Local Antimicrobial Therapy with Combined Aminoglycoside and Vancomycin Compared to Aminoglycoside Monotherapy in the Surgical Management of Osteomyelitis and Fracture-Related Infection
Abstract
1. Introduction
2. Results
2.1. Patient Demographics
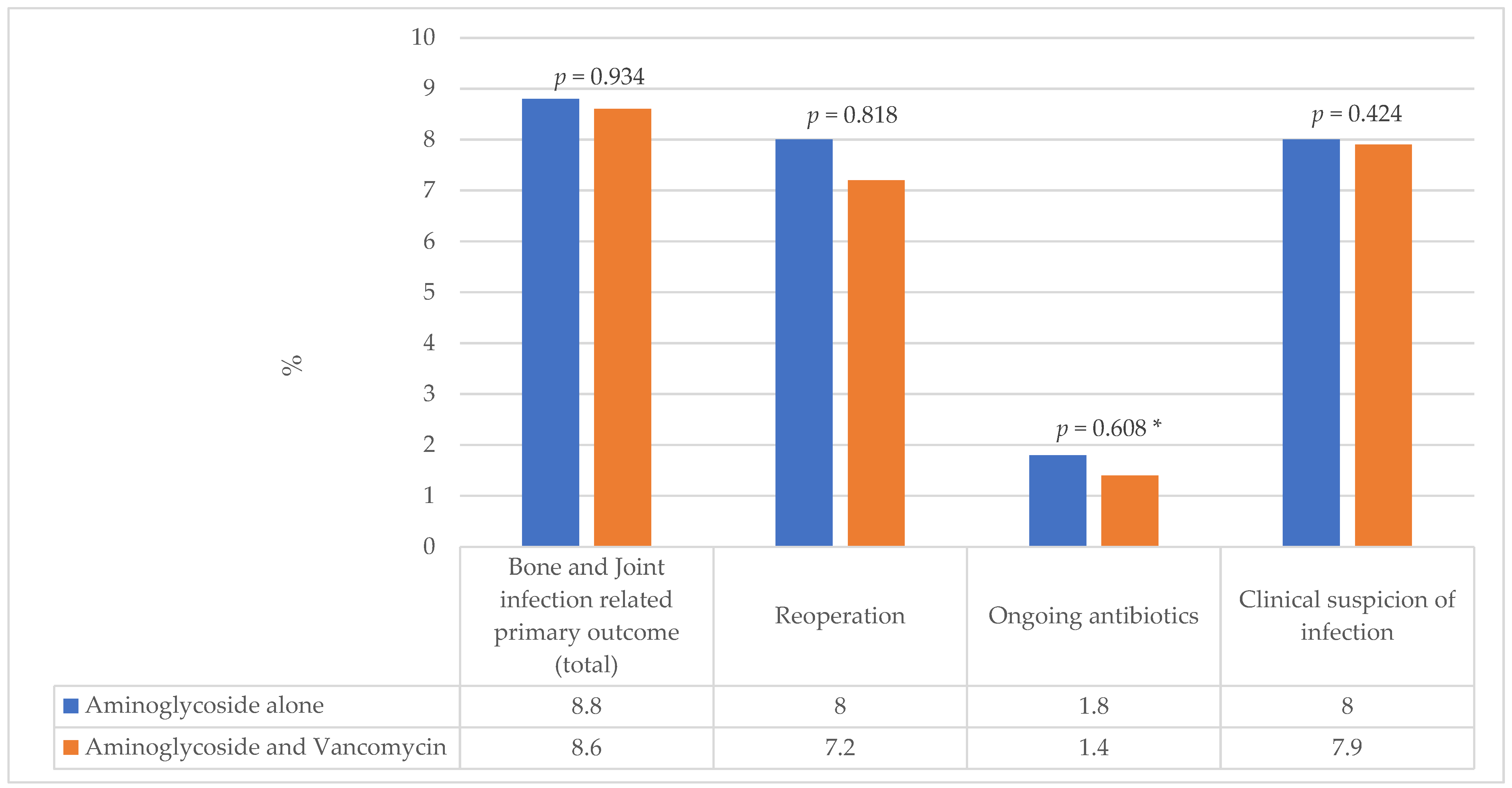
2.2. Patient Outcomes
2.3. Multivariate Analysis
2.4. Subgroup Analysis of Carrier Material Type
3. Discussion
Limitations
4. Materials and Methods
4.1. Recruitment and Inclusion Criteria
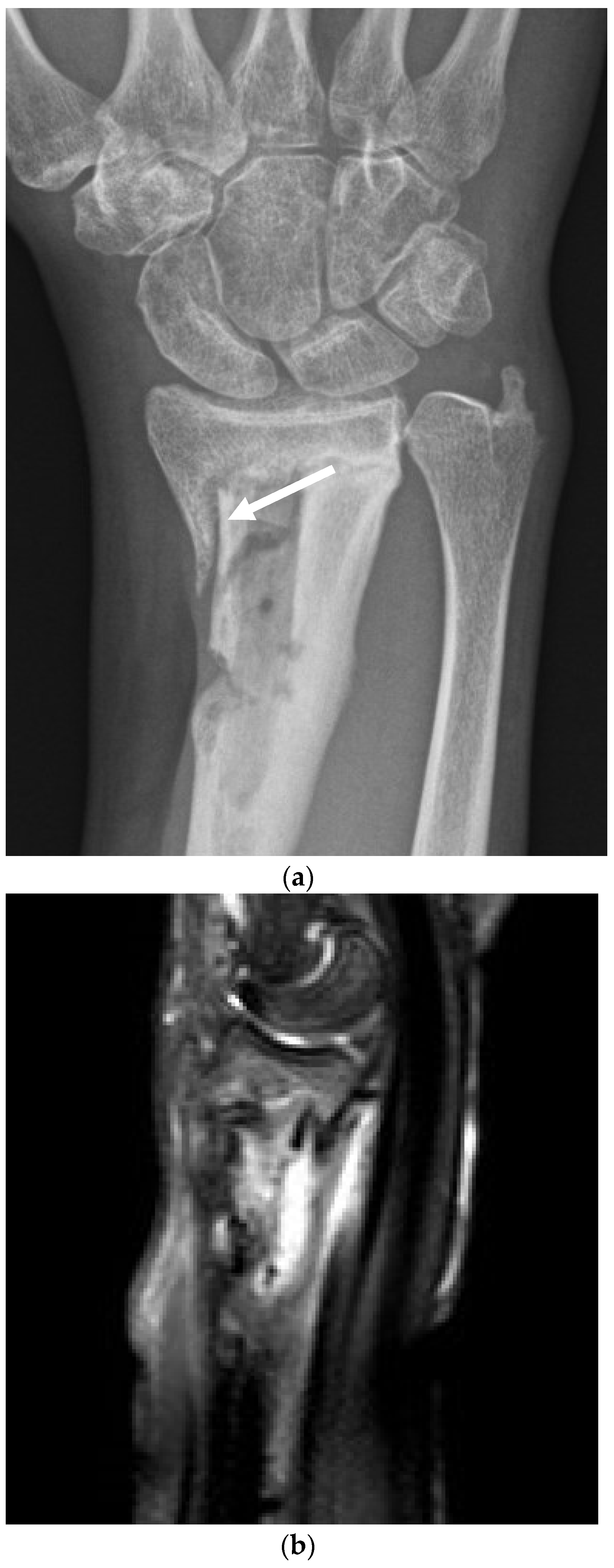
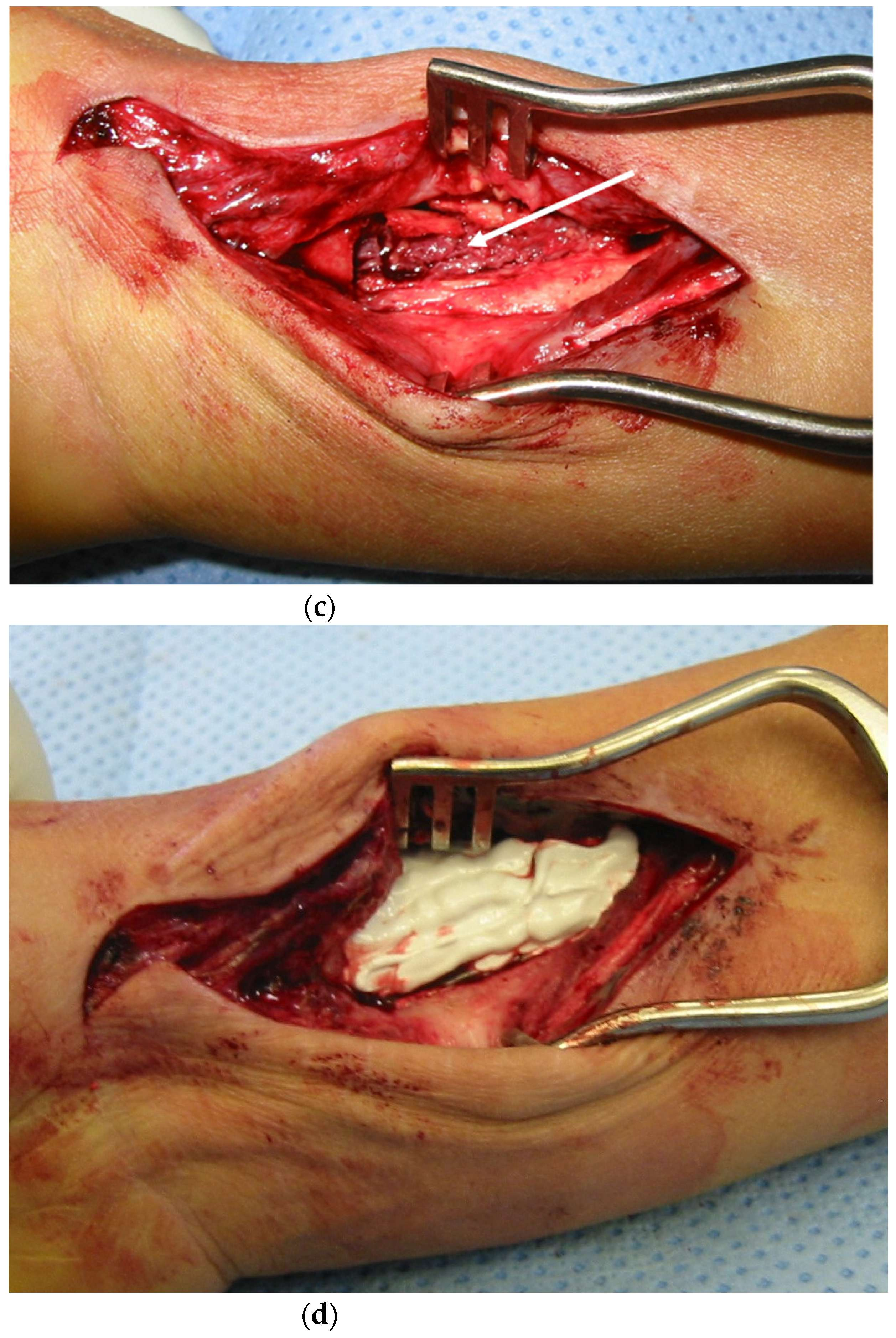
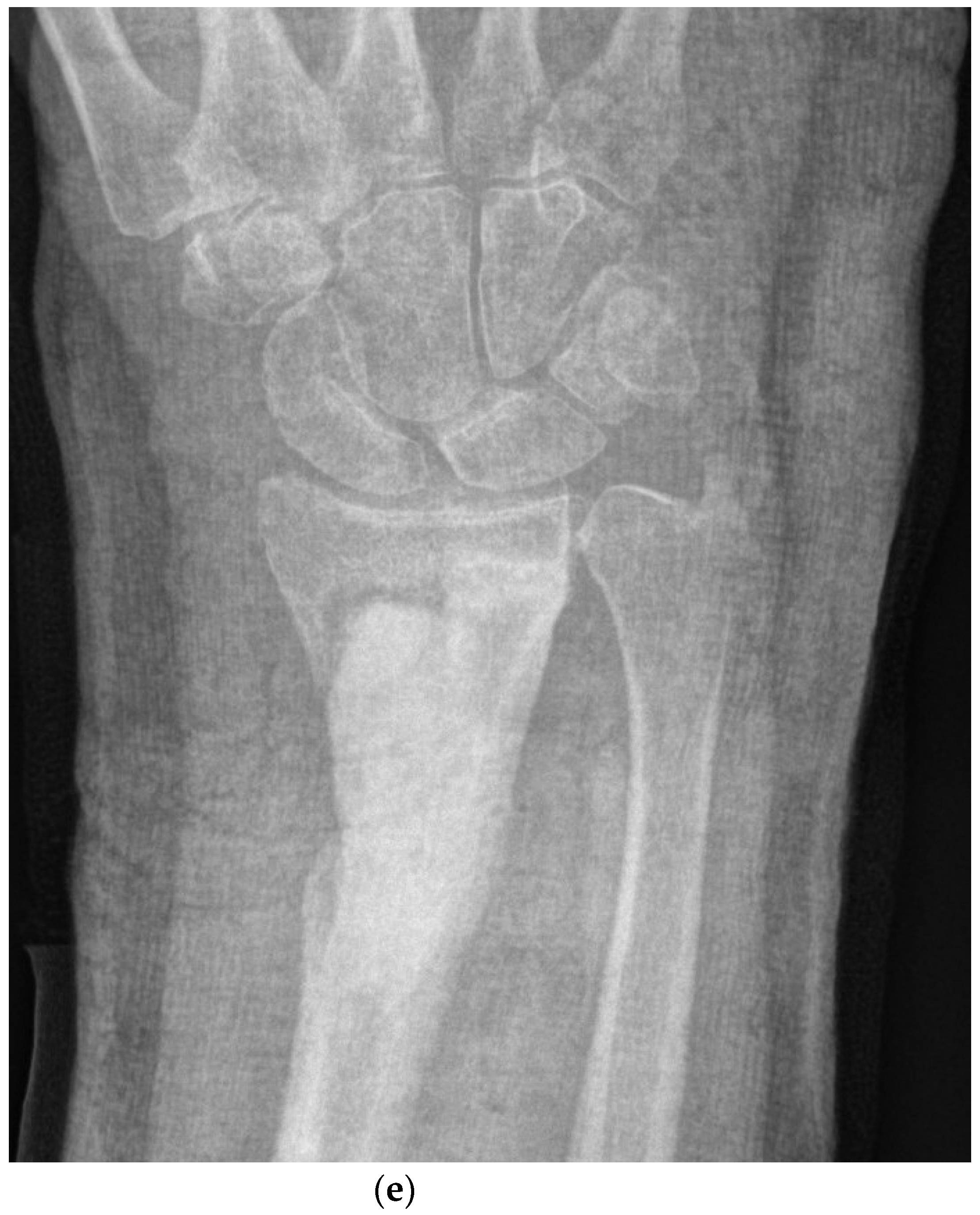
4.2. Surgical and Medical Treatment
4.3. Data Collection
4.4. Outcome
4.5. Data Management and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Sebastian, S.; Collin, M.; Tagil, M.; Lidgren, L.; Raina, D.B. A calcium sulphate/hydroxyapatite ceramic biomaterial carrier for the local delivery of tobramycin in bone infections: Analysis of rheology, drug release and antimicrobial efficacy. Ceram. Int. 2023, 49, 33725–33734. [Google Scholar] [CrossRef]
- Klemm, K.W. Antibiotic bead chains. Clin. Orthop. Relat. Res. 1993, 295, 63–76. [Google Scholar] [CrossRef]
- Pförringer, D.; Obermeier, A.; Kiokekli, M.; Büchner, H.; Vogt, S.; Stemberger, A.; Burgkart, R.; Lucke, M. Antimicrobial formulations of absorbable bone substitute materials as drug carriers based on calcium sulfate. Antimicrob. Agents Chemother. 2016, 60, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.H.; McCabe, E.M.; Priddy, L.B. Therapeutics and delivery vehicles for local treatment of osteomyelitis. J. Orthop. Res. 2020, 38, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Wahlig, H.; Dingeldein, E.; Bergmann, R.; Reuss, K. The release of gentamicin from polymethylmethacrylate beads. J. Bone Jt. Surg. 1978, 60-B, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, G.H. How I do it: Chronic osteomyelitis. Acta Orthop. Scand. 1997, 68, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Epstein, G.; Ferreira, N. Dead space management strategies in the treatment of chronic osteomyelitis. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Rodham, P.; Pantelli, M.; Qin, C.; Harwood, P.; Giannoudis, P.V. Long-term outcomes of lower limb post-traumatic osteomyelitis. Eur. J. Trauma Emerg. Surg. 2023, 49, 539–549. [Google Scholar] [CrossRef]
- Jiamton, C.; Apivatgaroon, A.; Aunaramwat, S.; Chawalitrujiwong, B.; Chuaychoosakoon, C.; Suwannaphisit, S.; Jeraveesal, C.; Iamsumang, C.; Kongmalai, P.; Sukvanich, P.; et al. Efficacy and Safety of Antibiotic Impregnated Microporous Nanohydroxyapatite Beads for Chronic Osteomyelitis Treatment: A Multicenter, Open-Label, Prospective Cohort Study. Antibiotics 2023, 12, 1049. [Google Scholar] [CrossRef]
- Ferguson, J.; Bourget-Murray, J.; Hotchen, A.J.; Stubbs, D.; McNally, M. A comparison of clinical and radiological outcomes between two different biodegradable local antibiotic carriers used in the single-stage surgical management of long bone osteomyelitis. Bone Jt. Res. 2023, 12, 412–422. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Ferguson, J.Y.; Scarborough, M.; Ramsden, A.; Stubbs, D.A.; Atkins, B.L. Mid-to long-term results of single-stage surgery for patients with chronic osteomyelitis using a bioabsorbable gentamicin-loaded ceramic carrier. Bone Jt. J. 2022, 104, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Corrigan, R.; Sliepen, J.; Dudareva, M.; Rentenaar, R.; IJpma, F.; Atkins, B.L.; Wouthuyzen-Bakker, M.; Govaert, G. What factors affect outcome in the treatment of fracture-related infection? Antibiotics 2022, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M.L.; Cosgrove, S.; Crowe, S.; Hope, W.; McCarthy, J.; Mills, J.; Mouton, J.W.; Paterson, D. (Eds.) Kucer’s the Use of Antibiotics; Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar]
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Hotchen, A.J.; Dudareva, M.; Ferguson, J.Y.; Sendi, P.; McNally, M.A. The BACH classification of long bone osteomyelitis. Bone Jt. Res. 2019, 8, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Ene, R.; Nica, M.; Ene, D.; Cursaru, A.; Cirstoiu, C. Review of calcium-sulphate-based ceramics and synthetic bone substitutes used for antibiotic delivery in PJI and osteomyelitis treatment. EFORT Open Rev. 2021, 6, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Zalavras, C.G.; Patzakis, M.J.; Holtom, P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin. Orthop. Relat. Res. 2004, 427, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Stravinskas, M.; Nilsson, M.; Horstmann, P.; Petersen, M.M.; Tarasevicius, S.; Lidgren, L. Antibiotic containing bone substitute in major hip surgery: A longterm gentamicin elution study. J. Bone Jt. Infect. 2018, 3, 68–72. [Google Scholar] [CrossRef][Green Version]
- Turnidge, J.; Paterson, D.L. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 2007, 20, 391–408. [Google Scholar] [CrossRef]
- Salvati, E.A.; Callaghan, J.J.; Brause, B.D.; Klein, R.F.; Small, R.D. Reimplantation in Infection: Elution of Gentamicin from Cement and Beads. Clin. Orthop. Relat. Res. 1986, 207, 83–93. [Google Scholar] [CrossRef]
- Muir, R.; Birnie, C.; Hyder-Wilson, R.; Ferguson, J.; McNally, M.A. Does local implantation of gentamicin impair renal function in patients undergoing surgery for chronic bone infection. Int. J. Res. Orthop. 2021, 7, 438. [Google Scholar] [CrossRef]
- Bonesupport, N.a.l. Gentamicin Release In Vitro from Setting CERAMENT™|G Paste. Data on File Report S009/2012. Available online: https://www.stoecklimedical.ch/wp-content/uploads/2022/11/PR-01118-01-en-EU-Value-Guide.pdf (accessed on 1 June 2024).
- Bezstarosti, H.; van Lieshout, E.M.M.; van den Hurt, M.J.B.; Kortram, K.; Oprel, P.; Kock, B.C.P.; Croughs, P.D.; Verhofstad, M.H.J. In vitro elution of gentamicin from CERAMENT G has an antimicrobial effect on bacterial with various levels of gentamicin resistance found in fracture-related infection. Clin. Orthop. Relat. Res. 2024, 482, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Bidossi, A.; Bottagisio, M.; Logoluso, N.; De Vecchi, E. In vitro evaluation of gentamicin or vancomycin containing bone graft substitute in the prevention of orthopedic implant-related infections. Int. J. Mol. Sci. 2020, 21, 9250. [Google Scholar] [CrossRef] [PubMed]
- Antoci, V., Jr.; Adams, C.S.; Hickok, N.J.; Shapiro, I.M.; Parvizi, J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Relat. Res. 2007, 462, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.-J.; Fragomen, A.T.; Moriarty, T.F.; Morgenstern, M.; Egol, K.A.; Zalavras, C.; Obremskey, W.T.; Raschke, M.; McNally, M.A. Evidence-based recommendations for local antimicrobial strategies and dead space management in fracture-related infection. J. Orthop. Trauma 2020, 34, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, M.; Kümin, M.; Vach, W.; Kaier, K.; Ferguson, J.; McNally, M.; Scarborough, M. Short or Long Antibiotic Regimes in Orthopaedics (SOLARIO): A randomised controlled open-label non-inferiority trial of duration of systemic antibiotics in adults with orthopaedic infection treated operatively with local antibiotic therapy. Trials 2019, 20, 693. [Google Scholar] [CrossRef] [PubMed]
- Govaert, G.A.M.; Kuhl, R.; Atkins, B.L.; Trampuz, A.; Morgenstern, M.; Obremsky, W.T.; Verhofstad, M.H.J.; McNally, M.A.; Metsemakers, W.-J. Diagnosing Fracture-related infections: Current concepts and recommendations. J. Orthop. Trauma 2020, 34, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sandbakken, E.T.; Witsø, E.; Sporsheim, B.; Egeberg, K.W.; Foss, O.A.; Hoang, L.; Bjerkan, G.; Løseth, K.; Bergh, K. Highly variable effect of sonication to dislodge biofilm-embedded taphylococcus epidermidis directly quantified by epifluorescence microscopy: An in vitro model study. J. Orthop. Surg. 2020, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Dudareva, M.; Vicentine, M.P.; Hotchen, A.J.; Ferguson, J.; McNally, M. Microbial Persistence, Replacement and Local Antimicrobial Therapy in Recurrent Bone and Joint Infection. Antibiotics 2023, 12, 708. [Google Scholar] [CrossRef]
- Wijendra, A.; Tsang, J.; Ferguson, J.; McNally, M.A. Managing fracture-related infection. Orthop. Trauma 2023, 37, 366–378. [Google Scholar] [CrossRef]
- Dudareva, M.; Barrett, L.; Oakley, S.; Jesuthasan, G.; Morgenstern, M.; Atkins, B.L.; Brent, A.J.; McNally, M.A. Providing an evidence base for tissue sampling and culture interpretation in suspected fracture-related infection. J. Bone Jt. Surg. 2021, 103, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, I.K.; Yeghiazaryan, L.; Luger, M.; Windhager, R.; Sulzbacher, I.; McNally, M.A. Three to six tissue specimens for histopathological analysis are most accurate for diagnosing periprosthetic joint infections. Bone Jt. J. 2023, 105-B, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, M.; Hotchen, A.J.; Ferguson, J.; Hodgson, S.; Scarborough, M.; Atkins, B.L.; McNally, M.A. The microbiology of osteomyelitis: Changes over ten years. J. Infect. 2019, 79, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Kuehl, R.; Metsemakers, W.J.; Senneville, E.; McNally, M.A.; Obremskey, W.T.; Zimmerli, W.; Atkins, B.L.; Trampuz, A. Recommendations for systemic antimicrobial therapy in fracture-related infection: A consensus from an international expert group. J. Orthop. Trauma 2020, 34, 30–41. [Google Scholar] [CrossRef]
- Michel, J.-P.; Klopfenstein, C.; Hoffmeyer, P.; Stern, R.; Grab, B. Hip fracture surgery: Is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin. Exp. Res. 2002, 14, 389–394. [Google Scholar] [CrossRef]
- Hotchen, A.J.; Wismayer, M.G.; Robertson-Waters, E.; McDonnell, S.M.; Kendrick, B.; Taylor, A.; Alvand, A.; McNally, M. The Joint-Specific BACH classification: A predictor of outcome in prosthetic joint infection. eClinicalMedicine 2021, 42, 101192. [Google Scholar] [CrossRef]
- The Sanford Guide to Antimicrobial Therapy 2023; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2023.
| Characteristics | Aminoglycoside Alone | Aminoglycoside and Vancomycin | ||
|---|---|---|---|---|
| Median | IQR * | Median | IQR * | |
| Age (years) | 57 | 40–66 | 52 | 37–62 |
| ASA Score * | 2 | 1–3 | 2 | 1–3 |
| BMI * | 26 | 22–30 | 28 | 24–32 |
| n | % | n | % | |
| Total | 113 | 100 | 139 | 100 |
| Male | 85 | 75.2 | 102 | 73.4 |
| Type of Surgery | ||||
| Osteomyelitis excision | 69 | 61.1 | 87 | 62.6 |
| Fracture-related infection with hardware removal | 38 | 33.6 | 44 | 31.7 |
| Fracture-related infection with hardware removal and reimplantation | 6 | 5.3 | 8 | 5.8 |
| BACH score * | ||||
| Uncomplicated | 32 | 28.3 | 55 | 39.6 |
| Complex | 77 | 68.1 | 84 | 60.4 |
| Limited options | 4 | 3.5 | 0 | 0 |
| Local antibiotic carrier § | ||||
| CERAMENT alone | 68 | 60.2 | 80 | 57.6 |
| OSTEOSET T only | 9 | 8 | 0 | 0 |
| Herafill G only | 9 | 8 | 0 | 0 |
| CERAMENT with Herafill G | 9 | 8 | 24 | 17.3 |
| CERAMENT and OSTEOSET T | 13 | 11.5 | 30 | 21.6 |
| Refobacin G | 4 | 3.5 | 0 | 0 |
| Stimulan | 0 | 0 | 4 | 2.9 |
| CERAMENT, Herafill G and Septopal G | 0 | 0 | 1 | 0.7 |
| Herafill G and Septopal G | 1 | 0.9 | 0 | 0 |
| Post-surgery characteristics | Median | IQR | Median | IQR |
| Duration of antibiotics (days) | 42 | 42–42 | 42 | 38.5–42 |
| IV * to oral switch (days) | 5 | 4–6 | 5 | 4–6 |
| n | % | n | % | |
| Organism | ||||
| Staphylococcus aureus | 53 | 46.9 | 92 | 66.2 |
| Coagulase negative Staphylococcus | 35 | 31.0 | 28 | 20.1 |
| Streptococcus species | 21 | 18.6 | 25 | 18.0 |
| Pseudomonas species | 11 | 9.7 | 7 | 5.0 |
| Other Gram-negative organisms | 42 | 37.2 | 30 | 21.6 |
| Other Gram-positive organisms | 29 | 25.7 | 17 | 12.2 |
| Polymicrobial infection | 38 | 33.6 | 44 | 31.7 |
| Confirmed gentamicin resistance | 12 | 10.6 | 14 | 10.1 |
| Presumed gentamicin resistance | 27 | 23.9 | 22 | 15.8 |
| Outcome | Category | OR | 95% CI | p Value |
|---|---|---|---|---|
| Primary Outcome | ASA (per 1 unit increase) | 1.05 | 0.61–1.82 | 0.86 |
| Age (per 1 year increase) | 1.00 | 0.98–1.03 | 0.76 | |
| BMI (per 1 unit increase) | 0.94 | 0.86–1.02 | 0.11 | |
| Confirmed and presumed gentamicin resistance | 0.39 | 0.05–3.01 | 0.37 | |
| Type of surgery: osteomyelitis resection | 0.88 | 0.35–2.19 | 0.79 | |
| Presence of polymicrobial infection | 1.51 | 0.62–3.69 | 0.37 | |
| Presence of Gram-negative organism | 2.35 | 0.96–5.71 | 0.06 | |
| BACH score (per 1 unit increase) | 3.29 | 1.12–9.23 | 0.02 | |
| IV to oral switch (per 1 day increase) | 0.95 | 0.82–1.11 | 0.53 | |
| Duration of antibiotics (per each week increase) | 1.00 | 0.99–1.01 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unsworth, A.; Young, B.; Ferguson, J.; Scarborough, M.; McNally, M. Local Antimicrobial Therapy with Combined Aminoglycoside and Vancomycin Compared to Aminoglycoside Monotherapy in the Surgical Management of Osteomyelitis and Fracture-Related Infection. Antibiotics 2024, 13, 703. https://doi.org/10.3390/antibiotics13080703
Unsworth A, Young B, Ferguson J, Scarborough M, McNally M. Local Antimicrobial Therapy with Combined Aminoglycoside and Vancomycin Compared to Aminoglycoside Monotherapy in the Surgical Management of Osteomyelitis and Fracture-Related Infection. Antibiotics. 2024; 13(8):703. https://doi.org/10.3390/antibiotics13080703
Chicago/Turabian StyleUnsworth, Annalise, Bernadette Young, Jamie Ferguson, Matthew Scarborough, and Martin McNally. 2024. "Local Antimicrobial Therapy with Combined Aminoglycoside and Vancomycin Compared to Aminoglycoside Monotherapy in the Surgical Management of Osteomyelitis and Fracture-Related Infection" Antibiotics 13, no. 8: 703. https://doi.org/10.3390/antibiotics13080703
APA StyleUnsworth, A., Young, B., Ferguson, J., Scarborough, M., & McNally, M. (2024). Local Antimicrobial Therapy with Combined Aminoglycoside and Vancomycin Compared to Aminoglycoside Monotherapy in the Surgical Management of Osteomyelitis and Fracture-Related Infection. Antibiotics, 13(8), 703. https://doi.org/10.3390/antibiotics13080703






