Matrix Metalloproteinase 9 (MMP-9) and Interleukin-8 (IL-8) in Gingival Crevicular Fluid after Minimally Invasive Periodontal Surgery with or without Er:YAG and Nd:YAG Laser Application
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population and Experimental Design
- -
- Presence of an intrabony defect, with a pocket depth (PD) ≥ 6 mm and a radiological defect depth of ≥3 mm and width of ≥2 mm;
- -
- Over 18 years of age;
- -
4.2. Clinical Examinations, Surgery, and Postoperative Care
4.3. GCF Sampling
4.4. GCF IL-8 and MMP-9 Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries:findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S130–S148. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Selikowitz, H.S.; Mathur, M.; Varenne, B. Strengthening oral health for universal health coverage. Lancet 2018, 392, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Tonetti, M.S. Diagnosis and epidemiology of periodontal osseous lesions. Periodontology 2000 2000, 22, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Choi, S.H.; Kim, T.S.; Kaltschmitt, J.; Eickholz, P. The infrabony defect and its determinants. J. Periodontal Res. 2006, 41, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Andreuccetti, G.; Blasi, A.; Matarasso, M.; Sculean, A.; Salvi, G.E. Clinical outcomes following regenerative therapy of non-contained intrabony defects using a deproteinized bovine bone mineral combined with either enamel matrix derivative or collagen membrane. J Periodontol. 2014, 85, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Blasi, A.; Nuzzolo, P.; Matarasso, M.; Isola, G.; Ramaglia, L. Treatment of periodontal intrabony defects using enamel matrix derivative: Surgical reentry after an observation period of at least 5 years. Int. J. Periodontics Restor. Dent. 2019, 39, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Bertl, K.; Spineli, L.M.; Sculean, A.; Cortellini, P.; Tonetti, M. Medium- and long-term clinical benefits of periodontal regenerative/reconstructive procedures in intrabony defects: Systematic review and network meta-analysis of randomized controlled clinical studies. J. Clin. Periodontol. 2021, 48, 410–430. [Google Scholar] [CrossRef]
- Thamaraiselvan, M.; Narayan, S.; Soundarajan, S. Minimally Invasive Surgery Periodontal Therapy for the Treatment of Intrabony Periodontal Defects: A Systematic Review. Contemp. Clin. Dent. 2022, 13, 101–107. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J. Clin. Periodontol. 2009, 36, 157–163. [Google Scholar] [CrossRef]
- Windisch, P.; Orban, K.; Salvi, G.E.; Sculean, A.; Molnar, B. Vertical-guided bone regeneration with a titanium-reinforced d-PTFE membrane utilizing a novel split-thickness flap design: A prospective case series. Clin Oral Investig. 2021, 25, 2969–2980. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A.A.; Mizutani, K.; Sasaki, K.M.; Izumi, Y. Application of lasers in periodontics: True innovation or myth? Periodontology 2000 2009, 50, 90–126. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, L.H.; Marcantonio, R.A.C.; Wainwright, M.; Garcia, V.G. LASER in periodontal treatment: Is it an effective treatment or science fiction? Braz. Oral Res. 2021, 35 (Suppl. S2), e099. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.T., Jr.; Deutsch, T.F. Er:YAG laser ablation of tissue: Measurement of ablation rates. Lasers Surg. Med. 1989, 9, 327–337. [Google Scholar] [CrossRef]
- Eberhard, J.; Ehlers, H.; Falk, W.; Açil, Y.; Albers, H.K.; Jepsen, S. Efficacy of subgingival calculus removal with Er:YAG laser compared to mechanical debridement: An in situ study. J. Clin. Periodontol. 2003, 30, 511–518. [Google Scholar] [CrossRef]
- Aoki, A.; Ando, Y.; Watanabe, H.; Ishikawa, I. In vitro studies on laser scaling of subgingival calculus with an erbium:YAG laser. J. Periodontol. 1994, 65, 1097–1106. [Google Scholar] [CrossRef]
- Crespi, R.; Romanos, G.E.; Cassinelli, C.; Gherlone, E. Effects of Er:YAG laser and ultrasonic treatment on fibroblast attachment to root surfaces: An in vitro study. J. Periodontol. 2006, 77, 1217–1222. [Google Scholar] [CrossRef]
- Roncati, M.; Gariffo, A. Systematic review of the adjunctive use of diode and Nd:YAG lasers for nonsurgical periodontal instrumentation. Photomed. Laser Surg. 2014, 32, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, R.; Lv, X.; Qu, C. Efficacy of a combined Er:YAG laser and Nd:YAG laser in non-surgical treatment for severe periodontitis. Lasers Med. Sci. 2022, 37, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Estrin, N.E.; Moraschini, V.; Zhang, Y.; Romanos, G.E.; Sculean, A.; Miron, R.J. Combination of Nd:YAG and Er:YAG lasers in non-surgical periodontal therapy: A systematic review of randomized clinical studies. Lasers Med. Sci. 2022, 37, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. Shifting the paradigm from inhibitors of inflammation to resolvers of inflammation in periodontitis. J. Periodontol. 2020, 91 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Lee, H.M. Periodontal therapeutics: Current host-modulation agents and future directions. Periodontology 2000 2020, 82, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S. Molecular factors associated with compartmentalization of gingival immune responses and transepithelial neutrophil migration. J. Periodontal Res. 1997, 32, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjäderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Elgezawi, M.; Haridy, R.; Almas, K.; Abdalla, M.A.; Omar, O.; Abuohashish, H.; Elembaby, A.; Wölfle, C.U.; Siddiqui, Y.; Kaisarly, D. Matrix metalloproteinases in dental and periodontal tissues and their current inhibitors: Developmental, degradational and pathological aspects. Int. J. Mol. Sci. 2022, 23, 8929. [Google Scholar] [CrossRef] [PubMed]
- Uitto, V.J.; Overall, C.M.; McCulloch, C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontology 2000 2003, 31, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Pozo, P.; Valenzuela, M.A.; Melej, C.; Zaldivar, M.; Puente, J.; Martinez, B.; Gamonal, J. Longitudinal analysis of metalloproteinases, tissue inhibitors of metalloproteinases and clinical parameters in gingival crevicular fluid from periodontitis-affected patients. J. Periodontal Res. 2005, 40, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, A.M.; Meschiari, C.A.; Zuardi, L.R.; de Sousa, T.S.; Taba, M.; Teofilo, J.M.; Jacob-Ferreira, A.L.B.; Tanus-Santos, J.E.; Novaes, A.B.; Gerlach, R.F. Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J. Clin.Periodontol. 2010, 37, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.A.; Docherty, A.J.; Bottomley, K.M.; O’Connell, J.P.; Morphy, J.R.; Reynolds, J.J.; Meikle, M.C. Inhibition of bone resorption in vitro by selective inhibitors of gelatinase and collagenase. Biochem. J. 1995, 308, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hernández Ríos, M.; Sorsa, T.; Obregón, F.; Tervahartiala, T.; Valenzuela, M.A.; Pozo, P.; Dutzan, N.; Lesaffre, E.; Molas, M.; Gamonal, J. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: Initial evidence for MMP-13/MMP-9 activation cascade. J. Clin. Periodontol. 2009, 36, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S149–S161. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Patel, R.A.; Wilson, R.F.; Palmer, R.M. The effect of smoking on periodontal bone regeneration: A systematic review and meta-analysis. J. Periodontol. 2012, 83, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal regeneration—Intrabony defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S105–S107. [Google Scholar] [CrossRef]
- Mizutani, K.; Aoki, A.; Coluzzi, D.; Yukna, R.; Wang, C.Y.; Pavlic, V.; Izumi, Y. Lasers in minimally invasive periodontal and peri-implant therapy. Periodontology 2000 2016, 71, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Koidou, V.P.; Chatzopoulos, G.S.; Tomas, I.; Nibali, L.; Donos, N. Expression of gingival crevicular fluid markers during early and late healing of intrabony defects after surgical treatment: A systematic review. Clin. Oral. Investig. 2020, 24, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000 2016, 70, 53–64. [Google Scholar] [CrossRef]
- Griffiths, G. Formation, collection and significance of gingival crevice fluid. Periodontology 2000 2003, 31, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Analysis of gingival crevicular fluid and risk of progression of Periodontitis. Periodontology 2000 2004, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kuru, L.; Griffiths, G.S.; Petrie, A.; Olsen, I. Changes in transforming growth factor-beta1 in gingival crevicular fluid following periodontal surgery. J. Clin. Periodontol. 2004, 31, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Miyazaki, A.; Momose, M.; Murata, M.; Nomura, T.; Kubota, T.; Wolff, L.F.; Yoshie, H. Levels of tissue inhibitor of metalloproteinases-1 and matrix metalloproteinases-1 and -8 in gingival crevicular fluid following treatment with enamel matrix derivative(Emdogain). J. Periodontal Res. 2001, 36, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Dolińska, E.; Pietruska, M.; Dymicka-Piekarska, V.; Milewski, R.; Sculean, A. Matrix metalloproteinase 9 (MMP-9) and interleukin-8 (IL-8) in gingival crevicular fluid after regenerative therapy in periodontal intrabony defects with and without systemic antibiotics-randomized clinical trial. Pathogens 2022, 11, 1184. [Google Scholar] [CrossRef] [PubMed]
- Koidou, V.P.; Hagi-Pavli, E.; Cross, S.; Nibali, L.; Donos, N. Molecular profiling of intrabony defects’ gingival crevicular fluid. J. Periodontal Res. 2022, 57, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Koidou, V.P.; Hagi-Pavli, E.; Nibali, L.; Donos, N. Elucidating the molecular healing of intrabony defects following non-surgical periodontal therapy: A pilot study. J. Periodontal Res. 2023, 59, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Rasperini, G.; Pagni, G.; Giannobile, W.V.; Milani, S.; Musto, F.; Dellavia, C. Local wound healing biomarkers for real-time assessment of periodontal regeneration: Pilot study. J. Periodontal Res. 2017, 52, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Rakmanee, T.; Calciolari, E.; Olsen, I.; Darbar, U.; Griffiths, G.S.; Petrie, A.; Donos, N. Expression of growth mediators in the gingival crevicular fluid of patients with aggressive periodontitis undergoing periodontal surgery. Clin. Oral. Investig. 2018, 23, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Salo, T.; Uitto, V.J.; Larjava, H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: Cellular origin and relationship to periodontal status. J. Dent. Res. 1994, 73, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Dutzan, N.; García-Sesnich, J.; Abusleme, L.; Dezerega, A.; Silva, N.; González, F.E.; Vernal, R.; Sorsa, T.; Gamonal, J. Host-pathogen interactions in progressive chronic periodontitis. J. Dent. Res. 2011, 90, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Valenzuela, M.A.; Lopez-Otin, C.; Alvarez, J.; Lopez, J.M.; Vernal, R.; Gamonal, J. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J. Periodontol. 2006, 77, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Sijari, Z.; Romano, F.; Ciardo, G.; Martella, V.; Maggiora, M.; Bottone, M.; Aimetti, M. Effect of two post-surgical cleansing protocols on early periodontal wound healing and cytokine levels following osseous resective surgery: A randomized controlled study. Int. J. Dent. Hyg. 2019, 17, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Opdenakker, G.; Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Van Coillie, E.; Masure, S.; Proost, P.; Van Damme, J. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 2001, 69, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Pini-Prato, G.; Tonetti, M.S. The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. J. Periodontol. 1995, 66, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Pini-Prato, G.; Tonetti, M.S. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int. J. Periodont. Rest. Dent. 1999, 19, 589–599. [Google Scholar]
- Cortellini, P.; Tonetti, M.S. Microsurgical approach to periodontal regeneration. Initial evaluation in a case cohort. J. Periodontol. 2001, 72, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Wassall, R.R.; Preshaw, P.M. Clinical and technical considerations in the analysis of gingival crevicular fluid. Periodontology 2000 2016, 70, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B.; Ahlo, J.K. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann. N. Y. Acad. Sci. 2007, 1098, 216–229. [Google Scholar] [CrossRef] [PubMed]


| Test (M-MIST + Er:YAG + Nd:YAG) | Control (M-MIST) | Significance | |
|---|---|---|---|
| Number of patients | 19 | 19 | - |
| Gender | 12F/7M | 9F/10M | NS (p * = 0.34) |
| Age (range) | 47 (30–73) | 43.5 (24–59) | NS (p * = 0.9) |
| Incisors/canines/premolars/molars | 3/3/12/1 | 3/1/9/6 | - |
| Mean intrasurgical defect depth | 4.03 ± 1.58 | 4.34 ± 1.13 | NS (p * = 0.14) |
| FMPS (%) | 10.8 ± 4.42 | 11.6 ± 4.68 | NS (p * = 0.53) |
| FMBOP (%) | 10.71 ± 3.89 | 10.34 ± 4.58 | NS (p * = 0.83) |
| PD (mm) | GR (mm) | CAL (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M-MIST + Er:YAG + Nd:YAG | M-MIST | p ** | M-MIST + Er:YAG + Nd:YAG | M-MIST | p ** | M-MIST + Er:YAG + Nd:YAG | M-MIST | p ** | |
| Baseline (mean) | 7.62 ± 1.44 | 7.15 ± 1.25 | 1.31 ± 1.34 | 0.89 ± 1.19 | 8.57 ± 2.16 | 8.05 ± 1.8 | |||
| 6 months (mean) | 4.42 ± 1.30 | 4.18 ± 1.14 | NS | 1.31 ± 1.00 | 1.13 ± 1.35 | NS | 5.73 ± 1.48 | 5.31 ± 2.02 | NS |
| p * = 0.0001 | p * = 0.0002 | NS | NS | p * = 0.0002 | p * = 0.0003 | ||||
| Baseline (median) | 7 (6–11) | 7 (5–9) | 1.5 (0–4) | 0 (0–4) | 8 (5–15) | 8 (5–12) | |||
| 6 months (median) | 4 (3–7) | 4 (3–7) | 1 (0–3) | 1 (0–3) | 6 (4–9) | 5 (3–10) | |||
| Diff. | 2.84 ± 0.96 | 2.97 ± 1.18 | NS | 0.00 ± 0.86 | −0.24 ± 0.79 | NS | 2.84 ± 1.45 | 2.74 ± 1.45 | NS |
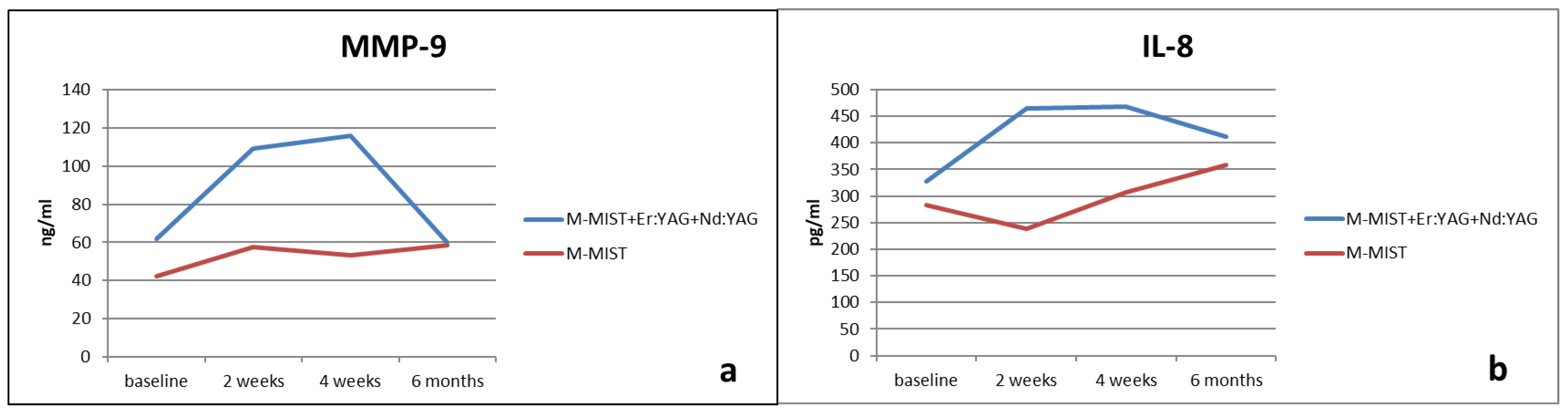
| MMP-9 | |||
|---|---|---|---|
| M-MIST + Er:YAG + Nd:YAG | M-MIST | p ** (Between Groups) | |
| Baseline | 61.96 ± 87.86 | 42.32 ± 30.52 | NS |
| 2 weeks | 109.04 ± 207.35 | 57.51 ± 65.86 | NS |
| 4 weeks | 115.96 ± 125.99 | 53.34 ± 46.71 | NS |
| 6 months | 59.78 ± 59.45 | 58.57 ± 49.44 | NS |
| p * (changes in time) | NS | NS | |
| Diff. 0–2 w | −47.08 ± 187.36 | −15.19 ± 70.34 | NS |
| Diff. 0–4 w | −54 ± 151.00 | −11.02 ± 54.02 | NS |
| Diff. 0–6 m | 2.18 ± 89.74 | −16.25 ± 52.14 | NS |
| IL-8 | |||
|---|---|---|---|
| M-MIST + Er:YAG + Nd:YAG | M-MIST | p ** (Between Groups) | |
| Baseline | 327.22 ± 281.55 | 283.20 ± 158.41 | NS |
| 2 weeks | 464.84 ± 477.98 | 238.03 ± 141.13 | NS |
| 4 weeks | 467.91 ± 313.12 | 307.77 ± 256.33 | NS |
| 6 months | 410.97 ± 300.62 | 357.77 ± 229.54 | NS |
| p * (changes in time) | NS | NS | |
| Diff. 0–2 w | −137.62± 448.06 | 45.17± 182.89 | NS |
| Diff. 0–4 w | −140.69± 387.93 | −24.57± 311.88 | NS |
| Diff. 0–6 m | −83.75 ± 341.86 | −74.56 ± 285.53 | NS |
| SFFR | |||
|---|---|---|---|
| M-MIST + Er:YAG + Nd:YAG | M-MIST | p ** (Between Groups) | |
| Baseline | 98.89 ± 30.14 | 98.42 ± 43.21 | NS |
| 2 weeks | 109.31 ± 33.65 | 102.00 ± 37.08 | NS |
| 4 weeks | 77.15 ± 28.67 | 75.78 ± 30.75 | NS |
| 6 months | 95.57 ± 46.96 | 87.00 ± 35.46 | NS |
| p * (changes in time) | NS | p = 0.046 | |
| Diff. 0–2 w | −10.42 ± 53.56 | −3.58 ± 53.40 | NS |
| Diff. 0–4 w | 21.74± 37.33 | 22.64 ± 46.15 | NS |
| Diff. 0–6 m | 3.32 ± 43.13 | 11.42 ± 51.02 | NS |
| IL-8 and MMP-9 | M-MIST + Er:YAG + Nd:YAG | M-MIST | ||
|---|---|---|---|---|
| R | p | R | p | |
| Baseline | - | NS | 0.62 | 0.0045 |
| 2 weeks | 0.68 | 0.0015 | 0.74 | 0.0003 |
| 4 weeks | - | NS | 0.59 | 0.0082 |
| 6 months | 0.58 | 0.0087 | 0.67 | 0.0016 |
| NS—non-significant | ||||
| Correlation | M-MIST + Er:YAG + Nd:YAG | M-MIST | |
|---|---|---|---|
| Baseline | IL-8 and PD | NS | NS |
| Baseline | IL-8 and SSFR | NS | NS |
| Baseline | IL-8 and intra-defect depth | NS | NS |
| Baseline | MMP-9 and PD | NS | NS |
| Baseline | MMP-9 and SSFR | NS | NS |
| Baseline | MMP-9 and intra-defect depth | NS | NS |
| 2 weeks | IL-8 and SFFR | NS | NS |
| 2 weeks | MMP-9 and SFFR | NS | NS |
| 4 weeks | IL-8 and SFFR | NS | NS |
| 4 weeks | MMP-9 and SFFR | NS | R = 0.5, p = 0.03 |
| 6 months | IL-8 and PD | NS | NS |
| 6 months | IL-8 and SSFR | NS | NS |
| 6 months | MMP-9 and PD | NS | R = 0.5, p = 0.026 |
| 6 months | MMP-9 and SSFR | R = 0.46, p = 0.047 | R = 0.5, p = 0.029 |
| NS—non-significant | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolińska, E.; Skurska, A.; Dymicka-Piekarska, V.; Milewski, R.; Pietruska, M. Matrix Metalloproteinase 9 (MMP-9) and Interleukin-8 (IL-8) in Gingival Crevicular Fluid after Minimally Invasive Periodontal Surgery with or without Er:YAG and Nd:YAG Laser Application. Antibiotics 2024, 13, 704. https://doi.org/10.3390/antibiotics13080704
Dolińska E, Skurska A, Dymicka-Piekarska V, Milewski R, Pietruska M. Matrix Metalloproteinase 9 (MMP-9) and Interleukin-8 (IL-8) in Gingival Crevicular Fluid after Minimally Invasive Periodontal Surgery with or without Er:YAG and Nd:YAG Laser Application. Antibiotics. 2024; 13(8):704. https://doi.org/10.3390/antibiotics13080704
Chicago/Turabian StyleDolińska, Ewa, Anna Skurska, Violetta Dymicka-Piekarska, Robert Milewski, and Małgorzata Pietruska. 2024. "Matrix Metalloproteinase 9 (MMP-9) and Interleukin-8 (IL-8) in Gingival Crevicular Fluid after Minimally Invasive Periodontal Surgery with or without Er:YAG and Nd:YAG Laser Application" Antibiotics 13, no. 8: 704. https://doi.org/10.3390/antibiotics13080704
APA StyleDolińska, E., Skurska, A., Dymicka-Piekarska, V., Milewski, R., & Pietruska, M. (2024). Matrix Metalloproteinase 9 (MMP-9) and Interleukin-8 (IL-8) in Gingival Crevicular Fluid after Minimally Invasive Periodontal Surgery with or without Er:YAG and Nd:YAG Laser Application. Antibiotics, 13(8), 704. https://doi.org/10.3390/antibiotics13080704








