Abstract
The skin microbiota, with its millions of bacteria, fungi, and viruses, plays a key role in balancing the health of the skin and scalp. Its continuous exposure to potentially harmful stressors can lead to abnormalities such as local dysbiosis, altered barrier function, pathobiont overabundance, and infections often sustained by multidrug-resistant bacteria. These factors contribute to skin impairment, deregulation of immune response, and chronic inflammation, with local and systemic consequences. In this scenario, according to the needs of the bio-circular-green economy model, novel harmless strategies, both for regulating the diverse epidermal infectious and inflammatory processes and for preserving or restoring the host skin eubiosis and barrier selectivity, are requested. Vitis vinifera L. leaves and their derived extracts are rich in plant secondary metabolites, such as polyphenols, with antioxidant, anti-inflammatory, antimicrobial, and immunomodulatory properties that can be further exploited through microbe-driven fermentation processes. On this premise, this literature review aims to provide an informative summary of the most updated evidence on their interactions with skin commensals and pathogens and on their ability to manage inflammatory conditions and restore microbial biodiversity. The emerging research showcases the potential novel beneficial ingredients for addressing various skincare concerns and advancing the cosmeceutics field as well.
1. Introduction
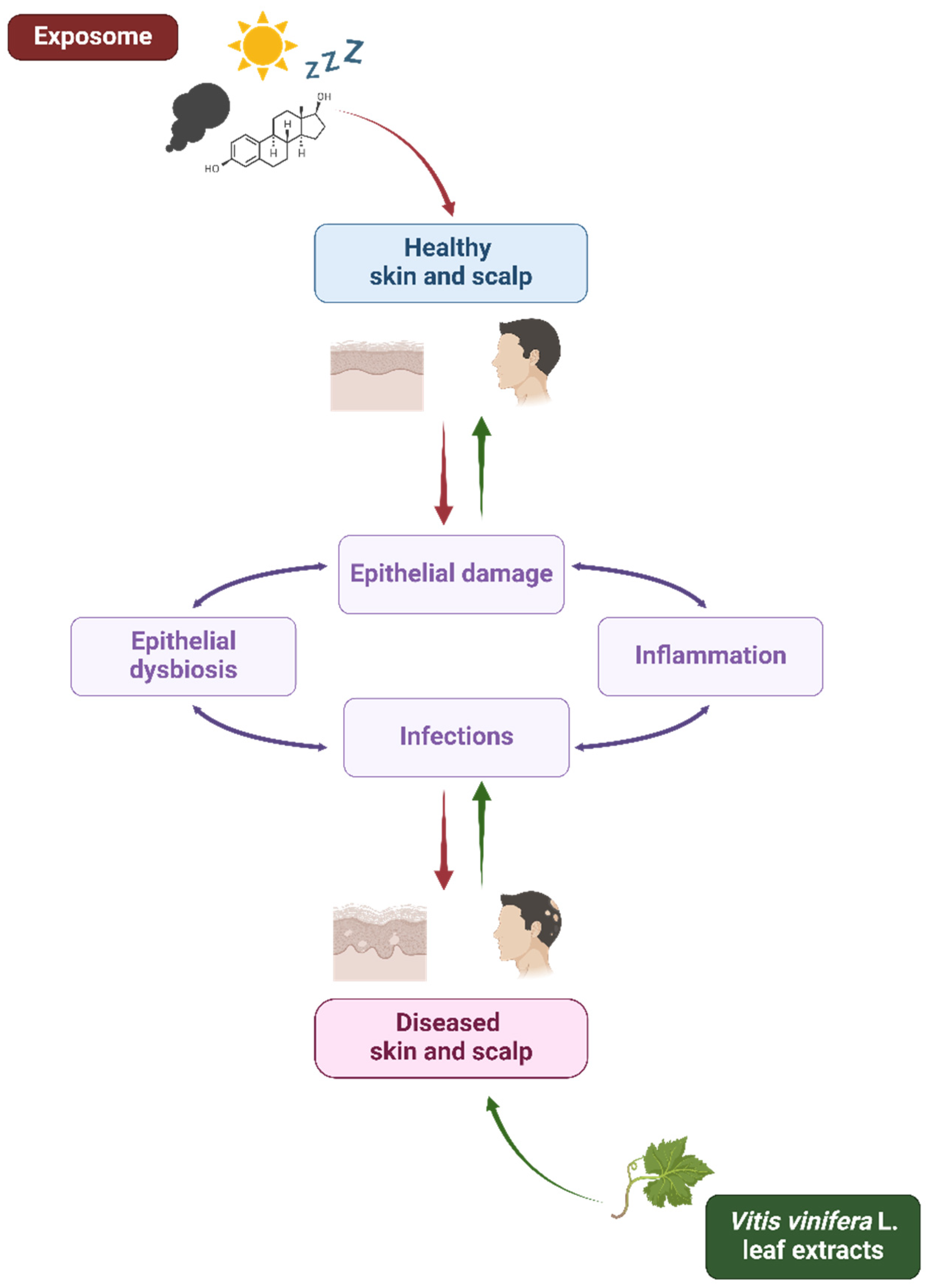
The skin, the outermost selective barrier of the human beings, exerts its protective role through physical, chemical, biochemical, and immunological mechanisms [1]. Exposure to harmful external stressors (such as pollutants, ultraviolet (UV) radiation, and tobacco), alongside the influence of physiological internal factors (like aging, hormones, and sleep) and the host genetic background, can be responsible for the disruption and function impairment of this tissue, as already deeply investigated by many authors (Table 1) [2,3]. According to the extended “epithelial barrier hypothesis” introduced by Cezmi Akdis, epithelial damage may be associated with the onset and worsening of local microbial dysbiosis and infectious and inflammatory conditions [4] interconnected by bidirectional dynamic interactions [5,6,7]. As a matter of fact, a weakened barrier means opportunistic pathogen overcolonization, virulence expression, and infectious events, thus contributing to microbiota alteration, host immune response dysregulation, and chronic inflammation, leading to a vicious cycle of aggravating pathological events (Figure 1) [8,9].
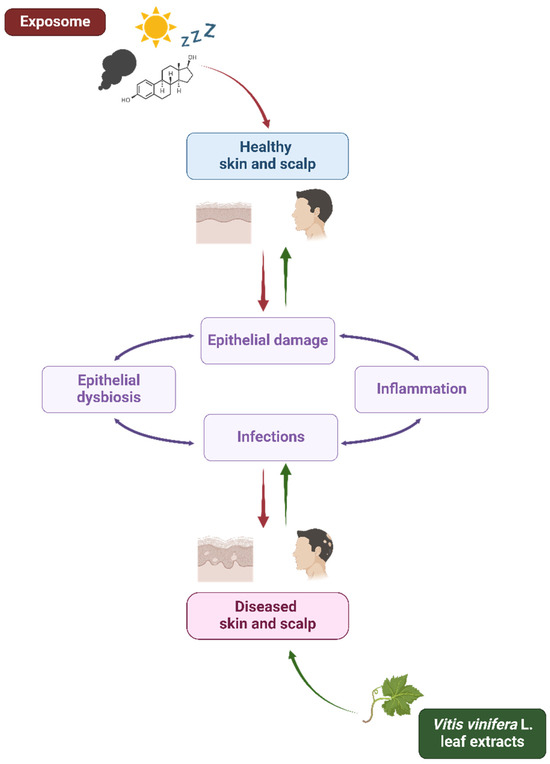
Figure 1.
Exposome and Vitis vinifera L. leaf extracts’ influence on skin and scalp health. Schematic representation of the interplay among the exposome, Vitis vinifera L. leaf extracts, and epithelial barrier in skin and scalp health. The exposome affects the epithelial integrity contributing to microbial dysbiosis, inflammation, and infections (red arrows). On the other hand, V. vinifera L. leaf extracts could contribute to microbial eubiosis and skin and scalp health (green arrows). Created with BioRender.com.
Gut epithelial disruption and dysbiosis have both local and systemic consequences, not only gastrointestinal [5,10] but also neurodegenerative, cutaneous, psychiatric, metabolic, and many others [4,11]. Likewise, epidermal impairment and microbiota alterations are linked to skin and scalp disorders like acne, atopic dermatitis (AD), psoriasis, vitiligo, hidradenitis suppurativa (HS), seborrheic dermatitis (SD), and alopecia areata (AA) [12,13,14,15,16,17,18] but also to systemic conditions such as systemic lupus erythematosus (SLE) [19], thanks to the gut–skin and gut–brain–skin axis relationship [20,21].
To manage these conditions, the preferred topical therapies may include anti-inflammatory drugs, such as corticosteroids [14,22]. Although they are not free from side effects, the risk is minimal when properly used [23]. However, ideal topical remedies should not interfere with the host skin eubiosis to avoid any possible pathological self-reinforcing vicious circuit [24,25]. In this scenario, novel effective strategies are needed.
Due to the presence of plant secondary metabolites (PSMs), plants and derived extracts have been effectively used for the treatment of several human health conditions [26]. Among the PSMs, polyphenols are the most promising ones because of their recognized antioxidant, anti-inflammatory, antimicrobial, anticancer, and immunomodulatory properties [27,28,29].
Vitis vinifera (VV) L. or grapevine is an emblematic example of polyphenols and other PSM-rich plants [30,31]. Agro-industrial biomasses such as grapevine leaves produced by the wine industry are an interesting raw material for the pharmaceutic, cosmeceutics, and nutraceutical markets, since they contain bioactive compounds that can be extracted and exploited in accordance with the bio-circular-green economy model needs [32,33,34]. Till now, only scarce research evidence has been available on both their anti-inflammatory activity, especially in reconstructed skin models, and on their interaction with the skin microbiota [35,36,37,38].


Table 1.
Overview of the main factors involved in skin impairment and disorders.
Table 1.
Overview of the main factors involved in skin impairment and disorders.
| General Domains | Specific Factors | References |
|---|---|---|
| External stressors | Solar radiations | [39,40,41] |
| Environmental factors (e.g., pollutants, allergens, particulate matter, ozone, industrial toxic gases, nano/microplastics, pesticides, tobacco …) | [8,40,42,43,44,45] | |
| Climate change | [8,41,42,43,44,45] | |
| Nutrition | [3,40,46,47] | |
| Internal factors | Hormones (e.g., sex hormones, thyroid hormones, glucocorticoids, …) | [40,48,49] |
| Psychological stress | [50,51,52,53] | |
| Sleep | [40,54] | |
| Aging | [55,56] | |
| Host microbiota/microbiome and microbial exposome | [57,58] | |
| Host genetic background (eukaryotic cells) | Polymorphisms and other mutations | [59,60,61] |
| Epigenetics | [60,61,62] | |
| Ethnicity | [41,63] | |
| Biological sex | [64,65,66] |
On theses bases, the aim of this review article is to provide an informative updated summary on the potential of VV, specifically of its leaf component, starting from its derivatives and active ingredients, including resveratrol and pterostilbene, in the prevention and management of inflammatory conditions affecting skin and scalp, and regarding its interactions with the skin microbiota as well as its activity against opportunistic infections. Future perspectives and limitations of this approach are reported as well.
2. Materials and Methods
In this literature review, the MEDLINE NIH National Library of Medicine (NLM)’s electronic free medical bibliographic PubMed and Scopus publicly available databases were consulted by looking for the keywords and terms “Vitis vinifera L. leaf extract”; “microbiota”; “microbiome”; “skin microbiota”; “skin microbiome”; “skin virota”; “skin bacteriota”; “skin mycobiota”; “gut-skin axis”; “bacteria”; “virus”; “epitheliotropic virus”; “mycetes”; “fungi”; “dermatophytes”; “Staphylococcus spp.”; “Human Papillomavirus”; “Human Herpes virus”; “Candida spp”; “Malassezia spp.”; “inflammation”; “inflammatory skin diseases”; “inflammatory scalp diseases”; “seborrheic dermatitis”; “ psoriasis”; “atopic dermatitis”; “hidradenitis suppurativa”; “acne”; “immune system”; “cytokines”; “cosmeceutics”; and “dermocosmetics”, both in single and/or in combination, and the Medical Subject Heading (MeSH) controlled vocabulary, in paper titles and abstracts to retrieve the highest quality indexed and most cited original peer reviewed scientific research studies and reviews.
The bibliographic literature search was focused on original scientific international studies written in the English language and recently published, with the older ones not exceeding the last 25 years (final consultation in mid July 2024).
3. Vitis vinifera L. Leaves
VV plays a prominent role in global agriculture as one of the most significant perennial crops. It has been acknowledged worldwide for its versatility, providing not only grapes for wine and alcoholic beverages but also a variety of other products, including raisins, juices, fermented foods, cosmeceutics, and nutraceuticals, among others [31,67].
Nowadays, it is imperative to harness all by-products of the VV production chain to achieve a sustainable approach from ethical, economic, and environmental perspectives. The reuse of these biomasses also contributes to waste reduction and promotes more ethical and environmentally friendly practices [68,69]. Among these winery discarded materials, grapevine leaves, similarly to other by-products such as pomace, are rich in PSMs, namely polyphenols, as well as lipids and organic acids [70]. Due to the health-promoting properties of said compounds, e.g., in terms of antioxidant and anti-inflammatory potential, their exploitation could guarantee many advantages, such as the production of novel active ingredients through biotechnological processes, while contributing to the green circular economy model [71,72]. Even though PSMs can potentially be found in every plant organ, some plant by-products are more likely to be investigated than others. Indeed, grape leaves have been studied less with respect to other raw materials, mainly in terms of extraction and utilization of PSMs [70,73].
3.1. Phytochemical Profile and Bioactive Compounds
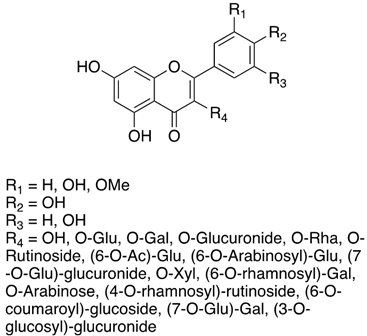
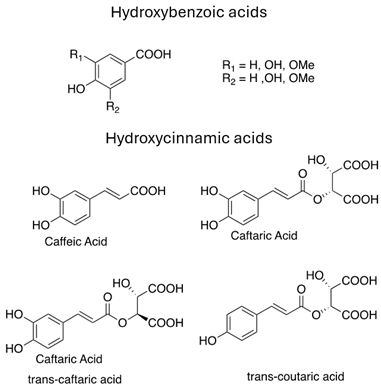
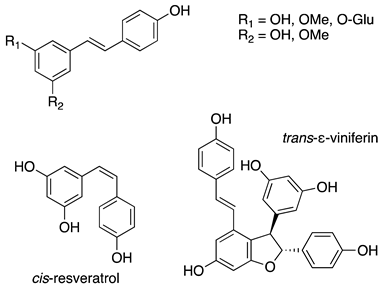
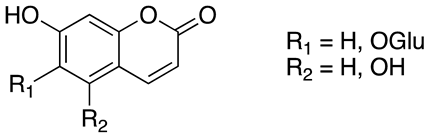
As previously introduced, Vitis vinifera L. leaf extracts (VVLEs) are rich sources of diverse bioactive compounds, mainly PSMs. These phytochemicals have health-promoting properties and are integral to the plant adaptive strategies, serving as defenses against infections and key contributors to the grapevine’s ability to thrive in its environment [74,75,76]. The extracts are particularly abundant in polyphenols, encompassing a wide spectrum of compounds such as anthocyanins, catechins (including catechin, epicatechin, gallocatechin, and epicatechin3-O-gallate), ellagitannins (like brevilagin-1, vitilagin, and isovitilagin), and flavones (traces of quercitrin, quercetin, kaempferol, rutin, isoquercitrin, and luteolin). The presence of various organic acids, such as tartaric, malic, oxalic, fumaric, succinic, citric, and glyceric, contributes to the overall chemical complexity. Phenol acids, such as the o- and p-hydroxybenzoic, protocatechuic, gallic, vanillic, syringic, and ellagic, further enhance the bioactive profile. Esters, featuring cinnamic and tartaric acids, add another layer of chemical diversity. The extracts also contain essential vitamins, including C, PP, B group, and folic acid. Carotenoids, volatile constituents, proteins, and mineral salts (ranging from 5% to 7%) overall offer a versatile array of bioactive compounds with potential applications in various health-related contexts [77,78].
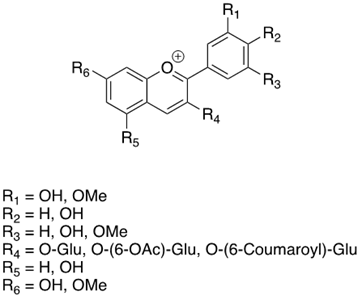
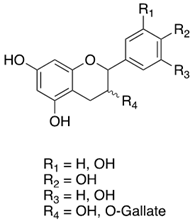
To sum up, the main classes of VVLE compounds include phenolic acids, flavonols, flavonoids, stilbenes, lignans, tannins, and anthocyanins (Table 2 and Table 3). However, in terms of qualitative and quantitative characterization, their phytochemical profile is strongly influenced by several factors. Environmental stimuli are involved, such as soil properties, weather conditions, and the season during which leaves are harvested. Grape cultivars and varieties, stage of maturation, and plant health status are responsible for the production of different PSMs, too [37,79,80,81,82,83]. Interestingly, although the phenolic compounds’ characterization in grapevine berries has been extensively studied, only limited information is available regarding the polyphenols profile in the leaves and their biological activities.

Table 2.
Phytochemical profile of Vitis vinifera L. leaves.

Table 3.
Chemical structure and mechanism of action of Vitis vinifera L. leaves’ phytochemical compounds.
3.2. Topical and Systemic Applications in Cosmeceutics
According to the World Health Organization (WHO), herbal medicines encompass herbs, herbal materials, preparations, and finished herbal products containing parts of plants or other plant materials, or combinations thereof. Their use is well established and widely recognized as safe and effective for the treatment of various health conditions [109]. The ancient tradition of herbal medicine has given rise to phytomedicines, appreciated for their low cost and reduced toxicity compared to other synthetic products [110,111].
In this context, VV leaves (VVLs) have been employed due to their diverse biological activities (e.g., anti-inflammatory, immunomodulatory, anticancer, and anti-infectious) and are widely recognized as natural antioxidants and chemopreventive agents [112]. The polyphenol content and the related biological activities make VVLEs interesting for many applications not only in the pharmaceutical market but also in the cosmetics one [113,114,115]. VVLEs in cosmetics products mainly serve as anticaries, antidandruff, antioxidant, and antipathogen agents [77,116]. In more detail, VVLEs help combat free radicals [117] while contributing to the maintenance of the natural microbiota of the skin and scalp [118]. Additionally, they protect against the harmful effects of UV radiation, positioning them as potential ingredients in sunscreen filters [119]. Although many products are currently available in the cosmetics and personal care markets, the underlying mechanisms of action and health-promoting properties of these raw materials and derived ingredients are still under investigation.
4. Skin Microbiota and Inflammatory Skin and Scalp Disorders
4.1. Skin Microbiota and Healthy Skin
The skin is globally recognized as a selective barrier. This function is guaranteed by the dynamic crosstalk between its microbiota and the below epidermal, dermal, and hypodermal strata, mainly through the secretion of mediators like antimicrobial peptides (AMPs), cytokines, and chemokines [57,120]. Being an interface, the skin coordinates the responses to external and internal both xenobiotic (e.g., toxic substances, UV rays, and opportunistic pathogens) and familiar stimuli (Figure 1) to maintain or restore skin microbial and immune balance, as well as to reduce the onset and progression of inflammatory local and systemic conditions. In this way, it is possible to avoid or solve skin disorders and ensure tissue homeostasis, appearance, and hydration [40]. To do this, the mechanisms underlying these processes should be investigated. A key role is played by the local microflora, recognized as one of the five functional levels of the skin as reviewed by Lefèvre-Utile et al. [121]. Since the launch of the Human Microbiome Project (HMP) in 2007, the microbial communities that colonize the skin are under investigation and the research on this topic has exponentially grown over the years [122]. Commensal and pathogenic microorganisms have been identified, while their relationships with the host and the changes during life are less known [123,124]. Human skin commensals (i.e., bacteria, fungi, and viruses) are in mutualistic relationships with their host, since they are involved in the development and regulation of the immune system, while hindering endogenous and exogenous infections [125,126]. However, in dysbiotic conditions, this fine orchestra no longer works [127,128].
4.2. Skin Microbiota and Cutaneous Diseases
The first evidence suggesting the role of microbiota in skin diseases dates to more than 20 years ago, with the so-called “hygiene hypothesis” stating that a low exposure to pathogens during childhood correlates with a high incidence of allergic disorders [129]. Subsequently, some attempts in preventing or lowering the incidence of atopic dermatitis (AD) and food allergies have been developed with oral probiotics’ administration in pregnant women and newborns, with no univocal results [130]. Through the years, this hypothesis has been further extended with the introduction of the “old friends hypothesis” by Graham Rook and the “biodiversity hypothesis” by Tari Haahtela. Nowadays, mechanisms described by these three theories are included in the more comprehensive “epithelial barrier hypothesis” by Cezmi Akdis, which could help understand the etiopathogenesis of several dermatological diseases, like AD and many others [4,131,132].
AD, a chronic and relapsing skin disorder characterized by intense itching and eczematous lesions, is a part of the so called “atopic march”, that involves different organs and develops sequentially with age [133]. Although its pathogenesis is multifactorial, skin dysbiosis is crucial, with a relative abundance of Staphylococcus aureus and local inflammation. Furthermore, the raised presence of the yeast Malassezia spp. contributes to its severity [134].
These species are also a causative agent of folliculitis in sebaceous gland-rich sites and are involved in seborrheic dermatitis (SD), a common and relapsing disease located in sebaceous body areas (i.e., scalp, central area of the face, chest, and upper part of the back), manifesting as yellowish and greasy scales on erythematous skin. Despite that its pathogenesis is not fully elucidated, cutaneous dysbiosis seem to be involved. Specifically, Malassezia spp. hydrolyze the sebum triglycerides into lipid peroxides and free fatty acids, thus favoring inflammation and the yeast growth itself. Moreover, S. aureus has also been found over-represented in SD subjects, thus underlying the role of microbiota changes in both such dermatitises [135,136].
A further chronic inflammatory dermatosis is acne, in which Cutibacterium acnes (previously known as Propionibacterium acnes) plays a key pathogenic role, since it promotes keratinocyte differentiation, autophagy, and response to different stimuli, thus leading to a cytokine-mediated pro-inflammatory pattern and further immune cell recruitment. Furthermore, specific C. acnes strains can induce biofilm formation and promote bacterial adhesion, thus reducing any antibiotic effect [137]. C. acnes is affected by the presence of the commensal Staphylococcus epidermidis, that, reducing inflammation, is thus linked to less severe forms of acne [138].
Several anaerobic microorganisms, such as Corynebacterium, Prevotella, Porphyromonas, and Peptoniphilus spp., are commonly detected in hidradenitis suppurativa (HS), a chronic and relapsing disease of the apocrine glands manifesting with abscesses leading to scarring and fistulae mainly in the axilla, groin, and gluteal regions. Such bacteria produce damage-associated molecular patterns (DAMPs), allowing the inflammatory process amplification through cytokines and pattern recognition receptors (PRRs). On the contrary, a reduced count of S. epidermidis, Staphylococcus hominis, and C. acnes has been reported, while their replenishing helps the recovery [139,140].
Psoriasis is a chronic and relapsing inflammatory skin disease characterized by occurrent scaling and erythematous plaques [141]. Its pathogenesis is multifactorial and is linked firstly to dendritic cell activation, followed by the production of cytokines and promotion of Th1 and Th17 patterns, followed by keratinocyte proliferation and dermal inflammation. The bacterial strains on plaques are mainly Corynebacterium, Propionibacterium, Staphylococcus, and Streptococcus spp.; accordingly, a causal interaction between streptococci and eruptive guttate psoriasis had been suggested. On the other side, even a possible role of Malassezia spp. has been hypothesized, especially in relapses; thus, the yeast might upregulate the expression of specific cytokines. Furthermore, the sebo-psoriasis variant could represent a further element supporting the role of Malassezia spp. in this context [142].
Concluding, the mentioned diseases have distinct patterns, which involve some specific microorganisms that are summarized in Table 4.

Table 4.
Skin and scalp disorders with microbial involvement.
5. Inflammatory Skin and Scalp Disorders and Vitis vinifera L. Leaf Extracts
The potential use of VV for skin and scalp diseases’ management, not only directly linked to microbial alterations, represents a current field of investigation in dermatology, despite that most of the studies are related to the grape seed extracts (GSEs). Recently, Soleymani et al. have reviewed the therapeutic effects of GSEs, and, mainly, of the nanoformulated resveratrol in some inflammatory skin disorders (i.e., acne, facial redness, chloasma, and chronic venous insufficiency), as well as in wound healing and skin aging [143]. Indeed, resveratrol possesses several health-promoting effects besides its anti-inflammatory potential, but its content in berries and leaves depends on the genetic background of the cultivars and on the plant organs as well [144,145].
To date, data on VVLEs are still scarce. Sangiovanni et al. recently reported their in vitro anti-inflammatory effect in cell cultures mimicking skin disorders. The authors found that in HaCaT cells, all the bioactive substances of water extract from VVL (VVWE), and not its singular main components, significantly reduced the IL-8 release induced by the NF-κB pathway following tumor necrosis factor-alpha (TNF-α), lipopolysaccharide (LPS), or ultraviolet B (UVB) stimulus, thus stating a synergism, while a lack of effect was detected in response to hydrogen peroxide (H2O2) [35].
As information regarding VVLEs is still poor, some authors investigated the effects of specific phytochemicals found in the grape leaves, as well as in other VV organs. Among them, in a recent review, Marko and Pawliczak properly evaluated the impact of resveratrol in psoriasis and AD. They reported the positive effects of local resveratrol in such skin diseases, but they also suggested that the in vitro results should be cautiously considered, given the difficulties in reproducing psoriasis and AD in animal models faithfully. Moreover, they did not consider any interaction between microbiota and resveratrol [146]. In addition, a clinical case on the effectiveness of oral isoquercetin (a flavonoid also contained in VV) in a man complaining of prurigo nodularis complicating AD has been reported by Pennesi et al. [147].
When considering the oral administration of grape derivatives, some authors reported their beneficial effects in different skin diseases, including AD in a mouse model and acne. In this latter disease, a randomized clinical trial involving patients treated with oral isotretinoin for severe acne showed that in subjects receiving both isotretinoin and a dietary supplement containing vitamins and VV, the acne clinical scores improved, and a greater adherence to the therapy with lower side effects was achieved [148,149]. Unfortunately, the precise composition of the administered supplementation was not specified.
Moreover, the beneficial effect of pterostilbene, found not only in VVLs but also in other plant sources such as blueberries, has been investigated in AD models. Interestingly, in an AD-induced mouse model, its topical application decreased skin thickness, evaluation scoring, and IgE levels [150].
When considering microbiota and acne, VVLE var. aglianico has been demonstrated as the most active plant derivative to inhibit the growth of C. acnes, but no effect was documented in countering the biofilm production [151]. Moreover, in HaCaT and THP-1 cells stimulated by C. acnes, quercetin suppressed pro-inflammatory cytokine production (i.e., IL-1β, IL-6, IL-8, and TNF-α) via TLR2 and MAPK pathways and decreased the MMP-9 expression. In addition, the anti-inflammatory effect of quercetin was tested in a mouse model intradermally injected with C. acnes, where it significantly reduced the cutaneous erythema and swelling [152].
Furthermore, VV derivatives demonstrated positive effects in numerous randomized clinical trials involving HS-affected patients, but the activity of the investigated product, represented by GSE administered orally, was only evaluated for the HS-associated metabolic syndrome improvement [153].
Only few reports exist on the use of VV derivatives in scalp disorders, namely in SD and hair loss. It has been reported that grape seed compounds of VV can counteract the Malassezia spp. activity in SD [154,155]. Accordingly, in hair loss, the potential use of GSE has been investigated both in vitro and in vivo. More in detail, proanthocyanidins and procyanidins from GSE were tested for local use both in hair cultures and in C3H mice, thus showing an increased hair proliferation both in cultures and after topical application in mice, probably due to the telogen-anagen phase conversion in the hair cell cycle [156]. The above-mentioned substances and their possible effects in dermatological disorders are summarized in Table 5.

Table 5.
Skin and scalp disorders and Vitis vinifera L.
From a molecular point of view, only few studies analyzed the effect of VVLEs in cutaneous inflammatory pathways, mainly focusing on their potential protective role towards UV damage [38]. Firstly, VVLE pre-treated HaCaT cells were separately irradiated with UVA and UVB. UVA increased DNA damage and cell cycle S phase block, thus leading to apoptosis even in the presence of VVLEs. UVB irradiation decreased cell viability and increased apoptosis not counteracted by VVLEs; this effect is probably due to the possible extract difficulties in acting as an antioxidant and the reduced pre-treatment time. However, these extracts demonstrated a protective activity against UV-induced damage [36]. Moreover, when evaluating keratin expression in UVB-induced skin damage (both in HaCaT and 3D normal skin organotypic cultures), keratin 17 (K17) expression induced by the pSTAT3 pathway significantly decreased in the presence of VVLEs. Since K17 can be considered as a reliable marker of UVB damage in skin, the authors concluded that VVLEs in vitro can protect from UVB-mediated epidermal injury [157]. Secondly, when pre-treated with VVLEs, UVA-irradiated normal human dermal fibroblasts showed an increased cell viability through the activation of SIRT1 and HSP47, two genes involved in the anti-aging processes, suggesting a further potential effect of the extracts in such condition [158]. Moreover, collagen XVII, a hemidesmosome transmembrane protein whose role has been recently studied both in skin and hair aging, as well as in wound repair and blistering skin diseases, increased in human skin models treated with pterostilbene and its glucoside [159,160]. Given the abundance of such chemicals in VVLEs, a further potential application in dermatology could be speculated.
Lastly, no data are currently available regarding the potential sensitization and irritant effect of VVLEs in skin, scalp, and mucosal surfaces (i.e., eyes and genitalia). More in general, a recent review reported that both VV fruit, juice, and seed extracts are non-irritant or only minimally irritant for the skin. Accordingly, fruit, seed, and grape skin extracts were classified as non-irritant or mildly irritant for the ocular surfaces [34].
6. Vitis vinifera L. Leaf Extracts, Microbiota, and Skin Opportunistic Pathogens
Among their properties, many authors highlight how polyphenols may inhibit or enhance microbial growth and survival, depending on the phytochemical structure and on the bacterial species [161]. Overall, they can exert a double beneficial effect, since they may counteract pathogen invasion and virulence, while supporting beneficial strains [162,163].
When used for human health, a key aspect to be stressed is that polyphenols establish a bidirectional interaction with the host microbiota, especially the gut one [37,164]. Due to their poor absorbability in the digestive system, once they arrive in the large intestine, they can act as prebiotics, thus modulating the human microbiota composition [162,165]. At the same time, polyphenols can be substrates of bacterial enzymes and undergo a process of biotransformation, being released in the bloodstream and thus conferring local and systemic beneficial effects also through the gut–brain and gut–skin axes [161,166,167,168].
In this context, several works focus on VV L. polyphenolic compounds and their activity. Different plant organs (i.e., fruits, leaves, seeds, skin, canes, stems, and roots) have distinct phytochemical profiles, as summarized by Sharafan et al. [34]. Consequently, extracts from different plant parts also have different antimicrobial effects: for instance, seed extracts have shown a greater antimicrobial potential compared to leaf ones [169]. In particular, a correlation between the total phenol content and the resulting antimicrobial activity has been highlighted, but several other factors (such as cultivar, environmental stimuli, and genotype) are also involved in determining the final effects [170,171,172].
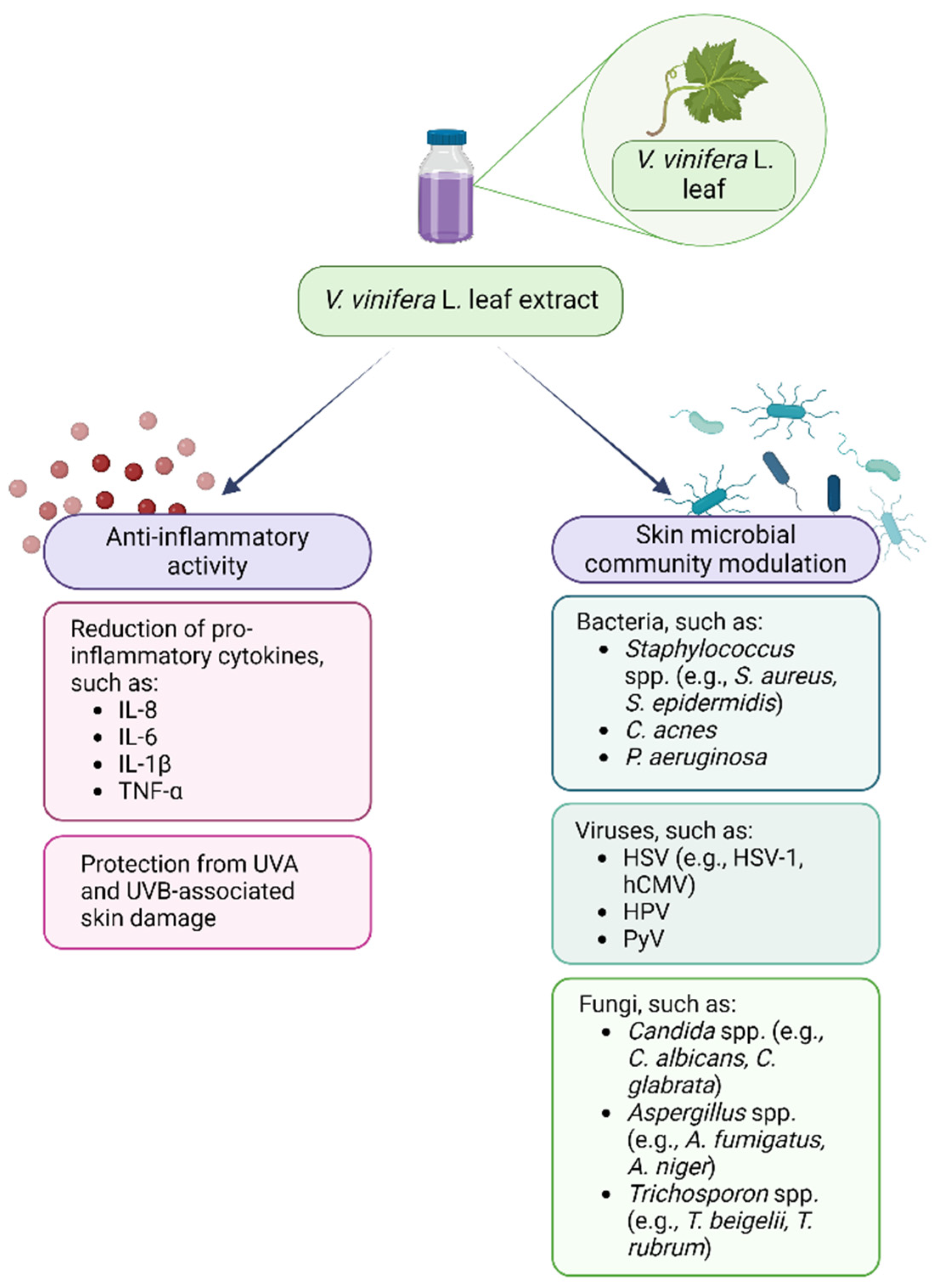
Therefore, plant polyphenols may be used in support to or as alternatives to the currently available antimicrobial drugs that nowadays have limited efficacy due to the global spread of antimicrobial resistance and to their ability to reduce the microbiota diversity [173,174]. Regarding the interaction between VVLEs and skin microorganisms, even if the antibacterial, antiviral, and antifungal properties of these extracts and polyphenols have been demonstrated in vitro on selected pathogenic microorganisms, the literature about the effects on the skin microbiota is very poor, so this topic needs to be further investigated. Lastly, the anti-inflammatory and prebiotic potential could synergistically enhance the beneficial effects of these compounds in the management of dysbiotic conditions (Figure 2). As a matter of fact, PSMs are known for their potential in restoring and improving gut dysbiosis by increasing the abundance of beneficial commensal that could counteract the inflammatory microenvironment [175,176]. This evidence, alongside the knowledge of their benefits on skin and hair health, could support the need for further investigation regarding their interaction with the skin microbiota [177].
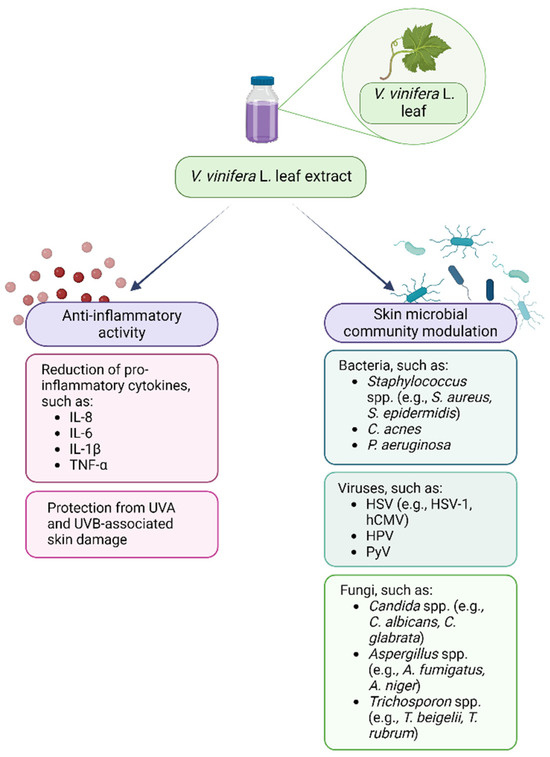
Figure 2.
Vitis vinifera L. leaf extract anti-inflammatory and skin microbial community modulatory activity. Created with BioRender.com.
6.1. Vitis vinifera L. Leaf Extracts and Bacteria
The antibacterial potential of VVLEs has been tested on different microorganisms, usually opportunistic pathogenic ones, some of which are also involved in skin infections. Gram-positive bacteria like Staphylococcus spp. seem to be more susceptible to the antibacterial activity of VVLEs if compared to Gram-negative bacteria, such as Pseudomonas aeruginosa [80,178,179]. The different sensitivity to phenolic-rich extracts has often been reported, regardless of the grape raw material [172,180,181]; nevertheless, other studies showed that there is not any statistically significant difference in response between Gram-positive and -negative bacteria [79,182,183]. Discording data may be attributable both to the extracts and the bacteria. On the one hand, as previously stressed, each extract has a distinct phytochemical profile which can result in different outcomes, and the solvents they are dissolved in may interfere with the biological response; on the other one, bacteria show species-specific and also strain-specific sensitivity to treatments [181,184,185]. The different susceptibility between Gram-positive and Gram-negative bacteria could also be linked to the bacterial surface and structure. Gram-negative double-layered cell envelope and periplasmic enzymes, e.g., some isoforms of metallo-β-lactamases (MBLs), protect the bacteria against many antimicrobial drugs by hindering their diffusion and degrading them, respectively [186,187].
Interesting results have been presented by Andelković et al. in 2015. Their study highlighted how extracts from grapevine leaves of two different varieties, Merlot and Vranac, had a significant antimicrobial activity towards Gram-positive bacteria more than Gram-negative ones. Notably, different activity depended not only on the grapevine variety, but also on the health status of the raw material itself. Extracts from Plasmapora viticola-infected leaves showed an increased antimicrobial effect when compared to those extracted from healthy leaves, probably due to the different PSM production of healthy and unhealthy plants [80].
In addition to the general antibacterial effects on planktonic forms, antibiofilm potential of VVLEs on skin pathogens is being investigated as well. Since currently available antimicrobial therapies are not always effective against biofilm forms, it is imperative to find new strategies for their prevention, treatment, and containment [188,189]. Grapevine leaves may have antibiofilm potential due to the presence of resveratrol and pterostilbene, of which antimicrobial and antibiofilm activity have been demonstrated on selected bacteria [190]. Stilbenoids, the phenolic group resveratrol and pterostilbene belong to, are classified as phytoalexins and as such are usually synthetized by the plant in response to infections or other damages [191]. These compounds seem to be more active against Gram-positive bacteria, but systematic studies are still needed to better understand their effects [192]. Lately, pterostilbene has gained more and more interest thanks to its pharmacological properties that make it a better candidate for therapeutic and clinical uses than resveratrol for the management not only of infectious disorders but also of dermatological issues [193,194].
Coenye et al. have identified the potential of resveratrol in counteracting the growth and biofilm formation of C. acnes, involved not only in the etiopathogenesis of acne vulgaris but also in implant-associated infections [195,196]. On the other hand, as previously reported in this review, Nelson et al. tested VV L. var. aglianico leaf extracts on C. acnes and highlighted their ability to inhibit bacterial growth without exerting any cytotoxicity on HaCaT cells, but they were not as effective in the eradication of mature biofilm forms. However, resveratrol was not detected in the selected extracts, despite the evidence in the literature [151].
Other studies were conducted on S. epidermidis, S. aureus, and P. aeruginosa, three of the main skin pathobionts involved in wound-associated biofilms, chronic wounds, and unpaired healing among other morbidities [197,198]. Even if antimicrobial effects are microorganism-dependent, both resveratrol and pterostilbene appeared to be more effective against Gram-positive bacteria, having shown low or no efficacy on P. aeruginosa [189,199]. Pterostilbene showed a greater potency than resveratrol not only in the treatment and prevention of biofilm but also in the inhibition of planktonic forms of Gram-positive cocci, namely, S. epidermidis, S. aureus, vancomycin-intermediate S. aureus (VISA), methicillin-resistant S. aureus (MRSA), and also Enterococcus faecalis [199,200]. Although none of these studies involved grapevine leaves or other winery by-products as sources for resveratrol and pterostilbene, it could be worthy to further delve into VVLEs and their phytochemical profile in order to understand the possible synergistic interactions among these and other secondary metabolites and the effects on the skin microbial communities.
6.2. Vitis vinifera L. Leaf Extracts and Viruses
Among VV-associated therapeutic properties exerted on microorganisms, both commensal and pathogenic ones, the antiviral effects have been investigated as well.
As reported in Viruses, Zannella et al., after examining the VVL content via an HPLC-MS/MS analysis, demonstrated the ability of the 40 identified phenolic compounds to inhibit the replication of the human opportunistic cutaneous Herpes simplex virus type 1 (HSV-1) pathogen (and also that of the severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) in vitro in the very early infection phases of an eukaryotic Vero cell line model. Through plaque reduction assays and different experimental conditions (i.e., co-treatment, virus pre- and post-treatment, and cell pre-treatment), the authors showed that the leaf extracts were able to inhibit the virus–host cell binding and all the succeeding infection stages, as shown by the lack of gene expression involved in the viral infection cycle [201]. In another research study, aimed to assess the antimicrobial and antioxidant properties of VV aqueous leaf extract fractions, the authors confirmed that the phenolic compounds (i.e., terpenoids, flavonoids, and other related molecules) contained in the chloroform (CHCl3) fraction have potential antiviral effects towards the HSV-1 DNA virus (and the RNA Parainfluenza virus, PIV). Experiments were conducted once again in the Vero cell line model, in which viral strain titers were assessed with the Frey and Liess protocol as 50% tissue culture infectious dose (TCID50) using acyclovir and oseltamivir as per internal controls. These data are really promising, thus also supporting the extract being employed in folk medicine [178].
In the study of Berardi et al. on murine Polyomavirus (Py), chosen as the model system, resveratrol showed dose- and time-dependent in vitro antiviral activity expressed after the virus’s entry into the target cells. The authors suggested that this effect could be linked to its ability to inhibit the murine Py DNA synthesis in mouse normal fibroblast 3T6 cells, probably due to the damage caused to the plasma membrane. As a result, the virus translocation from the endoplasmic reticulum to the nucleus, and hence the viral DNA production, were both hindered. This phenomenon was observed also in the human tumor HL60 cell line. Therefore, the resveratrol presence seems to be needed till the end of the productive cycle to completely inhibit the viral DNA synthesis, translation, and eventually the progeny production [202].
Despite not being specifically demonstrated for grape leaves, resveratrol also abrogated the DNA replication of the human Cytomegalovirus (hCMV), responsible for reactivating skin infections in immunocompromised elderly and/or transplanted people, as demonstrated by its ability of inactivating in vitro the epidermal growth factor in human embryonal fibroblasts [203].
Regarding SARS-CoV-2, even though it does not directly infect the skin, it may lead to mucocutaneous visible signs in infected patients, mainly in the form of hyperpigmentation, a common inflammatory dermatological disorder characterized by extra melanin production after injury, irritation, or antibiotic use. Since melanin production is regulated by tyrosinase and many bioactive compounds of VV act as tyrosinase inhibitors (e.g., resveratrol, gallic acid, chlorogenic acid, epicatechin, and rutin), these PSMs could be exploited for their antimelanogenic potential [204,205].
Notably, the skin virota also includes beta and gamma Human Papillomavirus (HPV), which may promote non-melanoma skin carcinogenesis in transplant and/or immunosuppressed patients [206]. The inhibitory potential of resveratrol against mucosal high-risk (HR) HPV16 and HPV18 E6 and E7 oncoproteins, involved in cervical carcinogenesis, was demonstrated in vitro on HeLa and CaSki cells [207]. Data on cutaneous HPVs are not yet available, but this could be worthy of further investigation.
6.3. Vitis vinifera L. Leaf Extracts and Fungi
The antifungal activity of VVLEs has been explored but, worthy of note, only sporadic clinical reports can be retrieved from the scientific literature. Indeed, the potential effect of VVLEs on dermatophytes (i.e., fungi affecting skin and appendages, mainly causing superficial infection), molds, and yeasts has been investigated in various studies evaluating different single chemical compounds. In particular, the analyzed groups of substances belonging to the VVLEs were mainly represented by flavonols and stilbenes, both abundant in grapevine leaves [83]. As already reported for the antibacterial and antiviral activities, pterostilbene and resveratrol are the two compounds that have showed interesting antifungal properties in vitro. Resveratrol was found to act as fungicidal agent on Candida albicans, by interfering with the ergosterol biosynthesis, but it demonstrated a lack of effectiveness towards other Candida spp., such as C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, and C. dubliniensis [208]. The high antifungal activity of pterostilbene has been demonstrated both in vitro and in vivo, being fivefold higher than resveratrol, and was suggested to be related to its better bioavailability and high capability to penetrate hydrophobic membranes, such as pathogen biofilm. In more detail, pterostilbene significantly inhibited biofilm formation in yeasts like Trichosporon cutaneum and, less weakly, C. krusei, while no effect was observed in C. albicans and C. parapsilosis [200]. In order to counteract this issue, pterostilbene was incorporated in poly-lactic-co-glycolic acid nanoparticles (PLGA NPs) and its effect on anti-C. albicans biofilm formation was compared with that of the free form and the crude pomace extract, demonstrating a significant superiority of PLGA NPS in allowing the delivery of antifungal drugs [209].
Resveratrol was found to be active in vitro against two other species of yeasts (Saccharomyces cerevisiae and Trichosporon beigelii), towards which its potency was comparable to Amphotericin B. Its mechanism of action was reported to induce apoptosis through the activation of metacaspases and cytochrome C release. Moreover, resveratrol was able to inhibit up to 80% of the growth of some of the most common human dermatophytes (i.e., Trichophyton mentagrophytes, Trichophyton rubrum, Trichophyton tonsurans, Microsporum gypseum, and Epidermophyton floccosum). When considering pathogenic molds, resveratrol was also tested against Aspergillus niger and Aspergillus fumigatus, responsible for various diseases among immunocompromised patients, demonstrating different degrees of activity depending on concentrations and VV cultivar [210].
Some studies have also reported the antifungal effect of other substances that can be found in the VVLEs, though these effects were observed through experiments involving different herbal plants. Indeed, epigallocathechin-3-gallate from green tea was found to inhibit the folate pathway and, also, ergosterol biosynthesis, both leading stopping the C. albicans growth, and a potential synergy with traditional treatments (azoles and terbinafine) was also suggested [211]. The same yeast was also targeted by caffeic acid, via inhibition of the isocitrate lyase enzyme and, consequently, lowering its virulence capability. Lastly, the dermatophyte T. rubrum development was demonstrated to be hindered by gallic acid and quercetin with a mechanism involving the activity of sterol 14-α-demethylase P450 and the expression of FAS1 and ERG6, respectively, thus interfering with the ergosterol synthesis [210].
7. Vitis vinifera L. Extract Prebiotic and Postbiotic Properties
The need for novel and/or alternative approaches for the management of skin inflammation in dysbiotic conditions has opened the doors to several new possibilities. As a matter of fact, due to the great health-promoting potential of grapevine and derived biomasses, their exploitation could provide novel active ingredients able to counteract pathogenic microorganisms and to support the local microbial flora. For instance, grapevine leaves could be converted into high-value-added products through biotechnological processes. In this context, the development of white biotechnology has emerged as a transformative force since it relies on enzymes and microorganisms to produce valuable components from renewable sources for the food, pharmaceutical, and cosmeceutics markets as well as other industries [212,213].
Recently, precision fermentation and the study of bioprocesses for the development of innovative active ingredients obtained through such procedures have been demonstrated to be a valid strategy to valorize many waste materials, satisfying the needs of the bio-circular-green economy model [214,215]. One application is represented by postbiotics, defined as “preparations of inanimate microorganisms and/or their components that confer a health benefit on the host” by the International Scientific Association for Probiotics and Prebiotics (ISAPP) in 2019 [216]. Postbiotics have unique properties that could be exploited in several sectors (e.g., food, dermocosmetics, and pharmaceutical markets), thus opening new scenarios and possibilities, such as the improvement in many skin issues, both pathological (i.e., AD) and aesthetic ones (i.e., wrinkles) [116,217].
Indeed, by understanding the basic scientific mechanisms of how these products work, it is possible to design more specific ones that address a wide range of functions, providing end users with reliable and effective solutions that could potentially prevent or improve pre-existing skin and scalp issues.
Due to their phenolic content, VVLEs could be exploited both for their prebiotic properties and for the development of postbiotics. Even if not completely understood yet, the interaction between polyphenols and microbiota is bidirectional, with the microbiota metabolizing polyphenols which themselves may modulate its quality, quantity, variety, and functions [37]. As a result, VVLEs are ideal prebiotic candidates for further processing in postbiotic production through probiotic-driven fermentation, as already reported in the literature for other raw materials [217,218]. Thanks to their greater specificity of action on the resident microbiota, and their interaction with the host cells, fermented extracts could better improve microbiome-based skin conditions compared to non-fermented ones [219]. Probiotic precise fermentation closely mimics the physiological processes of conversion of dietary polyphenols by the gut microbiota. In more detail, it leads to the ex novo or over-production of bioactive molecules, including phenolic-derived postbiotics and exopolysaccharides (EPSs), known for their health-promoting benefits, ranging from microbial restoration to immunomodulatory properties, anti-inflammatory, antiproliferative, and antioxidant ones [219,220,221]. Additionally, precision fermentation technology allows for more controlled bioprocesses and parameters, thus generating high-value-added products. Evidence suggests that fermented biomolecules, produced by the natural metabolism of microorganisms, also improve microbiome modulation [222,223]. However, despite productivity and yield being continuously optimized to ensure the quality and efficacy of the final products, this biotechnological approach still poses safety and standardization challenges, since many parameters can affect the final product features [224,225,226]. Lastly, their properties and phytochemical profile also depend on the selected fermenting microorganisms and on the natural substrate itself, here represented by VVLs [37,227].
8. Conclusions
The recent advances in dermatological and microbiota-related research highlight the importance of microbiota-friendly approaches to ensure eubiosis, while counteracting inflammatory conditions. For this reason, greater attention is devoted to plant-based bioactive compounds also because of their action dualism. If on one side they exert skin anti-inflammatory and antioxidant effects, on the other, they reduce pathogens, while sustaining beneficial microbial species at the same time. The exploitation of plant biomasses and the production of plant-derived ingredients through microbe-driven fermentation could represent a great opportunity for the management of many inflammatory dysbiosis-related skin and scalp issues (e.g., AD, SD, acnes, HS, and psoriasis).
Despite that Vitis vinifera L. and its leaves appear to be promising to help solve these conditions, the mechanisms of action, the effects on skin commensals and pathogens, and the synergistic forces underlying the observed health-promoting benefits are yet to be fully established. Moreover, their bioavailability, penetrating capability, and half-life, as well as the optimal administration route and standardization of extraction, isolation, and fermentation processes, also need to be investigated.
Author Contributions
Conceptualization, M.A. and B.A.; writing—original draft preparation, M.A., E.Z., C.F.T. and B.A.; writing—review and editing, M.A., B.A., E.Z., C.F.T., A.G. (Alessia Grossetti), A.G. (Alice Galeazzi), F.V. and P.S.; visualization, M.A., B.A., E.Z., C.F.T., A.G. (Alessia Grossetti), A.G. (Alice Galeazzi), F.V. and P.S.; supervision, B.A.; project administration, B.A.; funding acquisition, B.A. All authors have read and agreed to the published version of the manuscript.
Funding
B.A. has been partially supported by Fondi di Ricerca di Ateneo (FAR-2017) provided by UPO. M.A. holds a PhD career grant supported by Next Generation EU—MUR (Italy), for the PhD program in “Food, Health and Longevity Studies, XXXIX cycle”. The APC has been funded by Roelmi HPC srl.
Acknowledgments
The authors would like to thank Professor Roberta Rolla for the technical support in figure preparation and Aurora Tritto and Paolo Sonzini for their help in the phytochemical description.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Balato, A.; Cacciapuoti, S.; Di Caprio, R.; Marasca, C.; Masarà, A.; Raimondo, A.; Fabbrocini, G. Human Microbiome: Composition and Role in Inflammatory Skin Diseases. Arch. Immunol. Ther. Exp. 2019, 67, 1–18. [Google Scholar] [CrossRef]
- Pat, Y.; Ogulur, I.; Yazici, D.; Mitamura, Y.; Cevhertas, L.; Küçükkase, O.C.; Mesisser, S.S.; Akdis, M.; Nadeau, K.; Akdis, C.A. Effect of altered human exposome on the skin and mucosal epithelial barrier integrity. Tissue Barriers 2023, 11, 2133877. [Google Scholar] [CrossRef]
- Chopra, D.; Arens, R.A.; Amornpairoj, W.; Lowes, M.A.; Tomic-Canic, M.; Strbo, N.; Lev-Tov, H.; Pastar, I. Innate immunity and microbial dysbiosis in hidradenitis suppurativa—Vicious cycle of chronic inflammation. Front. Immunol. 2022, 13, 960488. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Lin, Q.; Panchamukhi, A.; Li, P.; Shan, W.; Zhou, H.; Hou, L.; Chen, W. Malassezia and Staphylococcus dominate scalp microbiome for seborrheic dermatitis. Bioprocess Biosyst. Eng. 2021, 44, 965–975. [Google Scholar] [CrossRef]
- Szabó, K.; Bolla, B.S.; Erdei, L.; Balogh, F.; Kemény, L. Are the Cutaneous Microbiota a Guardian of the Skin’s Physical Barrier? The Intricate Relationship between Skin Microbes and Barrier Integrity. Int. J. Mol. Sci. 2023, 24, 15962. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primer 2018, 4, 1. [Google Scholar] [CrossRef]
- Celoria, V.; Rosset, F.; Pala, V.; Dapavo, P.; Ribero, S.; Quaglino, P.; Mastorino, L. The Skin Microbiome and Its Role in Psoriasis: A Review. Psoriasis 2023, 13, 71–78. [Google Scholar] [CrossRef]
- Ganju, P.; Nagpal, S.; Mohammed, M.H.; Nishal Kumar, P.; Pandey, R.; Natarajan, V.T.; Mande, S.S.; Gokhale, R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016, 6, 18761. [Google Scholar] [CrossRef]
- Schell, S.L.; Schneider, A.M.; Nelson, A.M. Yin and Yang: A disrupted skin microbiome and an aberrant host immune response in hidradenitis suppurativa. Exp. Dermatol. 2021, 30, 1453–1470. [Google Scholar] [CrossRef]
- Pinto, D.; Sorbellini, E.; Marzani, B.; Rucco, M.; Giuliani, G.; Rinaldi, F. Scalp bacterial shift in Alopecia areata. PLoS ONE 2019, 14, e0215206. [Google Scholar] [CrossRef]
- Huang, C.; Yi, X.; Long, H.; Zhang, G.; Wu, H.; Zhao, M.; Lu, Q. Disordered cutaneous microbiota in systemic lupus erythematosus. J. Autoimmun. 2020, 108, 102391. [Google Scholar] [CrossRef]
- Savoia, P.; Azzimonti, B.; Rolla, R.; Zavattaro, E. Role of the Microbiota in Skin Neoplasms: New Therapeutic Horizons. Microorganisms 2023, 11, 2386. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.-M.; Fan, X.-Y.; Jin, Y.-L.; Li, X.; Wu, S.-R.; Ge, W.-W.; Lv, C.-H.; Wang, Y.-K.; Chen, J.-G. Gut-Brain-Skin Axis in Psoriasis: A Review. Dermatol. Ther. 2021, 11, 25–38. [Google Scholar] [CrossRef]
- Mehta, A.B.; Nadkarni, N.J.; Patil, S.P.; Godse, K.V.; Gautam, M.; Agarwal, S. Topical corticosteroids in dermatology. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 371–378. [Google Scholar] [CrossRef]
- Starbek Zorko, M.; Benko, M.; Rakuša, M.; Prunk Zdravković, T. Evaluation of corticophobia in patients with atopic dermatitis and psoriasis using the TOPICOP© score. Acta Dermatovenerol. Alp. Pannonica Adriat. 2023, 32, 135–139. [Google Scholar] [CrossRef]
- Yazici, D.; Ogulur, I.; Pat, Y.; Babayev, H.; Barletta, E.; Ardicli, S.; Bel Imam, M.; Huang, M.; Koch, J.; Li, M.; et al. The epithelial barrier: The gateway to allergic, autoimmune, and metabolic diseases and chronic neuropsychiatric conditions. Semin. Immunol. 2023, 70, 101846. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Dell’Agli, M. Special Issue: Anti-Inflammatory Activity of Plant Polyphenols 2.0. Biomedicines 2021, 10, 37. [Google Scholar] [CrossRef]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, e2100670. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Zajdel, K.; Kucharska, E.; Kowalczyk, T.; Zajdel, R. The Modulatory Influence of Plant-Derived Compounds on Human Keratinocyte Function. Int. J. Mol. Sci. 2021, 22, 12488. [Google Scholar] [CrossRef]
- Moldovan, M.L.; Carpa, R.; Fizeșan, I.; Vlase, L.; Bogdan, C.; Iurian, S.M.; Benedec, D.; Pop, A. Phytochemical Profile and Biological Activities of Tendrils and Leaves Extracts from a Variety of Vitis vinifera L. Antioxidants 2020, 9, 373. [Google Scholar] [CrossRef]
- Singh, J.; Rasane, P.; Kaur, R.; Kaur, H.; Garg, R.; Kaur, S.; Ercisli, S.; Choudhary, R.; Skrovankova, S.; Mlcek, J. Valorization of grape (Vitis vinifera) leaves for bioactive compounds: Novel green extraction technologies and food-pharma applications. Front. Chem. 2023, 11, 1290619. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols From Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.-D.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwaśniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a Valuable Cosmetic Raw Material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef]
- Marabini, L.; Melzi, G.; Lolli, F.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E.; Marinovich, M. Effects of Vitis vinifera L. leaves extract on UV radiation damage in human keratinocytes (HaCaT). J. Photochem. Photobiol. B 2020, 204, 111810. [Google Scholar] [CrossRef]
- Bogdan, C.; Pop, A.; Iurian, S.M.; Benedec, D.; Moldovan, M.L. Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care. Antioxidants 2020, 9, 502. [Google Scholar] [CrossRef]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34 (Suppl. S4), 4–25. [Google Scholar] [CrossRef]
- Khmaladze, I.; Leonardi, M.; Fabre, S.; Messaraa, C.; Mavon, A. The Skin Interactome: A Holistic “Genome-Microbiome-Exposome” Approach to Understand and Modulate Skin Health and Aging. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1021–1040. [Google Scholar] [CrossRef]
- Cecchi, L.; D’Amato, G.; Annesi-Maesano, I. External exposome and allergic respiratory and skin diseases. J. Allergy Clin. Immunol. 2018, 141, 846–857. [Google Scholar] [CrossRef]
- Prieux, R.; Eeman, M.; Rothen-Rutishauser, B.; Valacchi, G. Mimicking cigarette smoke exposure to assess cutaneous toxicity. Toxicol. Vitr. 2020, 62, 104664. [Google Scholar] [CrossRef]
- Lima, C.; Falcão, M.A.P.; Rosa, J.G.S.; Disner, G.R.; Lopes-Ferreira, M. Pesticides and Their Impairing Effects on Epithelial Barrier Integrity, Dysbiosis, Disruption of the AhR Signaling Pathway and Development of Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 12402. [Google Scholar] [CrossRef]
- Belzer, A.; Parker, E.R. Climate Change, Skin Health, and Dermatologic Disease: A Guide for the Dermatologist. Am. J. Clin. Dermatol. 2023, 24, 577–593. [Google Scholar] [CrossRef]
- Aşkın, Ö.; Uzunçakmak, T.K.Ü.; Altunkalem, N.; Tüzün, Y. Vitamin deficiencies/hypervitaminosis and the skin. Clin. Dermatol. 2021, 39, 847–857. [Google Scholar] [CrossRef]
- Joshi, M.; Hiremath, P.; John, J.; Ranadive, N.; Nandakumar, K.; Mudgal, J. Modulatory role of vitamins A, B3, C, D, and E on skin health, immunity, microbiome, and diseases. Pharmacol. Rep. PR 2023, 75, 1096–1114. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef]
- Gratton, R.; Del Vecchio, C.; Zupin, L.; Crovella, S. Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases. Int. J. Mol. Sci. 2022, 23, 3132. [Google Scholar] [CrossRef]
- Orion, E.; Wolf, R. Psychological stress and epidermal barrier function. Clin. Dermatol. 2012, 30, 280–285. [Google Scholar] [CrossRef]
- Chen, Y.; Lyga, J. Brain-Skin Connection: Stress, Inflammation and Skin Aging. Inflamm. Allergy Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.J.; Kim, D.; Kim, E.J.; Ahn, J.-S.; Choi, E.-J.; Son, E.D.; Lee, T.R.; Choi, E.H. Psychological Stress Deteriorates Skin Barrier Function by Activating 11β-Hydroxysteroid Dehydrogenase 1 and the HPA Axis. Sci. Rep. 2018, 8, 6334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, M.; Zhao, X.; Wang, Y.; Chen, X.; Su, J. Role of stress in skin diseases: A neuroendocrine-immune interaction view. Brain Behav. Immun. 2024, 116, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin improves skin barrier damage caused by sleep restriction through gut microbiota. J. Pineal Res. 2023, 75, e12874. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.R. The impact of aging on epithelial barriers. Tissue Barriers 2017, 5, e1343172. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Hu, A.; Bollag, W.B. The Skin and Inflamm-Aging. Biology 2023, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Nicolas, G.; Amara, A.; Salek, R.M.; Scalbert, A. The human microbial exposome: Expanding the Exposome-Explorer database with gut microbial metabolites. Sci. Rep. 2023, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.D.; McLean, W.H.I.; Leung, D.Y.M. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef]
- Schleimer, R.P.; Berdnikovs, S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J. Allergy Clin. Immunol. 2017, 139, 1752–1761. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Ozdemir, C.; Yazici, D.; Pat, Y.; Mitamura, Y.; Li, M.; Sun, N.; D’Avino, P.; Bu, X.; Zhu, X.; et al. The epithelial barrier theory: Development and exacerbation of allergic and other chronic inflammatory diseases. Asia Pac. Allergy 2023, 13, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.N.; Piñón Hofbauer, J.; Wally, V.; Kofler, B.; Schmuth, M.; De Rosa, L.; De Luca, M.; Bauer, J.W. Epigenetic and metabolic regulation of epidermal homeostasis. Exp. Dermatol. 2021, 30, 1009–1022. [Google Scholar] [CrossRef]
- Kimball, A.B. Skin differences, needs, and disorders across global populations. J. Investig. Dermatol. Symp. Proc. 2008, 13, 2–5. [Google Scholar] [CrossRef]
- Andersen, L.K.; Davis, M.D.P. Sex differences in the incidence of skin and skin-related diseases in Olmsted County, Minnesota, United States, and a comparison with other rates published worldwide. Int. J. Dermatol. 2016, 55, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Rahrovan, S.; Fanian, F.; Mehryan, P.; Humbert, P.; Firooz, A. Male versus female skin: What dermatologists and cosmeticians should know. Int. J. Womens Dermatol. 2018, 4, 122–130. [Google Scholar] [CrossRef]
- Lagacé, F.; D’Aguanno, K.; Prosty, C.; Laverde-Saad, A.; Cattelan, L.; Ouchene, L.; Oliel, S.; Genest, G.; Doiron, P.; Richer, V.; et al. The Role of Sex and Gender in Dermatology—From Pathogenesis to Clinical Implications. J. Cutan. Med. Surg. 2023, 27, NP1–NP36. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.I.; Hong, G.; Albornoz, K.; Berlanga, M. Fresh grapevine (Vitis vinifera L.) leaves: Postharvest biology and handling recommendations. Sci. Hortic. 2022, 292, 110627. [Google Scholar] [CrossRef]
- Vinci, G.; Prencipe, S.A.; Abbafati, A.; Filippi, M. Environmental Impact Assessment of an Organic Wine Production in Central Italy: Case Study from Lazio. Sustainability 2022, 14, 15483. [Google Scholar] [CrossRef]
- Wong, M.C.; Hendrikse, S.I.S.; Sherrell, P.C.; Ellis, A.V. Grapevine waste in sustainable hybrid particleboard production. Waste Manag. 2020, 118, 501–509. [Google Scholar] [CrossRef]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef]
- Lacerda, D.D.S.; Santos, C.F.; Oliveira, A.S.; Zimmermann, R.; Schneider, R.; Agostini, F.; Dani, C.; Funchal, C.; Gomez, R. Antioxidant and hepatoprotective effects of an organic grapevine leaf (Vitis labrusca L.) extract in diabetic rats. RSC Adv. 2014, 4, 52611–52619. [Google Scholar] [CrossRef]
- Gonçalves, D.A.; González, A.; Roupar, D.; Teixeira, J.A.; Nobre, C. How prebiotics have been produced from agro-industrial waste: An overview of the enzymatic technologies applied and the models used to validate their health claims. Trends Food Sci. Technol. 2023, 135, 74–92. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- Kortekamp, A. Expression analysis of defence-related genes in grapevine leaves after inoculation with a host and a non-host pathogen. Plant Physiol. Biochem. PPB 2006, 44, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of Vitis vinifera (grape)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2014, 33, 48S–83S. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Mehri, S.; Shaebani Behbahani, F.; Hosseinzadeh, H. Protective effects of Vitis vinifera (grapes) and one of its biologically active constituents, resveratrol, against natural and chemical toxicities: A comprehensive review. Phytother. Res. 2018, 32, 2164–2190. [Google Scholar] [CrossRef]
- Katalinić, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis vinifera L. Varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Anđelković, M.; Radovanović, B.; Anđelković, A.M.; Radovanović, V. Phenolic Compounds and Bioactivity of Healthy and Infected Grapevine Leaf Extracts from Red Varieties Merlot and Vranac (Vitis vinifera L.). Plant Foods Hum. Nutr. 2015, 70, 317–323. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J. In Situ Visible and Near-Infrared Spectroscopy Applied to Vineyards as a Tool for Precision Viticulture. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 80, pp. 253–279. ISBN 978-0-444-64048-2. [Google Scholar]
- Gülcü, M.; Ghafoor, K.; Al-Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M.; Azmi, I.U. Effect of grape (Vitis vinifera L.) varieties and harvest periods on bioactive compounds, antioxidant activity, phenolic composition, mineral contents, and fatty acid compositions of Vitis leave and oils. J. Food Process. Preserv. 2020, 44, e14890. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhao, Y.; Ma, X.; Jiao, X.; Fang, Y.; Zhang, Z.; Ju, Y. Effects of leaf removal on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in Cabernet Sauvignon (Vitis vinifera L.) grapes. J. Sci. Food Agric. 2021, 101, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Harb, J.; Alseekh, S.; Tohge, T.; Fernie, A.R. Profiling of primary metabolites and flavonols in leaves of two table grape varieties collected from semiarid and temperate regions. Phytochemistry 2015, 117, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-H.; Bi, W.-L.; Hao, X.-Y.; Li, P.-M.; Duan, Y.; Walker, M.A.; Xu, Y.; Wang, Q.-C. Drought Stress Enhances Up-Regulation of Anthocyanin Biosynthesis in Grapevine leafroll-associated virus 3-Infected in vitro Grapevine (Vitis vinifera) Leaves. Plant Dis. 2017, 101, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, L.; Gambuti, A.; De Cicco, P.; Ercolano, G.; Ianaro, A.; Taglialatela-Scafati, O.; Moio, L.; Forino, M. NMR-based phytochemical analysis of Vitis vinifera cv Falanghina leaves. Characterization of a previously undescribed biflavonoid with antiproliferative activity. Fitoterapia 2018, 125, 13–17. [Google Scholar] [CrossRef]
- Lima, M.R.M.; Felgueiras, M.L.; Cunha, A.; Chicau, G.; Ferreres, F.; Dias, A.C.P. Differential phenolic production in leaves of Vitis vinifera cv. Alvarinho affected with esca disease. Plant Physiol. Biochem. PPB 2017, 112, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Djemaa-Landri, K.; Hamri-Zeghichi, S.; Valls, J.; Cluzet, S.; Tristan, R.; Boulahbal, N.; Kadri, N.; Madani, K. Phenolic content and antioxidant activities of Vitis vinifera L. leaf extracts obtained by conventional solvent and microwave-assisted extractions. J. Food Meas. Charact. 2020, 14, 3551–3564. [Google Scholar] [CrossRef]
- Jediyi, H.; Naamani, K.; Ait Elkoch, A.; Dihazi, A.; Lemjiber, N. A comparative study of phenols composition, antioxidant, and antifungal potency of leaves extract from five Moroccan Vitis vinifera L. varieties. J. Food Saf. 2020, 40, e12756. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.D.; Ćirić, I.Ž.; Pergal, M.V.; Relić, D.J.; Todić, S.R.; Natić, M.M. Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia. J. Food Compos. Anal. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Farhadi, K.; Esmaeilzadeh, F.; Hatami, M.; Forough, M.; Molaie, R. Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chem. 2016, 199, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Aouey, B.; Samet, A.M.; Fetoui, H.; Simmonds, M.S.J.; Bouaziz, M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomed. Pharmacother. 2016, 84, 1088–1098. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Guella, G.; Stefanini, M.; Velasco, R.; Mattivi, F. Profiling and accurate quantification of trans-resveratrol, trans-piceid, trans-pterostilbene and 11 viniferins induced by Plasmopara viticola in partially resistant grapevine leaves. Aust. J. Grape Wine Res. 2012, 18, 11–19. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of Resveratrol Oligomers, Important Stress Metabolites, Accumulating in the Leaves of Hybrid Vitis vinifera (Merzling × Teroldego) Genotypes Infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
- Rätsep, R.; Karp, K.; Maante-Kuljus, M.; Aluvee, A.; Bhat, R. Polyphenols and Resveratrol from Discarded Leaf Biomass of Grapevine (Vitis sp.): Effect of Cultivar and Viticultural Practices in Estonia. Agriculture 2020, 10, 393. [Google Scholar] [CrossRef]
- Sun, H.; Lin, Q.; Wei, W.; Qin, G. Ultrasound-assisted extraction of resveratrol from grape leaves and its purification on mesoporous carbon. Food Sci. Biotechnol. 2018, 27, 1353–1359. [Google Scholar] [CrossRef]
- Dadáková, K.; Jurasová, L.; Kašparovský, T.; Průšová, B.; Baroň, M.; Sochor, J. Origin of Wine Lignans. Plant Foods Hum. Nutr. 2021, 76, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Tylewicz, U.; Nowacka, M.; Martín-García, B.; Wiktor, A.; Gómez Caravaca, A.M. 5—Target sources of polyphenols in different food products and their processing by-products. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 135–175. ISBN 978-0-12-813572-3. [Google Scholar]
- Li, X.; Liu, C.; Li, Y.; Yuan, K.; Zhang, W.; Cai, D.; Peng, Z.; Hu, Y.; Sun, J.; Bai, W. Bioactivity and application of anthocyanins in skin protection and cosmetics: An extension as a functional pigment. Phytochem. Rev. 2023, 22, 1441–1467. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological Applications and Health-Promoting Properties of Flavonols: An Updated View. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Shetty, K. Biological Functionality of Ellagic Acid: A Review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, K.; Erkiert-Polguj, A.; Budzisz, E. Sunscreening and Photosensitizing Properties of Coumarins and their Derivatives. Lett. Drug Des. Discov. 2016, 13, 465–474. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, O.A.; Andreev, Y.A.; Kozlov, S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022, 23, 6031. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO Traditional Medicine Strategy: 2014–2023. Available online: http://web.archive.org/web/20220912054716/https://apps.who.int/iris/handle/10665/92455 (accessed on 25 March 2024).
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Su, Z.; Pan, X.; Zheng, X.; Li, H.; Ye, Z.; Tang, B.; Lu, Y.; Zheng, G.; Lu, C. Phytochemicals: Targeting autophagy to treat psoriasis. Phytomedicine 2023, 120, 155041. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Khan, M.K.; Hassan, S.; Paniwnyk, L. Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs. In Plant Based “Green Chemistry 2.0”; Li, Y., Chemat, F., Eds.; Green Chemistry and Sustainable Technology; Springer: Singapore, 2019; pp. 197–235. ISBN 9789811338090. [Google Scholar]
- Castro, M.L.; Ferreira, J.P.; Pintado, M.; Ramos, O.L.; Borges, S.; Baptista-Silva, S. Grape By-Products in Sustainable Cosmetics: Nanoencapsulation and Market Trends. Appl. Sci. 2023, 13, 9168. [Google Scholar] [CrossRef]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and Topical Probiotics and Postbiotics in Skincare and Dermatological Therapy: A Concise Review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, A.; Bisignano, C.; Mandalari, G.; Navarra, M. In Vitro Antimicrobial Activity and Effect on Biofilm Production of a White Grape Juice (Vitis vinifera) Extract. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 856243. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Sousa, I.M.d.O.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Foglio, M.A.; Mazzola, P.G. In vitro solar protection factor, antioxidant activity, and stability of a topical formulation containing Benitaka grape (Vitis vinifera L.) peel extract. Nat. Prod. Res. 2020, 34, 2677–2682. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five Functional Aspects of the Epidermal Barrier. Int. J. Mol. Sci. 2021, 22, 11676. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef]
- Callewaert, C.; Ravard Helffer, K.; Lebaron, P. Skin Microbiome and its Interplay with the Environment. Am. J. Clin. Dermatol. 2020, 21, 4–11. [Google Scholar] [CrossRef]
- Luna, P.C. Skin Microbiome as Years Go By. Am. J. Clin. Dermatol. 2020, 21, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Azzimonti, B.; Ballacchino, C.; Zanetta, P.; Cucci, M.A.; Monge, C.; Grattarola, M.; Dianzani, C.; Barrera, G.; Pizzimenti, S. Microbiota, Oxidative Stress, and Skin Cancer: An Unexpected Triangle. Antioxidants 2023, 12, 546. [Google Scholar] [CrossRef]
- Pinto, D.; Ciardiello, T.; Franzoni, M.; Pasini, F.; Giuliani, G.; Rinaldi, F. Effect of commonly used cosmetic preservatives on skin resident microflora dynamics. Sci. Rep. 2021, 11, 8695. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, P.; Ballacchino, C.; Squarzanti, D.F.; Amoruso, A.; Pane, M.; Azzimonti, B. Lactobacillus johnsonii LJO02 (DSM 33828) Cell-Free Supernatant and Vitamin D Improve Wound Healing and Reduce Interleukin-6 Production in Staphylococcus aureus-Infected Human Keratinocytes. Pharmaceutics 2023, 16, 18. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Betsi, G.I.; Papadavid, E.; Falagas, M.E. Probiotics for the treatment or prevention of atopic dermatitis: A review of the evidence from randomized controlled trials. Am. J. Clin. Dermatol. 2008, 9, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.a.W.; Adams, V.; Hunt, J.; Palmer, R.; Martinelli, R.; Brunet, L.R. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin. Immunopathol. 2004, 25, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T.; Holgate, S.; Pawankar, R.; Akdis, C.A.; Benjaponpitak, S.; Caraballo, L.; Demain, J.; Portnoy, J.; von Hertzen, L. WAO Special Committee on Climate Change and Biodiversity The biodiversity hypothesis and allergic disease: World allergy organization position statement. World Allergy Organ. J. 2013, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, M.; Ikeda, M.; Matsumoto, N.; Yorifuji, T.; Tsukahara, H. Current Insights into Atopic March. Children 2021, 8, 1067. [Google Scholar] [CrossRef]
- Kim, K.; Jang, H.; Kim, E.; Kim, H.; Sung, G.Y. Recent advances in understanding the role of the skin microbiome in the treatment of atopic dermatitis. Exp. Dermatol. 2023, 32, 2048–2061. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Borda, L.J.; Miteva, M.; Paus, R. Seborrheic dermatitis-Looking beyond Malassezia. Exp. Dermatol. 2019, 28, 991–1001. [Google Scholar] [CrossRef]
- Jackson, J.M.; Alexis, A.; Zirwas, M.; Taylor, S. Unmet needs for patients with seborrheic dermatitis. J. Am. Acad. Dermatol. 2024, 90, 597–604. [Google Scholar] [CrossRef]
- Huang, C.; Zhuo, F.; Han, B.; Li, W.; Jiang, B.; Zhang, K.; Jian, X.; Chen, Z.; Li, H.; Huang, H.; et al. The updates and implications of cutaneous microbiota in acne. Cell Biosci. 2023, 13, 113. [Google Scholar] [CrossRef]
- Dreno, B.; Dekio, I.; Baldwin, H.; Demessant, A.L.; Dagnelie, M.-A.; Khammari, A.; Corvec, S. Acne microbiome: From phyla to phylotypes. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 657–664. [Google Scholar] [CrossRef]
- Świerczewska, Z.; Lewandowski, M.; Surowiecka, A.; Barańska-Rybak, W. Microbiome in Hidradenitis Suppurativa-What We Know and Where We Are Heading. Int. J. Mol. Sci. 2022, 23, 11280. [Google Scholar] [CrossRef]
- Rosi, E.; Guerra, P.; Silvi, G.; Nunziati, G.; Scandagli, I.; Di Cesare, A.; Prignano, F. Consistency of Bacterial Triggers in the Pathogenesis of Hidradenitis Suppurativa. Vaccines 2023, 11, 179. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and Microbiota: A Systematic Review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef]
- Soleymani, S.; Iranpanah, A.; Najafi, F.; Belwal, T.; Ramola, S.; Abbasabadi, Z.; Momtaz, S.; Farzaei, M.H. Implications of grape extract and its nanoformulated bioactive agent resveratrol against skin disorders. Arch. Dermatol. Res. 2019, 311, 577–588. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.-Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid.-Based Complement. Altern. Med. ECAM 2020, 2020, 2416837. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.-D.; Wang, Y.-D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Marko, M.; Pawliczak, R. Resveratrol and Its Derivatives in Inflammatory Skin Disorders-Atopic Dermatitis and Psoriasis: A Review. Antioxidants 2023, 12, 1954. [Google Scholar] [CrossRef]
- Pennesi, C.M.; Neely, J.; Marks, A.G.; Basak, S.A. Use of Isoquercetin in the Treatment of Prurigo Nodularis. J. Drugs Dermatol. 2017, 16, 1156–1158. [Google Scholar]
- Fabbrocini, G.; Cameli, N.; Lorenzi, S.; De Padova, M.P.; Marasca, C.; Izzo, R.; Monfrecola, G. A dietary supplement to reduce side effects of oral isotretinoin therapy in acne patients. G Ital. Dermatol. Venereol. 2014, 149, 441–445. [Google Scholar]
- Singh, C.K.; Mintie, C.A.; Ndiaye, M.A.; Chhabra, G.; Roy, S.; Sullivan, R.; Longley, B.J.; Schieke, S.M.; Ahmad, N. Protective effects of dietary grape against atopic dermatitis-like skin lesions in NC/NgaTndCrlj mice. Front. Immunol. 2022, 13, 1051472. [Google Scholar] [CrossRef]
- Bangash, Y.; Saleem, A.; Akhtar, M.F.; Anwar, F.; Akhtar, B.; Sharif, A.; Khan, M.I.; Khan, A. Pterostilbene reduces the progression of atopic dermatitis via modulating inflammatory and oxidative stress biomarkers in mice. Inflammopharmacology 2023, 31, 1289–1303. [Google Scholar] [CrossRef]
- Nelson, K.; Lyles, J.T.; Li, T.; Saitta, A.; Addie-Noye, E.; Tyler, P.; Quave, C.L. Anti-Acne Activity of Italian Medicinal Plants Used for Skin Infection. Front. Pharmacol. 2016, 7, 425. [Google Scholar] [CrossRef]
- Lim, H.-J.; Kang, S.-H.; Song, Y.-J.; Jeon, Y.-D.; Jin, J.-S. Inhibitory Effect of Quercetin on Propionibacterium acnes-induced Skin Inflammation. Int. Immunopharmacol. 2021, 96, 107557. [Google Scholar] [CrossRef]
- Witte, K.; Wolk, K.; Witte-Händel, E.; Krause, T.; Kokolakis, G.; Sabat, R. Targeting Metabolic Syndrome in Hidradenitis Suppurativa by Phytochemicals as a Potential Complementary Therapeutic Strategy. Nutrients 2023, 15, 3797. [Google Scholar] [CrossRef]
- Simonetti, G.; D’Auria, F.D.; Mulinacci, N.; Innocenti, M.; Antonacci, D.; Angiolella, L.; Santamaria, A.R.; Valletta, A.; Donati, L.; Pasqua, G. Anti-Dermatophyte and Anti-Malassezia Activity of Extracts Rich in Polymeric Flavan-3-ols Obtained from Vitis vinifera Seeds. Phytother. Res. PTR 2017, 31, 124–131. [Google Scholar] [CrossRef]
- Mustarichie, R.; Rostinawati, T.; Pitaloka, D.A.E.; Saptarini, N.M.; Iskandar, Y. Herbal Therapy for the Treatment of Seborrhea Dermatitis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2391–2405. [Google Scholar] [CrossRef]
- Takahashi, T.; Kamiya, T.; Hasegawa, A.; Yokoo, Y. Procyanidin oligomers selectively and intensively promote proliferation of mouse hair epithelial cells in vitro and activate hair follicle growth in vivo. J. Investig. Dermatol. 1999, 112, 310–316. [Google Scholar] [CrossRef]
- Lombardo, G.; Melzi, G.; Indino, S.; Piazza, S.; Sangiovanni, E.; Baruffaldi Preis, F.; Marabini, L.; Donetti, E. Keratin 17 as a Marker of UVB-Induced Stress in Human Epidermis and Modulation by Vitis vinifera Extract. Cells Tissues Organs 2022, 211, 611–627. [Google Scholar] [CrossRef]
- Letsiou, S.; Kapazoglou, A.; Tsaftaris, A.; Spanidi, E.; Gardikis, K. Transcriptional and epigenetic effects of Vitis vinifera L. leaf extract on UV-stressed human dermal fibroblasts. Mol. Biol. Rep. 2020, 47, 5763–5772. [Google Scholar] [CrossRef]
- Hamada, H.; Shimoda, K.; Horio, Y.; Onoa, T.; Hosoda, R.; Nakayama, N.; Uranod, K. Pterostilbene and Its Glucoside Induce Type XVII Collagen Expression. Nat. Prod. Commun. 2017, 12, 85–86. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, C.; Wen, D.; Sun, J.; Huang, L.; Gao, Y.; Li, Q.; Zhang, Y. Targeting the stem cell niche: Role of collagen XVII in skin aging and wound repair. Theranostics 2022, 12, 6446–6454. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols with Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef]
- Chakkalakal, M.; Nadora, D.; Gahoonia, N.; Dumont, A.; Burney, W.; Pan, A.; Chambers, C.J.; Sivamani, R.K. Prospective Randomized Double-Blind Placebo-Controlled Study of Oral Pomegranate Extract on Skin Wrinkles, Biophysical Features, and the Gut-Skin Axis. J. Clin. Med. 2022, 11, 6724. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.-D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Pozzo, L.; Grande, T.; Raffaelli, A.; Longo, V.; Weidner, S.; Amarowicz, R.; Karamać, M. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species. Molecules 2023, 28, 4924. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Tyagi, A.; Elahi, F.; Aloo, S.O.; Oh, D.-H. The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease. Antioxidants 2021, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef]
- Orhan, D.; Orhan, N.; Özçelik, B.; Ergun, F. Biological Screening of Vitis vinifera L. Leaf Fractions. Turk. J. Biol. 2009, 33, 341–348. [Google Scholar] [CrossRef]
- Ceyhan Guvensen, N.; Keskin, D.; Zorlu, Z.; Ugur, A. In-vitro antimicrobial activities of different extracts of grapevine leaves (Vitis vinifera L.) from West Anatolia against some pathogenic microorganisms. J. Pure Appl. Microbiol. 2012, 6, 1303–1308. [Google Scholar]
- Leal, C.; Santos, R.A.; Pinto, R.; Queiroz, M.; Rodrigues, M.; José Saavedra, M.; Barros, A.; Gouvinhas, I. Recovery of bioactive compounds from white grape (Vitis vinifera L.) stems as potential antimicrobial agents for human health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards. Plants 2020, 9, 1470. [Google Scholar] [CrossRef]
- Oskay, M.; Oskay, D. Antimicrobial Screening of Some Turkish Medicinal Plants. Pharm. Biol. 2007, 45, 176–181. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional constituents, phytochemical profiles, in vitro antioxidant and antimicrobial properties, and gas chromatography-mass spectrometry analysis of various solvent extracts from grape seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544. [Google Scholar] [CrossRef]
- Brochado, A.R.; Telzerow, A.; Bobonis, J.; Banzhaf, M.; Mateus, A.; Selkrig, J.; Huth, E.; Bassler, S.; Zamarreño Beas, J.; Zietek, M.; et al. Species-specific activity of antibacterial drug combinations. Nature 2018, 559, 259–263. [Google Scholar] [CrossRef]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef]
- López, C.; Ayala, J.A.; Bonomo, R.A.; González, L.J.; Vila, A.J. Protein determinants of dissemination and host specificity of metallo-β-lactamases. Nat. Commun. 2019, 10, 3617. [Google Scholar] [CrossRef]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef]
- Jensen, G.S.; Cruickshank, D.; Hamilton, D.E. Disruption of Established Bacterial and Fungal Biofilms by a Blend of Enzymes and Botanical Extracts. J. Microbiol. Biotechnol. 2023, 33, 715–723. [Google Scholar] [CrossRef]
- Kolouchová, I.; Maťátková, O.; Paldrychová, M.; Kodeš, Z.; Kvasničková, E.; Sigler, K.; Čejková, A.; Šmidrkal, J.; Demnerová, K.; Masák, J. Resveratrol, pterostilbene, and baicalein: Plant-derived anti-biofilm agents. Folia Microbiol. 2018, 63, 261–272. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.-A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef]
- Lim, Y.R.I.; Preshaw, P.M.; Lim, L.P.; Ong, M.M.A.; Lin, H.-S.; Tan, K.S. Pterostilbene complexed with cyclodextrin exerts antimicrobial and anti-inflammatory effects. Sci. Rep. 2020, 10, 9072. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Brackman, G.; Rigole, P.; De Witte, E.; Honraet, K.; Rossel, B.; Nelis, H.J. Eradication of Propionibacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine Int. J. Phytother. Phytopharm. 2012, 19, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.; Grout, E.; Morris, T.; Brown, H.L. Cutibacterium acnes biofilm forming clinical isolates modify the formation and structure of Staphylococcus aureus biofilms, increasing their susceptibility to antibiotics. Anaerobe 2022, 76, 102580. [Google Scholar] [CrossRef] [PubMed]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020, 21, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef] [PubMed]
- Vaňková, E.; Paldrychová, M.; Kašparová, P.; Lokočová, K.; Kodeš, Z.; Maťátková, O.; Kolouchová, I.; Masák, J. Natural antioxidant pterostilbene as an effective antibiofilm agent, particularly for gram-positive cocci. World J. Microbiol. Biotechnol. 2020, 36, 101. [Google Scholar] [CrossRef] [PubMed]
- Zannella, C.; Giugliano, R.; Chianese, A.; Buonocore, C.; Vitale, G.A.; Sanna, G.; Sarno, F.; Manzin, A.; Nebbioso, A.; Termolino, P.; et al. Antiviral Activity of Vitis vinifera Leaf Extract against SARS-CoV-2 and HSV-1. Viruses 2021, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Berardi, V.; Ricci, F.; Castelli, M.; Galati, G.; Risuleo, G. Resveratrol exhibits a strong cytotoxic activity in cultured cells and has an antiviral action against polyomavirus: Potential clinical use. J. Exp. Clin. Cancer Res. CR 2009, 28, 96. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, H.-J.; Huang, J.-P.; Lee, P.-C.; Tsai, C.-R.; Hsu, T.-F.; Huang, W.-Y. Kinetics of Tyrosinase Inhibitory Activity Using Vitis vinifera Leaf Extracts. BioMed Res. Int. 2017, 2017, 5232680. [Google Scholar] [CrossRef]
- Sil, A.; Panigrahi, A.; Chandra, A.; Pramanik, J.D. “COVID nose”—A unique post-COVID pigmentary sequelae reminiscing Chik sign: A descriptive case series. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, e419–e421. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Madupu, R.; Karaoz, U.; Nossa, C.W.; Yang, L.; Yooseph, S.; Yachimski, P.S.; Brodie, E.L.; Nelson, K.E.; Pei, Z. Human Papillomavirus Community in Healthy Persons, Defined by Metagenomics Analysis of Human Microbiome Project Shotgun Sequencing Data Sets. J. Virol. 2014, 88, 4786–4797. [Google Scholar] [CrossRef]
- Sun, X.; Fu, P.; Xie, L.; Chai, S.; Xu, Q.; Zeng, L.; Wang, X.; Jiang, N.; Sang, M. Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int. J. Mol. Med. 2021, 47, 335–345. [Google Scholar] [CrossRef]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P.; et al. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Palocci, C.; Valletta, A.; Kolesova, O.; Chronopoulou, L.; Donati, L.; Di Nitto, A.; Brasili, E.; Tomai, P.; Gentili, A.; et al. Anti-Candida Biofilm Activity of Pterostilbene or Crude Extract from Non-Fermented Grape Pomace Entrapped in Biopolymeric Nanoparticles. Molecules 2019, 24, 2070. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis vinifera L. against Human Pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martínez, M.D.; García-Cánovas, F.; Rodríguez-López, J.N. Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. J. Antimicrob. Chemother. 2006, 57, 1083–1092. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Sousa Lobo, J.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Kordi, M.; Salami, R.; Bolouri, P.; Delangiz, N.; Asgari Lajayer, B.; van Hullebusch, E.D. White biotechnology and the production of bio-products. Syst. Microbiol. Biomanuf. 2022, 2, 413–429. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market—An update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Seara, M.; Carvalho, M.J.; de Carvalho, N.M.; Costa, E.M.; Silva, S.; Duarte, M.; Pintado, M.; Oliveira, C.; Madureira, A.R. Production of Sustainable Postbiotics from Sugarcane Straw for Potential Food Applications. Appl. Sci. 2023, 13, 3391. [Google Scholar] [CrossRef]
- Ciardiello, T.; Pinto, D.; Marotta, L.; Giuliani, G.; Rinaldi, F. Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota. Cosmetics 2020, 7, 34. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Yan, F.; Liu, L.; Dempsey, P.J.; Tsai, Y.-H.; Raines, E.W.; Wilson, C.L.; Cao, H.; Cao, Z.; Liu, L.; Polk, D.B. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J. Biol. Chem. 2013, 288, 30742–30751. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J. Honest nutraceuticals, cosmetics, therapies, and foods (NCTFs): Standardization and safety of natural products. Crit. Rev. Food Sci. Nutr. 2022, 62, 4326–4341. [Google Scholar] [CrossRef] [PubMed]
- Antignac, E.; Nohynek, G.J.; Re, T.; Clouzeau, J.; Toutain, H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011, 49, 324–341. [Google Scholar] [CrossRef]
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. 2023, 30, 25148–25169. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Biological Activity of Fermented Plant Extracts for Potential Dermal Applications. Pharmaceutics 2023, 15, 2775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).