases the expression of specific genes related to glucan synthesis, remodeling, and binding, leading to variations in matrix construction and biofilm maintenance [41]. This study constructed a cariogenic biofilm with dominant bacterial species implicated in the initiation and progression of root caries [5,46,47,48]. A previous study indicates that the flow variable, simulating the continuous flow of saliva or gingival crevicular fluid, is an important factor that may create differences in both the biofilm structure and composition [49]. The MRD is a reproducible flow-cell system for mixed-species cariogenic biofilm formation, useful for evaluating the efficacy of antibiofilm agents before clinical trials [49,50,51]. This system allows the growth of mature biofilms, preventing the overgrowth of planktonic cells and the accumulation of bacterial metabolites. However, no model fully replicates the dynamics of the oral environment, and the limitations of each biofilm model must be considered when interpreting the research findings [51].
Nevertheless, this model provides valuable information for predicting clinical outcomes. While this biofilm model is an in vitro model providing a stable environment that differs from in vivo conditions, the limitations of such an in vitro study must be considered when interpreting the findings, as natural oral biofilms are more complex and dynamic ecosystems. Further research is needed to validate the efficacy of SDF combined with KI in clinical settings. An in situ biofilm model, reflecting intra-oral situations, is considered as an alternative for further validation.
4. Materials and Methods
4.1. Study Design and Specimen Preparation
This study assessed the effect of 38% SDF with and without KI on cariogenic biofilm grown on root dentin surfaces for 24 h in terms of biofilm morphology, bacterial viability, and bacterial DNA quantity. For ion incorporation into root dentins, silver and fluoride distribution profiles were assessed using EPMA analysis.
Rectangular root dentin slabs (3 × 3 × 2 mm) were prepared from roots of human upper premolar teeth (Niigata University Research Ethics Committee Approval No. 2022-0069) as previously described, with slight modifications [39]. Briefly, a human root was cut along the vertical plane (thickness) using a low-speed diamond saw to create two pieces of dentin specimens from the buccal and palatal sides. Thereafter, pieces of dentin were cut along the second plane (width and length) using tapered diamond burs. The specimen surfaces were further polished using a 2000-grit silicon carbide paper under water irrigation. To remove the organic tissue, specimens were immersed in 2.5% sodium hypochlorite (NaOCl) for 1 min, followed by ultrasonication with 17% ethylenediaminetetraacetic acid for 1 min and re-immersion in 2.5% NaOCl for 1 min. The specimens were mounted on MRD sampling plugs using a silicone ring (10 mm), and the MRD was sterilized using ethylene oxide.
The number of samples for biofilm analysis was defined by considering an effect size of 2, 0.05 level of significance, and 80% power. The total of 28 paired sampling plugs (N = 56) were then randomly assigned to the test materials in either the SDF or SDF + KI groups. One of the paired sampling plugs was allocated to the experimental group, and the other served as a control (n = 14 per group; 5 samples for viable bacterial count and ATP analysis; 5 samples for DNA quantification; 4 samples for morphological observations) (Figure 7). The samples in the SDF group were treated with 38% SDF (Saforide; Bee Brand Medico Dental, Osaka, Japan) for 4 min, followed by washing with distilled water for 30 s prior to the biofilm challenge. The specimens in the SDF + KI group were subjected to a layer of 38% SDF and agitated using a microbrush for 10 s, instantly followed by the application of a saturated KI solution in accordance with the manufacturer’s instructions (Riva star; SDI, Bayswater, Australia) until the creamy white solution became clear. Next, the reaction products were washed off with distilled water for 30 s. The control group did not receive any treatment.
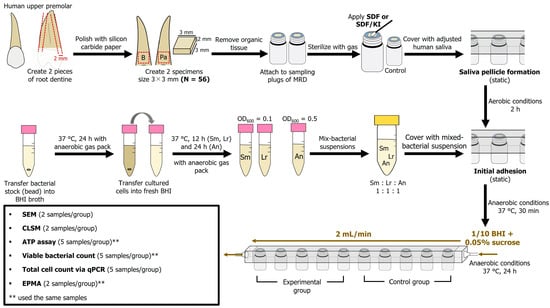
Figure 7.
Experimental flow chart. MRD: modified Robbins device; SDF: silver diamine fluoride; KI: potassium iodide; Sm: S. mutans; Lr: L. rhamnosus; An: A. naeslundii; BHI: brain–heart infusion; SEM: scanning electron microscopy; CLSM: confocal laser scanning microscopy; ATP: adenosine triphosphate; qPCR: quantitative polymerase chain reaction; EPMA: electron probe microanalyzer.
4.2. Bacterial Strains and Culture Conditions
In this study, three cariogenic bacterial strains, S. mutans (ATCC 25175), L. rhamnosus (ATCC 7469), and A. naeslundii (ATCC 12104), were used to create biofilms. Cariogenic bacterial strains were grown overnight from frozen stocks in brain–heart infusion (BHI) broth (Difco Laboratories, Sparks, MD, USA) at 37 °C under anaerobic conditions (80% N2, 10% H2, and 10% CO2). Starter cultures were inoculated into fresh media and cultured for 12 or 24 h under the same conditions. Overnight cultures of the three cariogenic strains in BHI were adjusted and diluted to an optimal optical density at 600 nm of approximately 107 colony-forming units (CFUs) per mL prior to inoculation.
4.3. Adjusted Saliva Preparation and Saliva Pellicle Formation
Unstimulated saliva was collected from one of the authors. Saliva samples were diluted (1:10) with sterile Ringer’s solution containing 0.05% cysteine (Sigma-Aldrich; St. Louis, MO, USA). Then, the dilute solution was centrifuged at 10,000× g, 4 °C for 10 min, and the supernatant was filter-sterilized [52]. The specimen surfaces were covered with adjusted human saliva (15 mL) for 2 h at 37 °C under aerobic conditions.
4.4. Biofilm Formation
The MRD method described in previous studies [50,52,53] was used to assess cariogenic biofilm formation. Following saliva pellicle formation, the mixed suspension containing equal amounts of each organism was pumped into the chamber and kept static for 30 min at 37 °C under anaerobic conditions to encourage initial adhesion. After 30 min, the biofilm developed on the specimen in 1/10 strength BHI broth containing 0.05% sucrose for 24 h under continuous flow at a flow rate of 2 mL/min at 37 °C under anaerobic conditions (Figure 7). After the incubation period, the samples were removed from the chamber and gently washed twice with sterile phosphate-buffered saline (PBS; pH = 7.0).
4.5. SEM Observation
Two biofilm samples in each group were fixed with 2.5% glutaraldehyde overnight at 4 °C. Subsequently, the samples were washed twice with PBS and dehydrated with an ascending ethanol series (50–100% [v/v]). The samples were then dried in a desiccator and sputtered with gold palladium [52]. The biofilms were observed using SEM (EPMA-1610; Shimadzu, Kyoto, Japan) at 300× and 1000× magnifications in a beam scan mode at 12 kV.
4.6. CLSM Observation
Two biofilm samples in each group were stained with a fluorescent bacterial viability kit (LIVE/DEAD BacLight Bacterial Viability Kit; Thermo Fisher Scientific, Waltham, MA, USA) at room temperature in the dark for 30 min [53]. Biofilm samples were imaged using CLSM (LSM 700; Carl Zeiss, Oberkochen, Germany) with Ar 488 nm and He-Ne 543 nm lasers. The 510–530 nm and ≥610 nm filters were used to detect SYTO 9 and propidium iodide, respectively. Three-dimensional reconstructed images were created using Imaris software version 9.6.0 (Bitplane AG, Zurich, Switzerland), and the dead and live bacterial cells were assessed. Biofilm samples formed on untreated specimen surfaces were used as control.
4.7. ATP Bioluminescence Assay
Specimens were transferred to an Eppendorf tube containing 1 mL PBS, ultrasonicated for 5 min, shaken vigorously for 1 min, and ultrasonicated again for 5 min to detach the biofilm from the dentin surfaces. Following biofilm detachment, the ATP level of the collected biofilm suspension was determined using the BacTiter-Glo microbial cell viability assay (Promega, Madison, WI, USA). Luminescence was measured using a GloMaxVR microplate reader (Promega, Madison, WI, USA). The relative ATP content of each test group was calculated using the following formula:
4.8. Viable Bacterial Count
The suspension with detached biofilm cells was homogenized, serially diluted tenfold, and plated on selective agar medium. The number of viable bacteria (CFU/mL) was determined after incubation for 48 h at 37 °C under anaerobic conditions. Selective media for S. mutans, L. rhamnosus, and A. naeslundii included Mitis Salivarius agar with bacitracin [54], modified LBS agar [55], and cadmium sulfate–fluoride–acridine trypticase agar [56,57], respectively, with slight modifications. Log reduction in viable cells of each bacterial species was calculated using the following formula:
4.9. DNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
After washing the biofilm samples, DNA was isolated using the DNeasy PowerSoil Pro Kit (Qiagen, Limburg, The Netherlands) according to the manufacturer’s instructions. Total genomic DNA was quantified and stored at −20 °C before further processing. The quantities of total bacterial DNA and three cariogenic strain DNA were evaluated using real-time PCR.
The sequences of primers used were as follows: total bacteria (16S rDNA) (5′-TCCTACGGGAGGCAGCAGT-3′ and 5′-GGACTACCAGGGTATCTAATCCTGTT-3′) [58,59], S. mutans (5′-AGCGTTGTCCGGATTTATTG-3′ and 5′-CTACGCATTTCACCGCTACA-3′) [60], L. rhamnosus (5′-AGGTGCTTGCATCTTGATTT-3′ and 5′-CGCCATCTTTCAGCCAAGAA-3′) [61], and A. naeslundii (5′-CTGCTGCTGACATCGCCGCTCGTA-3′ and 5′-TCCGCTCGCGCCACCTCTCGTTA-3′) [59,62]. Each reaction mixture (final volume 10 µL) contained 0.5 µL template DNA, 5 µL Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), forward/reverse primers at a final concentration of 500 nM, and 3.5 µL of ultra-pure water. A StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Foster City, CA, USA) was used to estimate the numbers of biofilm-forming bacteria via a calibration curve method. Calibration curves were prepared using S. mutans (ATCC 25175), L. rhamnosus (ATCC 7469), and A. naeslundii (ATCC 12104) genomic DNA. Log reduction in DNA concentration (copies/mL) was calculated using the following formula:
4.10. EPMA Analysis
Two dentin discs from the SDF and SDF + KI groups were sectioned longitudinally after removing the biofilm and were embedded in a chemically polymerized resin. The cut surface was serially polished with 2400-grit and 4000-grit SiC papers (Marumoto Struers KK, Tokyo, Japan). Element mappings of silver and fluoride ions were performed to evaluate the penetration of silver and fluoride ions into root dentin according to a previous protocol [53]. A root dentin specimen without treatment and incubation served as a negative control.
4.11. Statistical Analyses
All statistical analyses were performed using SPSS version 11.0 (SPSS, Chicago, IL, USA). Statistical significance was set at p < 0.05. The assumption of normal distribution of the data was tested using the Shapiro–Wilk test. The Mann-Whitney U test was used to compare the biofilm cell viability (relative ATP content, log reduction in viable bacteria) and log reduction in DNA concentration between SDF and SDF + KI groups.
Author Contributions
Conceptualization, J.M., M.S. and S.T.; Methodology, J.M., M.S. and S.T.; Investigation, J.M., M.S., N.K., R.N., T.I., R.T. and R.S.; Formal Analysis, J.M., M.S. and S.T.; Visualization, J.M. and M.S.; Writing—Original Draft Preparation, J.M.; Writing—Review and Editing, M.S.; Supervision, Y.N.; Project Administration, Y.N.; Funding Acquisition, M.S. and Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI, Grant Numbers JP21H03117B and 21K16990.
Institutional Review Board Statement
The study design was reviewed and approved by the Ethics Committee of Niigata University, Niigata, Japan (Approval No. 2022-0069). All procedures performed in studies involving human participants were conducted in accordance with ethical guidelines for human medical science studies.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suzuki, S.; Onose, Y.; Yoshino, K.; Takayanagi, A.; Kamijo, H.; Sugihara, N. Factors associated with development of root caries in dentition without root caries experience in a 2-year cohort study in Japan. J. Dent. 2020, 95, 103304. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, K.; Kimura-Ono, A.; Mino, T.; Osaka, S.; Numoto, K.; Koyama, E.; Kurosaki, Y.; Nakagawa, S.; Amano, Y.; Nguyen, H.T.T.; et al. Risk factors for root caries annual incidence and progression among older people requiring nursing care: A one-year prospective cohort study. J. Prosthodont. Res. 2022, 66, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health 2006, 6 (Suppl. 1), S14. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Edanami, N.; Komatsu, Y.; Nagata, R.; Naksagoon, T.; Sotozono, M.; Ida, T.; Noiri, Y. Periodontal pathogens inhabit root caries lesions extending beyond the gingival margin: A next-generation sequencing analysis. Microorganisms 2021, 9, 2349. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the use of mouthwash for the control of supragingival biofilm and its potential adverse effects. Antibiotics 2022, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.P.; Lo, E.C.; Dyson, J.E.; Luo, Y.; Corbet, E.F. A randomized trial on root caries prevention in elders. J. Dent. Res. 2010, 89, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; McGrath, C.; Lo, E.C.; Li, J.Y. Silver diamine fluoride and education to prevent and arrest root caries among community-dwelling elders. Caries Res. 2013, 47, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lo, E.C.; Liu, B.Y.; Wong, M.C.; Chu, C.H. Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J. Dent. 2016, 51, 15–20. [Google Scholar] [CrossRef]
- Zhang, J.; Sardana, D.; Li, K.Y.; Leung, K.C.M.; Lo, E.C.M. Topical fluoride to prevent root caries: Systematic review with network meta-analysis. J. Dent. Res. 2020, 99, 506–513. [Google Scholar] [CrossRef]
- Takahashi, M.; Matin, K.; Matsui, N.; Shimizu, M.; Tsuda, Y.; Uchinuma, S.; Hiraishi, N.; Nikaido, T.; Tagami, J. Effects of silver diamine fluoride preparations on biofilm formation of Streptococcus mutans. Dent. Mater. J. 2021, 40, 911–917. [Google Scholar] [CrossRef]
- Yu, O.Y.; Mei, M.L.; Zhao, I.S.; Li, Q.L.; Lo, E.C.; Chu, C.H. Remineralisation of enamel with silver diamine fluoride and sodium fluoride. Dent. Mater. 2018, 34, e344–e352. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Mayanagi, G.; Azumi, M.; Otani, H.; Fukushima, A.; Sasaki, K.; Takahashi, N. Sodium fluoride and silver diamine fluoride-coated tooth surfaces inhibit bacterial acid production at the bacteria/tooth interface. J. Dent. 2019, 84, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Lo, E.C. Promoting caries arrest in children with silver diamine fluoride: A review. Oral Health Prev. Dent. 2008, 6, 315–321. [Google Scholar] [PubMed]
- Duangthip, D.; Fung, M.H.T.; Wong, M.C.M.; Chu, C.H.; Lo, E.C.M. Adverse Effects of silver diamine fluoride treatment among preschool children. J. Dent. Res. 2018, 97, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Al Marzooq, F.; Mohamad, S.; Abd Rahman, N.; Rani, K.G.A.; Chi Ngo, H.; Samaranayake, L.P. The antibacterial efficacy of silver diamine fluoride (SDF) is not modulated by potassium iodide (KI) supplements: A study on in-situ plaque biofilms using viability real-time PCR with propidium monoazide. PLoS ONE 2020, 15, e0241519. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Bradley, J.; Merkley, S.; Pachal, T.; Gopal, J.V.; Sharma, D. Does potassium iodide application following silver diamine fluoride reduce staining of tooth? A systematic review. Aust. Dent. J. 2020, 65, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Haiat, A.; Ngo, H.C.; Samaranayake, L.P.; Fakhruddin, K.S. The effect of the combined use of silver diamine fluoride and potassium iodide in disrupting the plaque biofilm microbiome and alleviating tooth discoloration: A systematic review. PLoS ONE 2021, 16, e0252734. [Google Scholar] [CrossRef]
- Lou, Y.L.; Botelho, M.G.; Darvell, B.W. Reaction of silver diamine [corrected] fluoride with hydroxyapatite and protein. J. Dent. 2011, 39, 612–618. [Google Scholar] [CrossRef]
- Sayed, M.; Matsui, N.; Hiraishi, N.; Inoue, G.; Nikaido, T.; Burrow, M.F.; Tagami, J. Evaluation of discoloration of sound/demineralized root dentin with silver diamine fluoride: In-vitro study. Dent. Mater. J. 2019, 38, 143–149. [Google Scholar] [CrossRef]
- dos Santos Junior, V.E.; Targino, A.G.R.; Flores, M.A.P.; Rodríguez-Díaz, J.M.; Teixeira, J.A.; Heimer, M.V.; Pessoa, H.d.L.F.; Galembeck, A.; Rosenblatt, A. Antimicrobial activity of silver nanoparticle colloids of different sizes and shapes against Streptococcus mutans. Res. Chem. Intermed. 2017, 43, 5889–5899. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Koo, H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv. Dent. Res. 2008, 20, 17–21. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Janal, M.N.; Hamilton, D.S.; Niederman, R. Parental perceptions and acceptance of silver diamine fluoride staining. J. Am. Dent. Assoc. 2017, 148, 510–518.e4. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Niederman, R. Evidence-based dentistry update on silver diamine fluoride. Dent. Clin. N. Am. 2019, 63, 45–68. [Google Scholar] [CrossRef]
- Garg, S.; Sadr, A.; Chan, D. Potassium iodide reversal of silver diamine fluoride staining: A case report. Oper Dent. 2019, 44, 221–226. [Google Scholar] [CrossRef]
- Zhao, I.S.; Mei, M.L.; Burrow, M.F.; Lo, E.C.; Chu, C.H. Effect of silver diamine fluoride and potassium iodide treatment on secondary caries prevention and tooth discolouration in cervical glass ionomer cement restoration. Int. J. Mol. Sci. 2017, 18, 340. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; López, L.A.; Quock, R.L. Silver diamine fluoride, potassium iodide, and esthetic perception: An in vitro pilot study. Am J. Dent. 2016, 29, 248–250. [Google Scholar]
- Hamama, H.H.; Yiu, C.K.; Burrow, M.F. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust. Dent. J. 2015, 60, 80–87. [Google Scholar] [CrossRef]
- Alvarez-Marín, C.A.; Robles-Bermeo, N.L.; Hassan Moustafa, W.H.; Medina-Solís, C.E. Antibacterial effects of silver diamine fluoride with and without potassium iodide against Streptococcus mutans. Contemp. Clin. Dent. 2024, 15, 22–26. [Google Scholar] [CrossRef] [PubMed]
- García-Bernal, D.; Pecci-Lloret, M.P.; López-García, S. The cytocompatibility of silver diamine fluoride on mesenchymal stromal cells from human exfoliated deciduous teeth: An in vitro study. Materials 2022, 15, 2104. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.O.; Mendes Soares, I.P.; Anselmi, C.; Pires, M.; Ribeiro, R.A.O.; Peruchi, V.; de Souza Costa, C.A.; Hebling, J. Pulp cell response to the application of silver diamine fluoride and potassium iodide on caries-like demineralized dentin. Clin. Oral Investig. 2023, 27, 7295–7306. [Google Scholar] [CrossRef] [PubMed]
- Göstemeyer, G.; Woike, H.; Paris, S.; Schwendicke, F.; Schlafer, S. Root caries preventive effect of varnishes containing fluoride or fluoride + chlorhexidine/cetylpyridinium chloride in vitro. Microorganisms 2021, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D. Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res. 1998, 32, 456–462. [Google Scholar] [CrossRef]
- Horiuchi, M.; Washio, J.; Mayanagi, H.; Takahashi, N. Transient acid-impairment of growth ability of oral Streptococcus, Actinomyces, and Lactobacillus: A possible ecological determinant in dental plaque. Oral Microbiol. Immunol. 2009, 24, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Nikaido, T.; Abdou, A.; Burrow, M.F.; Tagami, J. Potential use of silver diammine fluoride in detection of carious dentin. Dent. Mater. J. 2021, 40, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Klanliang, K.; Asahi, Y.; Maezono, H.; Sotozono, M.; Kuriki, N.; Machi, H.; Ebisu, S.; Hayashi, M. An extensive description of the microbiological effects of silver diamine fluoride on dental biofilms using an oral in situ model. Sci. Rep. 2022, 12, 7435. [Google Scholar] [CrossRef]
- Mei, M.L.; Li, Q.L.; Chu, C.H.; Lo, E.C.; Samaranayake, L.P. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 4. [Google Scholar] [CrossRef]
- Klein, M.I.; Xiao, J.; Lu, B.; Delahunty, C.M.; Yates, J.R., 3rd; Koo, H. Streptococcus mutans protein synthesis during mixed-species biofilm development by high-throughput quantitative proteomics. PLoS ONE 2012, 7, e45795. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.G.; Ellepolla, A.; Drobiova, H.; Karched, M. Biofilm growth and IL-8 & TNF-α-inducing properties of Candida albicans in the presence of oral gram-positive and gram-negative bacteria. BMC Microbiol. 2020, 20, 156. [Google Scholar] [CrossRef]
- Xu, T.; Xiao, Y.; Wang, H.; Zhu, J.; Lee, Y.; Zhao, J.; Lu, W.; Zhang, H. Characterization of mixed-species biofilms formed by four gut microbiota. Microorganisms 2022, 10, 2332. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Preza, D.; Olsen, I.; Aas, J.A.; Willumsen, T.; Grinde, B.; Paster, B.J. Bacterial profiles of root caries in elderly patients. J. Clin. Microbiol. 2008, 46, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, Y.; Lin, Y.; Du, M.; Li, Y.; Fan, M. Salivary levels of five microorganisms of root caries in nursing home elderly: A preliminary investigation. BMC Oral Health 2023, 23, 355. [Google Scholar] [CrossRef] [PubMed]
- Schüpbach, P.; Osterwalder, V.; Guggenheim, B. Human root caries: Microbiota of a limited number of root caries lesions. Caries Res. 1996, 30, 52–64. [Google Scholar] [CrossRef]
- Blanc, V.; Isabal, S.; Sánchez, M.C.; Llama-Palacios, A.; Herrera, D.; Sanz, M.; León, R. Characterization and application of a flow system for in vitro multispecies oral biofilm formation. J. Periodontal. Res. 2014, 49, 323–332. [Google Scholar] [CrossRef]
- Naksagoon, T.; Ohsumi, T.; Takenaka, S.; Nagata, R.; Hasegawa, T.; Maeda, T.; Noiri, Y. Effect of water aging on the anti-biofilm properties of glass ionomer cement containing fluoro-zinc-silicate fillers. Biofouling 2020, 36, 1090–1099. [Google Scholar] [CrossRef]
- Kohno, T.; Kitagawa, H.; Tsuboi, R.; Nishimura, Y.; Imazato, S. Establishment of novel in vitro culture system with the ability to reproduce oral biofilm formation on dental materials. Sci. Rep. 2021, 11, 21188. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Oda, M.; Domon, H.; Ohsumi, T.; Suzuki, Y.; Ohshima, H.; Yamamoto, H.; Terao, Y.; Noiri, Y. Vizantin inhibits bacterial adhesion without affecting bacterial growth and causes Streptococcus mutans biofilm to detach by altering its internal architecture. Biochem. Biophys. Res. Commun. 2016, 480, 173–179. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takenaka, S.; Ohsumi, T.; Ida, T.; Ohshima, H.; Terao, Y.; Naksagoon, T.; Maeda, T.; Noiri, Y. Effect of a novel glass ionomer cement containing fluoro-zinc-silicate fillers on biofilm formation and dentin ion incorporation. Clin. Oral Investig. 2020, 24, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Gold, O.G.; Jordan, H.V.; Van Houte, J. A selective medium for Streptococcus mutans. Arch. Oral Biol. 1973, 18, 1357–1364. [Google Scholar] [CrossRef]
- Kinoshita, H.; Ohuchi, S.; Arakawa, K.; Watanabe, M.; Kitazawa, H.; Saito, T. Isolation of lactic acid bacteria bound to the porcine intestinal mucosa and an analysis of their moonlighting adhesins. Biosci. Microbiota Food Health 2016, 35, 185–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellen, R.P.; Balcerzak-Raczkowski, I.B. Differential medium for detecting dental plaque bacteria resembling Actinomyces viscosus and Actinomyces naeslundii. J. Clin. Microbiol. 1975, 2, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Zylber, L.J.; Jordan, H.V. Development of a selective medium for detection and enumeration of Actinomyces viscosus and Actinomyces naeslundii in dental plaque. J. Clin. Microbiol. 1982, 15, 253–259. [Google Scholar] [CrossRef]
- Pahumunto, N.; Sophatha, B.; Piwat, S.; Teanpaisan, R. Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: A double-blind, randomized, controlled study. J. Dent. Sci 2019, 14, 178–184. [Google Scholar] [CrossRef]
- Neves, B.G.; Stipp, R.N.; Bezerra, D.D.S.; Guedes, S.F.F.; Rodrigues, L.K.A. Quantitative analysis of biofilm bacteria according to different stages of early childhood caries. Arch. Oral Biol. 2018, 96, 155–161. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch. Oral Biol. 2012, 57, 678–683. [Google Scholar] [CrossRef]
- Chen, Z.; Schlafer, S.; Göstemeyer, G.; Schwendicke, F. Probiotic effects on multispecies biofilm composition, architecture, and caries activity in vitro. Microorganisms 2020, 8, 1272. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lim, Y.K.; Kook, J.K. Development of quantitative real-time PCR primers for detecting 42 oral bacterial species. Arch. Microbiol. 2013, 195, 473–482. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).