Adverse Events Comparison of Double Beta-Lactam Combinations for Bloodstream Infections: Ampicillin plus Ceftriaxone and Ampicillin/Cloxacillin
Abstract
1. Introduction
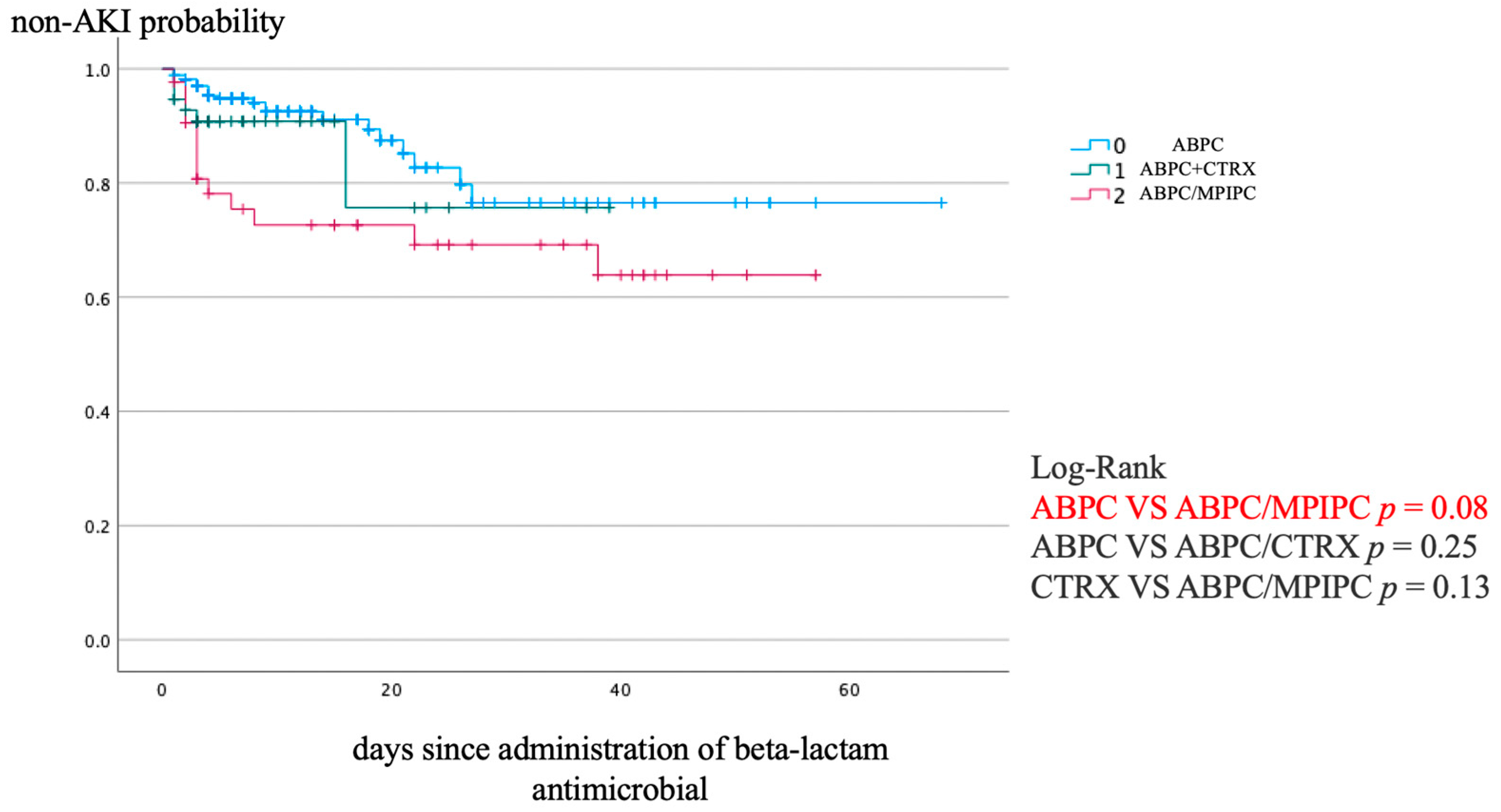
2. Results
3. Discussion
| 1 | A. Ramos-Martinez, et al., 2020 [20]/non-randomized prospective cohort study/E. faecalis NVE | ABPC 2 g q4h plus CTRX 2 g q12h for 4 weeks (39) | 2 g q4h plus CTRX 2 g q12h for 6 weeks (70) | |
| Previous renal failure | 11/39(28.2%) | 17/70(24.3%) | ||
| Renal impairment/glomerulonephritis | 10/39(25.6%)/0/39(0%) | 20/70(28.6%)/1/39(3%) | ||
| Leukopenia | 0/39(0%) | 2/70(2.8%) | ||
| In-hospital mortality | 4/39(10.3%) | 8/70(11.4%) | ||
| 2 | A. El Rafei, et al., 2018 [21]/retrospective cohort study/E. faecalis NVE, PVE | ABPC 2 g q4h plus CTRX 2 g q12h for 4–6 weeks (18) | ABPC 2 g q4h plus GM 3 mg/kg/day for 4–6 weeks (67) | |
| AKI | 2/18(11%) | 17/67(25%) | ||
| Leukopenia | 0/18(0%) | 0/67(0%) | ||
| 1-year mortality | 2/18(11%) | 6/67(9%) | ||
| ABPC + CTRX | ||||
| 3 | J. M. Pericas, et al., 2018 [22]/retrospective analysis of a prospective collected data/E. faecalis NVE, PVE | ABPC 2 g q4h plus CTRX 2 g q12h for 4–6 weeks (46) | ABPC 2 g q4h plus GM 3 mg/kg/day for 4–6 weeks (32) | |
| CKD/HD | 12/46(26%)/1/46(2%) | 8/32(25%)/3/32(9%) | ||
| AKI | 15/46(33%) | 20/32(63%) | ||
| myelotoxicity | 1/46(2%) | 0/32(0%) | ||
| 1-year mortality | 11/46(24%) | 10/32(31%) | ||
| 4 | N. H. Shah, et al., 2021 [23]/propensity score-matched retrospective cohort study/E. faecalis NVE, PVE | ABPC plus CTRX(100) | ABPC plus GM(90) | |
| CKD/HD | NP | NP | ||
| AKI | 6/100(6%) | 13/90(14%) | ||
| Leukopenia | 1/100(1%) | 4/90(4%) | ||
| 90-day mortality | 6/56(10.7%) after PS | 4/56(7.1%) after PS | ||
| 5 | J. Gavalda, et al., 2007 [24]/observational, open-label, non-randomized cohort study/E. faecalis NVE, PVE | ABPC 2 g q4h plus CTRX 2 g q12h for 6 weeks (43) | ||
| CKD | 8/43(19%) | |||
| AKI | 0/43(0%) | |||
| Leukopenia | 1/43(2%) | |||
| All-cause mortality | 12/43(28%) | |||
| 6 | N. Fernández-Hidalgo, et al., 2013 [25]/non-randomized, non-blinded cohort study/E. faecalis NVE, PVE | ABPC 2 g q4h plus CTRX 2 g q12h for 4–6 weeks (159) | ABPC 2 g q4h plus GM 3 mg/kg/day for 4–6 weeks (87) | |
| CKD/HD | 53/159(33%)/12/159(8%) | 14/87(16%)/3/87(3%) | ||
| AKI | 53/159(33%) | 40/87(46%) | ||
| Leukopenia | 1%(only treatment interruption) | 0% | ||
| All-cause mortality | 42/159(26%) | 22/87(25%) |
4. Materials and Methods
4.1. Study Design and Setting
4.2. Ethics and Informed Consent
4.3. Inclusion and Exclusion Criteria
4.4. Study Outcomes
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Patel, N.; O’Donnell, J.N. Optimal treatment of MSSA bacteraemias: A meta-analysis of cefazolin versus antistaphylococcal penicillins. J. Antimicrob. Chemother. 2018, 73, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Bakare, L.; Casapao, A.M.; Klinker, K.; Childs-Kean, L.M.; Pomputius, A.F. Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with methicillin-susceptible Staphylococcus aureus infection: A meta-analysis with trial sequential analysis. Infect. Dis. Ther. 2019, 8, 671–686. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- van de Beek, D.; Cabellos, C.; Dzupova, O.; Esposito, S.; Klein, M.; Kloek, A.T.; Leib, S.L.; Mourvillier, B.; Ostergaard, C.; Pagliano, P.; et al. ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clin. Microbiol. Infect. 2016; 22, (Suppl. 3), S37–S62. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective endocarditis in adults: Diagnosis, Antimicrobial Therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Nishita, Y.; Taga, M.; Kawai, Y.; Noda, Y.; Nakagawa, Y.; Iinuma, Y.; Niwa, O. Feasibility and value of developing an age-stratified antibiogram. Jpn. J. Environ. Infect. 2019, 34, 115–121. [Google Scholar] [CrossRef]
- Japan Nosocomial Infections Surveillance: JANIS. Available online: https://janis.mhlw.go.jp/ (accessed on 25 February 2024).
- Gagneux-Brunon, A.; Pouvaret, A.; Maillard, N.; Berthelot, P.; Lutz, M.F.; Cazorla, C.; Tulane, C.; Fuzellier, J.F.; Verhoeven, P.O.; Frésard, A.; et al. Acute kidney injury in infective endocarditis: A retrospective analysis. Med. Mal. Infect. 2019, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Von Tokarski, F.; Lemaignen, A.; Portais, A.; Fauchier, L.; Hennekinne, F.; Sautenet, B.; Halimi, J.M.; Legras, A.; Patat, F.; Bourguignon, T.; et al. Risk factors and outcomes of early acute kidney injury in infective endocarditis: A retrospective cohort study. Int. J. Infect. Dis. 2020, 99, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.; Rhodes, N.J.; Lee, B.J.; Scheetz, M.H.; Hanson, A.P.; Segreti, J.; Crank, C.W.; Wang, S.K. Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob. Agents Chemother. 2015, 59, 5232–5238. [Google Scholar] [CrossRef]
- Bai, A.D.; Showler, A.; Burry, L.; Steinberg, M.; Ricciuto, D.R.; Fernandes, T.; Chiu, A.; Raybardhan, S.; Science, M.; Fernando, E.; et al. Comparative effectiveness of cefazolin versus cloxacillin as definitive antibiotic therapy for MSSA bacteraemia: Results from a large multicentre cohort study. J. Antimicrob. Chemother. 2015, 70, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, K.H.; Jung, S.I.; Park, W.B.; Lee, S.H.; Kim, Y.S.; Kwak, Y.G.; Kim, Y.K.; Kiem, S.M.; Kim, H.I.; et al. Comparative outcomes of cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bacteraemia: A prospective multicentre cohort study in Korea. Clin. Microbiol. Infect. 2018, 24, 152–158. [Google Scholar] [CrossRef] [PubMed]
- McDanel, J.S.; Roghmann, M.C.; Perencevich, E.N.; Ohl, M.E.; Goto, M.; Livorsi, D.J.; Jones, M.; Albertson, J.P.; Nair, R.; O’Shea, A.M.J.; et al. Comparative effectiveness of cefazolin versus nafcillin or oxacillin for treatment of methicillin-susceptible Staphylococcus aureus infections complicated by bacteremia: A nationwide cohort study. Clin. Infect. Dis. 2017, 65, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Shenoy, E.S.; Hooper, D.C.; Nelson, S.B. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin. Infect. Dis. 2014, 59, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.T.; Teng, C.B.; Bergin, S.; Hon, P.Y.; Lye, D.C.; De, P.P.; Vasoo, S. Treatment outcomes with benzylpenicillin and non-benzylpenicillin antibiotics, and the performance of the penicillin zone-edge test versus molecular detection of blaZ in penicillin-susceptible Staphylococcus aureus (PSSA) bacteraemia. J. Antimicrob. Chemother. 2023, 78, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Turlej-Rogacka, A.; Lerner, A.; Adler, A.; Tacconelli, E.; Mouton, J.W. Amplification of Antimicrobial Resistance in Gut Flora of Patients Treated with Ceftriaxone. Antimicrob. Agents Chemother. 2017, 61, e00473-17, Erratum in Antimicrob. Agents Chemother. 2018, 62, e02184-18. [Google Scholar] [CrossRef] [PubMed]
- Rottier, W.C.; Bamberg, Y.R.; Dorigo-Zetsma, J.W.; van der Linden, P.D.; Ammerlaan, H.S.; Bonten, M.J. Predictive value of prior colonization and antibiotic use for third-generation cephalosporin-resistant enterobacteriaceae bacteremia in patients with sepsis. Clin. Infect. Dis. 2015, 60, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martínez, A.; Pericàs, J.M.; Fernández-Cruz, A.; Muñoz, P.; Valerio, M.; Kestler, M.; Montejo, M.; Fariñas, M.C.; Sousa, D.; Domínguez, F.; et al. Four weeks versus six weeks of ampicillin plus ceftriaxone in Enterococcus faecalis native valve endocarditis: A prospective cohort study. PLoS ONE 2020, 15, e0237011. [Google Scholar] [CrossRef] [PubMed]
- El Rafei, A.; DeSimone, D.C.; Narichania, A.D.; Sohail, M.R.; Vikram, H.R.; Li, Z.; Steckelberg, J.M.; Wilson, W.R.; Baddour, L.M. Comparison of Dual beta-Lactam therapy to penicillin-aminoglycoside combination in treatment of Enter-ococcus faecalis infective endocarditis. J. Infect. 2018, 77, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Pericas, J.; Cervera, C.; del Rio, A.; Moreno, A.; de la Maria, C.G.; Almela, M.; Falces, C.; Ninot, S.; Castañeda, X.; Armero, Y.; et al. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: From ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin. Microbiol. Infect. 2014, 20, O1075–O1083. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; Shutt, K.A.; Doi, Y. Ampicillin-Ceftriaxone vs Ampicillin-Gentamicin for Definitive Therapy of Enterococcus faecalis Infective Endocarditis: A Propensity Score-Matched, Retrospective Cohort Analysis. Open Forum Infect. Dis. 2021, 8, ofab102. [Google Scholar] [CrossRef] [PubMed]
- Gavaldà, J.; Len, O.; Miró, J.M.; Muñoz, P.; Montejo, M.; Alarcón, A.; de la Torre-Cisneros, J.; Peña, C.; Martínez-Lacasa, X.; Sarria, C.; et al. Brief Communication: Treatment of Enterococcus Faecalis Endocarditis with Ampicillin plus Ceftriaxone. Ann. Intern. Med. 2007, 146, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hidalgo, N.; Almirante, B.; Gavaldà, J.; Gurgui, M.; Peña, C.; de Alarcón, A.; Ruiz, J.; Vilacosta, I.; Montejo, M.; Vallejo, N.; et al. Ampicillin Plus Ceftriaxone Is as Effective as Ampicillin Plus Gentamicin for Treating Enterococcus faecalis Infective Endocarditis. Clin. Infect. Dis. 2013, 56, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
| n | ABPC(277) | ABPC plus CTRX(57) | ABPC/MCIPC(43) | p |
|---|---|---|---|---|
| age(SD) (years) | 71.1(16.5) | 64.0(16.9) | 56.6(17.5) | <0.01 |
| female(%) | 173(62.5) | 27(47.4) | 31(72.1) | 0.031 |
| congestive heart failure(%) | 69(24.9) | 9(15.8) | 11(25.6) | 0.32 |
| diabetes(%) | 87(31.4) | 20(35.1) | 7(16.3) | 0.091 |
| respiratory disease(%) | 135(48.7) | 29(50.9) | 17(39.5) | 0.48 |
| myocadiac infarction(%) | 24(8.7) | 1(1.8) | 2(4.7) | 0.15 |
| collagen disease(%) | 4(1.4) | 1(1.8) | 2(4.7) | 0.35 |
| liver dysfunction(%) | 30(10.8) | 10(17.5) | 4(9.3) | 0.31 |
| cancer(%) | 110(39.7) | 17(29.8) | 6(14.0) | 0.003 |
| chronic kidney disease(%) | 108(39.0) | 20(35.1) | 8(18.6) | 0.034 |
| cerebrovascular(%) | 35(12.6) | 9(15.3) | 6(14.0) | 0.81 |
| hypertension(%) | 150(54.2) | 20(35.1) | 20(46.5) | 0.028 |
| HIV(%) | 0(0) | 0(0) | 2(4.7) | <0.01 |
| qSofa ≥ 2(%) | 63(23.1) | 38(64.4) | 18(41.9) | <0.01 |
| CLDM(%) | 53(19.1) | 14(24.6) | 19(44.2) | 0.001 |
| VCM(%) | 21(7.6) | 16(28.1) | 18(41.9) | <0.01 |
| AG(%) | 37(13.4) | 4(7.0) | 4(9.3) | 0.35 |
| L-AMPHB(%) | 0(0) | 0(0) | 1(2.3) | 0.02 |
| ACV(%) | 1(0.4) | 5(8.8) | 0(0) | <0.01 |
| NSAIDs(%) | 59(21.3) | 5(8.8) | 10(23.3) | 0.078 |
| ACE inhibitor(%) | 18(6.5) | 3(5.3) | 3(7.0) | 0.92 |
| ARB(%) | 34(12.3) | 5(8.8) | 10(23.3) | 0.081 |
| ACE inhibitor/ARB(%) | 50(18.1) | 8(14.0) | 12(27.9) | 0.19 |
| diuretics(%) | 49(17.7) | 4(7.0) | 10(23.3) | 0.069 |
| chemotherapy(%) | 6(2.2) | 0(0) | 0(0) | 0.34 |
| calcineurin inhibitor(%) | 0(0) | 1(1.8) | 0(0) | 0.06 |
| contrast CT(%) | 75(27.1) | 28(49.1) | 26(60.5) | <0.001 |
| on admission, eGFR(SD) (mL/min/1.73 m2) | 78.8(46.3) | 80.8(43.0) | 102.1(54.6) | 0.011 |
| T-bil(SD) (mg/dL) | 0.78(0.65) | 1.2(0.96) | 0.73(0.50) | <0.001 |
| ALP(SD) (U/L) | 314.8(271.0) | 370.2(245.7) | 328.9(243.7) | 0.4 |
| AST(SD) (U/L) | 42.6(81.7) | 120.4(493.0) | 54.1(68.5) | 0.035 |
| ALT(SD) (U/L) | 33.1(36.0) | 98.2(380.0) | 46.6(67.6) | 0.014 |
| GTP(SD) (U/L) | 76.2(107.6) | 101.1(135.8) | 75.5(70.5) | 0.47 |
| CRP(SD) (mg/dL) | 10.9(9.3) | 15.3(12.3) | 13.5(10.1) | 0.005 |
| WBC(SD) (103/μL) | 10.9(6.1) | 11.9(8.9) | 11.3(5.1) | 0.53 |
| Hgb(SD) (g/dL) | 10.8(2.1) | 11.6(2.5) | 11.9(2.8) | 0.002 |
| PLT(SD) (103/μL) | 203.6(105.0) | 154.7(116.0) | 255.9(160.0) | <0.001 |
| duration of treatment(SD) (day) | 14.0(12.1) | 9.3(8.8) | 29.0(19.2) | <0.01 |
| use of mechanical ventilation(%) | 15(5.4) | 14(24.6) | 9(20.9) | <0.001 |
| use of vasopressor(%) | 29(10.5) | 18(31.6) | 10(23.3) | <0.001 |
| n | ABPC(277) | ABPC plus CTRX(57) | ABPC/MCIPC(43) | p |
|---|---|---|---|---|
| Length of stay(SD) (day) | 34.2(31.1) | 45.8(47.4) | 51.6(32.5) | 0.03 |
| AKI KDIGO Grade 2–3(%) | 25(9.0) | 7(12.3) | 13(30.2) | <0.001 |
| Stage 1(%) | 18(6.5) | 3(5.3) | 5(11.6) | |
| Stage 2(%) | 4(1.4) | 1(1.8) | 3(7.0) | |
| Stage 3(%) | 3(1.1) | 3(5.3) | 5(11.6) | |
| Grade 2–4 leukopenia(%) | 21(7.6) | 7(12.3) | 2(4.7) | 0.35 |
| Grade 2(%) | 11(4.0) | 4(7.0) | 2(4.7) | |
| Grade 3(%) | 7(2.5) | 2(3.5) | 0(0) | |
| Grade 4(%) | 3(1.1) | 1(1.8) | 0(0) | |
| Grade 2–4 anemia(%) | 143(51.8) | 29(50.9) | 23(53.5) | 0.97 |
| Grade 2(%) | 103(37.3) | 16(28.1) | 13(30.2) | |
| Grade 3(%) | 31(11.2) | 10(17.5) | 7(16.3) | |
| Grade 4(%) | 9(3.3) | 3(5.3) | 3(7.0) | |
| Grade 2–4 thrombocytopenia(%) | 30(10.9) | 21(36.8) | 7(16.3) | <0.001 |
| Grade 2(%) | 8(2.9) | 6(10.5) | 5(11.6) | |
| Grade 3(%) | 12(4.3) | 6(10.5) | 1(2.3) | |
| Grade 4(%) | 10(3.6) | 9(15.8) | 1(2.3) | |
| Grade 2–4 T-bil elevation(%) | 13(5.1) | 5(8.9) | 3(7.1) | 0.6 |
| Grade 2(%) | 12(4.7) | 2(3.6) | 3(7.1) | |
| Grade 3(%) | 1(0.4) | 3(5.4) | 0(0) | |
| Grade 4(%) | 0(0) | 0(0) | 0(0) | |
| Grade 2–4 ALP elevation(%) | 17(8.7) | 5(9.6) | 4(9.8) | 0.97 |
| Grade 2(%) | 14(7.2) | 5(9.6) | 4(9.8) | |
| Grade 3(%) | 3(1.5) | 0(0) | 0(0) | |
| Grade 4(%) | 0(0) | 0(0) | 0(0) | |
| Grade 2–4 G-GTP elevation(%) | 55(24.6) | 19(37.3) | 12(28.6) | 0.21 |
| Grade 2(%) | 33(14.7) | 12(23.5) | 5(11.9) | |
| Grade 3(%) | 22(9.8) | 7(13.7) | 7(16.7) | |
| Grade 4(%) | 0(0) | 0(0) | 0(0) | |
| Grade 2–4 AST elevation(%) | 28(10.2) | 13(22.8) | 9(20.9) | 0.014 |
| Grade 2(%) | 13(4.7) | 8(14.0) | 2(4.7) | |
| Grade 3(%) | 13(4.7) | 3(5.3) | 7(16.3) | |
| Grade 4(%) | 2(0.7) | 2(3.5) | 0(0) | |
| Grade 2–4 ALT elevation(%) | 26(9.5) | 11(19.3) | 7(16.3) | 0.07 |
| Grade 2(%) | 16(5.8) | 6(10.5) | 3(7.0) | |
| Grade 3(%) | 10(3.6) | 3(5.3) | 4(9.3) | |
| Grade 4(%) | 0(0) | 2(3.5) | 0(0) | |
| 30-day mortality(%) | 12(4.3) | 4(7.0) | 1(2.3) | 0.51 |
| 90-day mortality(%) | 23(8.3) | 8(14.0) | 3(7.0) | 0.35 |
| 30-day ICU admission(%) | 18(6.5) | 24(42.1) | 6(14.0) | <0.001 |
| Hazard Ratio | 95% CI | p | |
|---|---|---|---|
| Age | 0.992 | 0.971–1.01 | 0.474 |
| sex (male:0, female:1) | 1.04 | 0.469–2.29 | 0.929 |
| use of mechanical ventilation | 1.79 | 0.591–5.42 | 0.303 |
| ABPC/MCIPC (ABPC:0, ABPC/MCIPC:2) | 1.83 | 1.22–2.74 | 0.003 |
| vasopressor | 3.52 | 1.22–10.1 | 0.020 |
| qSOFA more than 2 | 0.493 | 0.189–1.29 | 0.148 |
| chronic kidney disease | 1.99 | 0.907–4.35 | 0.086 |
| duration of therapy | 0.976 | 0.950–1.00 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, K.; Kobayashi, D.; Mori, N. Adverse Events Comparison of Double Beta-Lactam Combinations for Bloodstream Infections: Ampicillin plus Ceftriaxone and Ampicillin/Cloxacillin. Antibiotics 2024, 13, 696. https://doi.org/10.3390/antibiotics13080696
Ishikawa K, Kobayashi D, Mori N. Adverse Events Comparison of Double Beta-Lactam Combinations for Bloodstream Infections: Ampicillin plus Ceftriaxone and Ampicillin/Cloxacillin. Antibiotics. 2024; 13(8):696. https://doi.org/10.3390/antibiotics13080696
Chicago/Turabian StyleIshikawa, Kazuhiro, Daiki Kobayashi, and Nobuyoshi Mori. 2024. "Adverse Events Comparison of Double Beta-Lactam Combinations for Bloodstream Infections: Ampicillin plus Ceftriaxone and Ampicillin/Cloxacillin" Antibiotics 13, no. 8: 696. https://doi.org/10.3390/antibiotics13080696
APA StyleIshikawa, K., Kobayashi, D., & Mori, N. (2024). Adverse Events Comparison of Double Beta-Lactam Combinations for Bloodstream Infections: Ampicillin plus Ceftriaxone and Ampicillin/Cloxacillin. Antibiotics, 13(8), 696. https://doi.org/10.3390/antibiotics13080696






