Synergistic Antimicrobial Effects of Phage vB_AbaSi_W9 and Antibiotics against Acinetobacter baumannii Infection
Abstract
1. Introduction
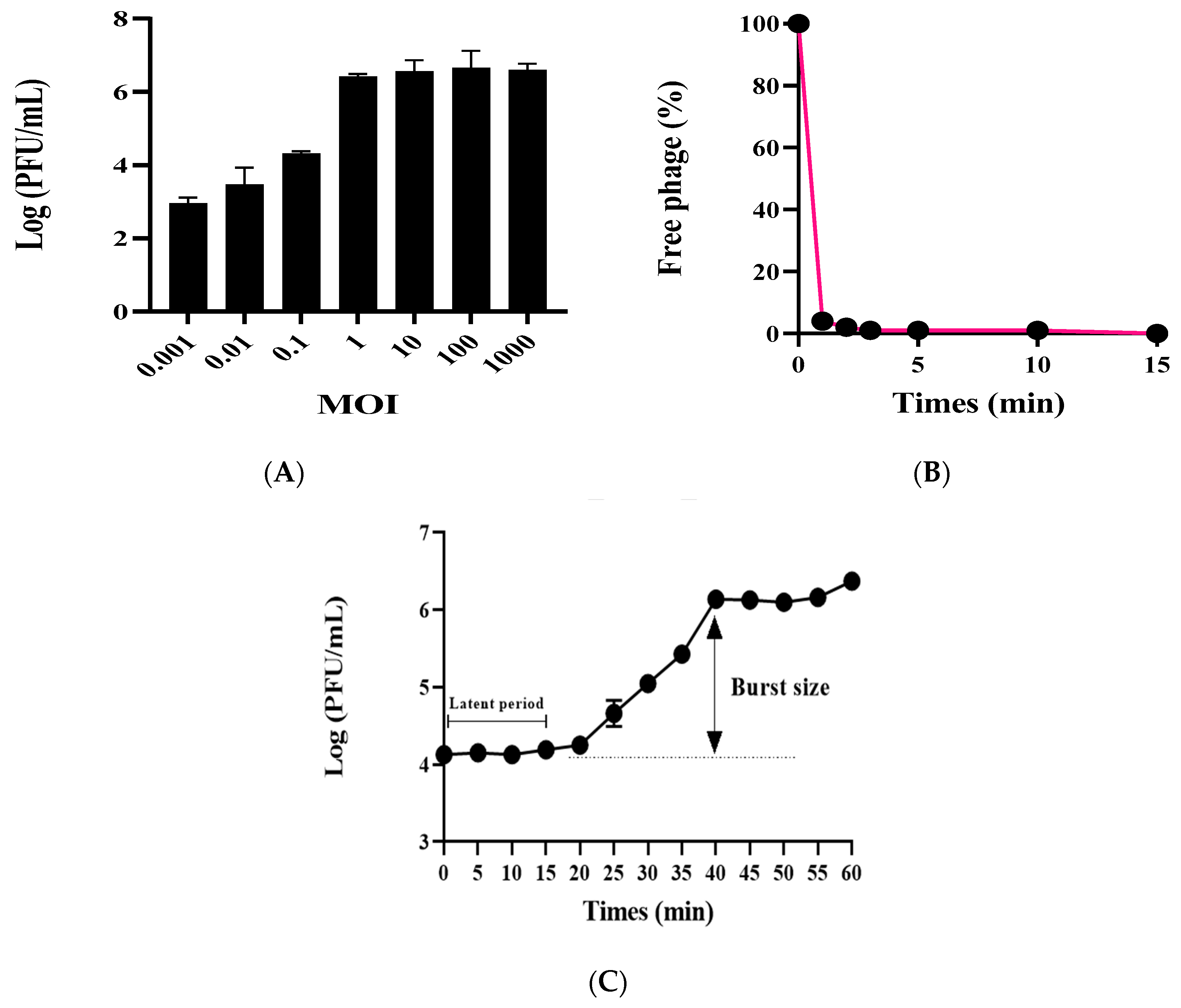
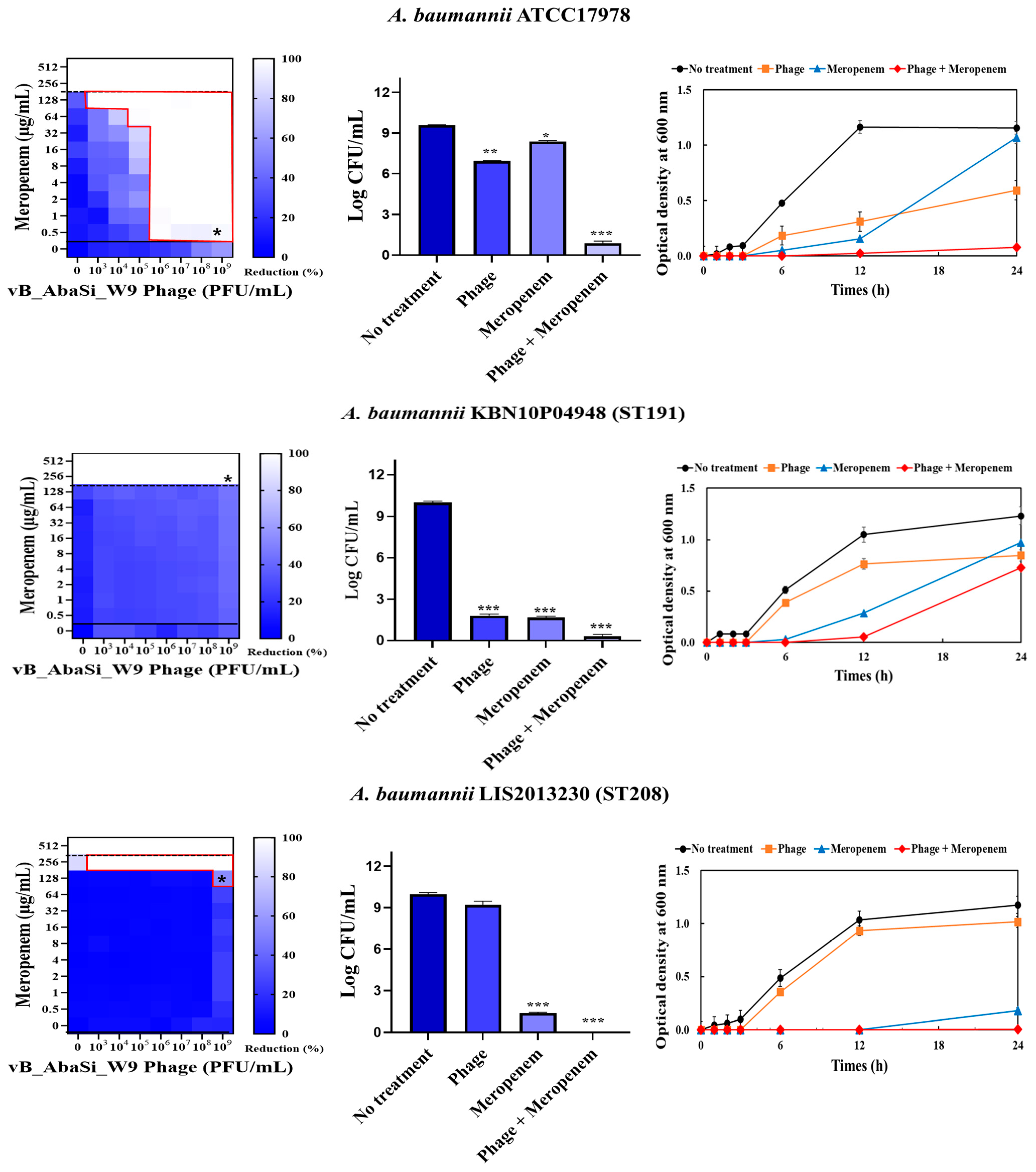
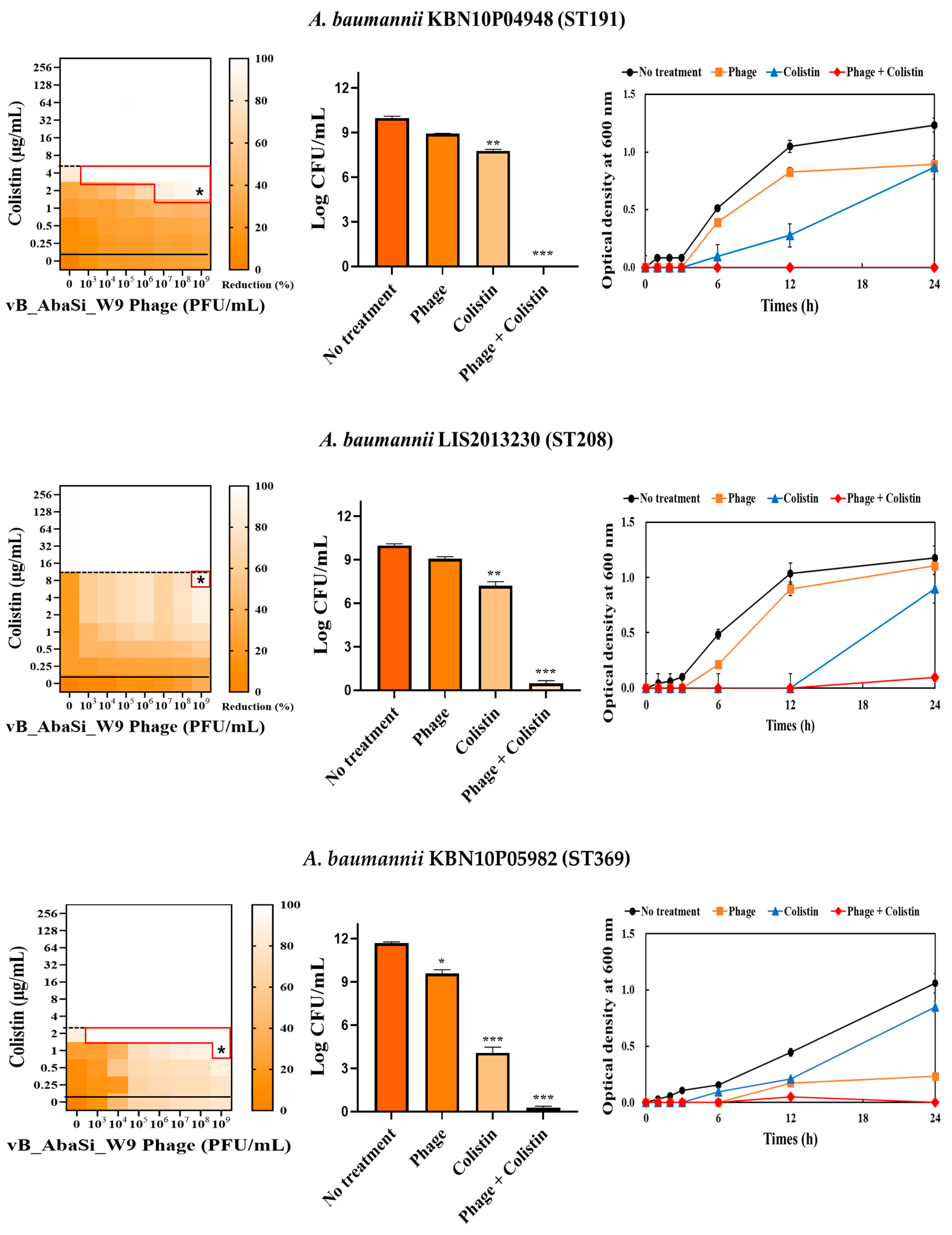
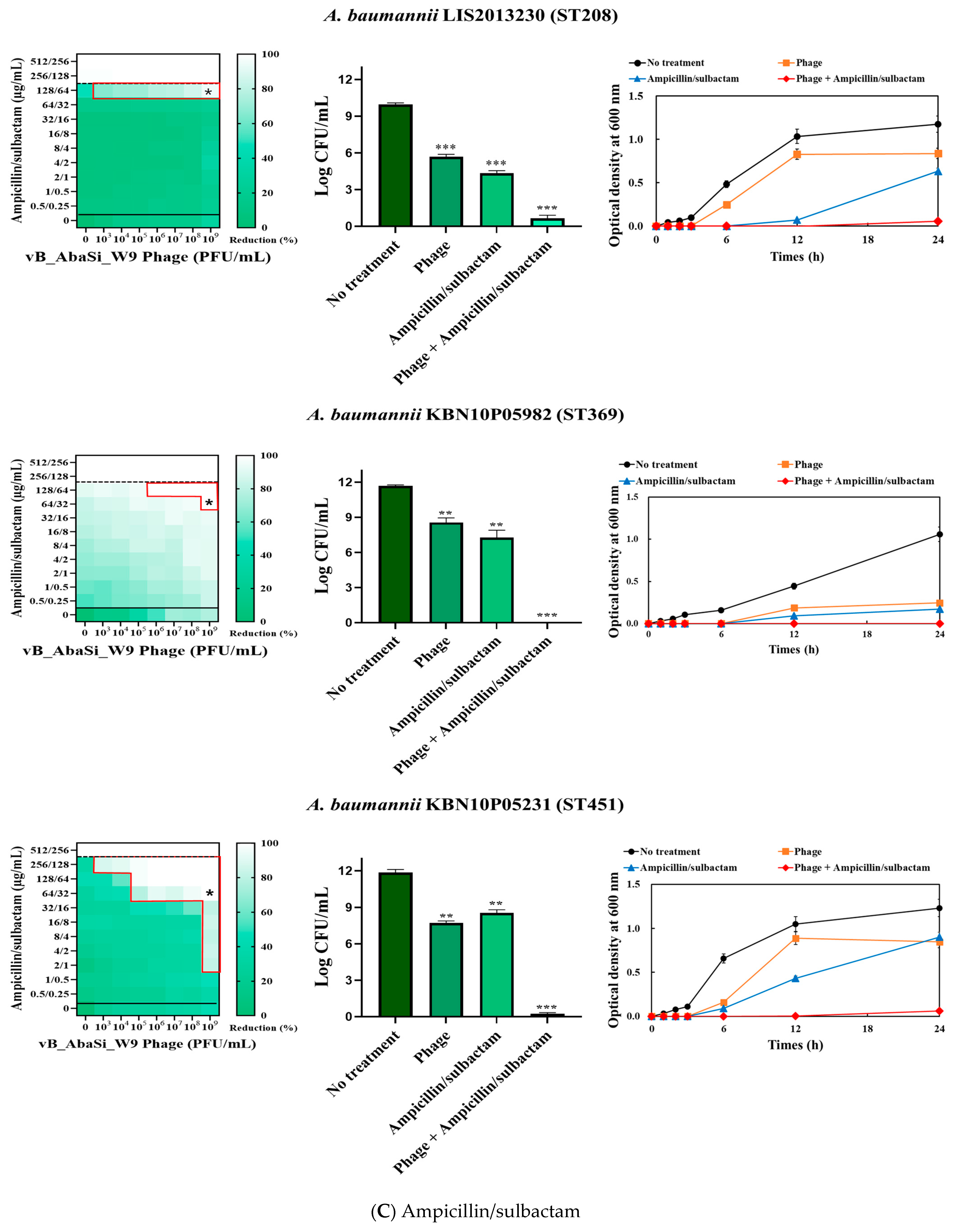
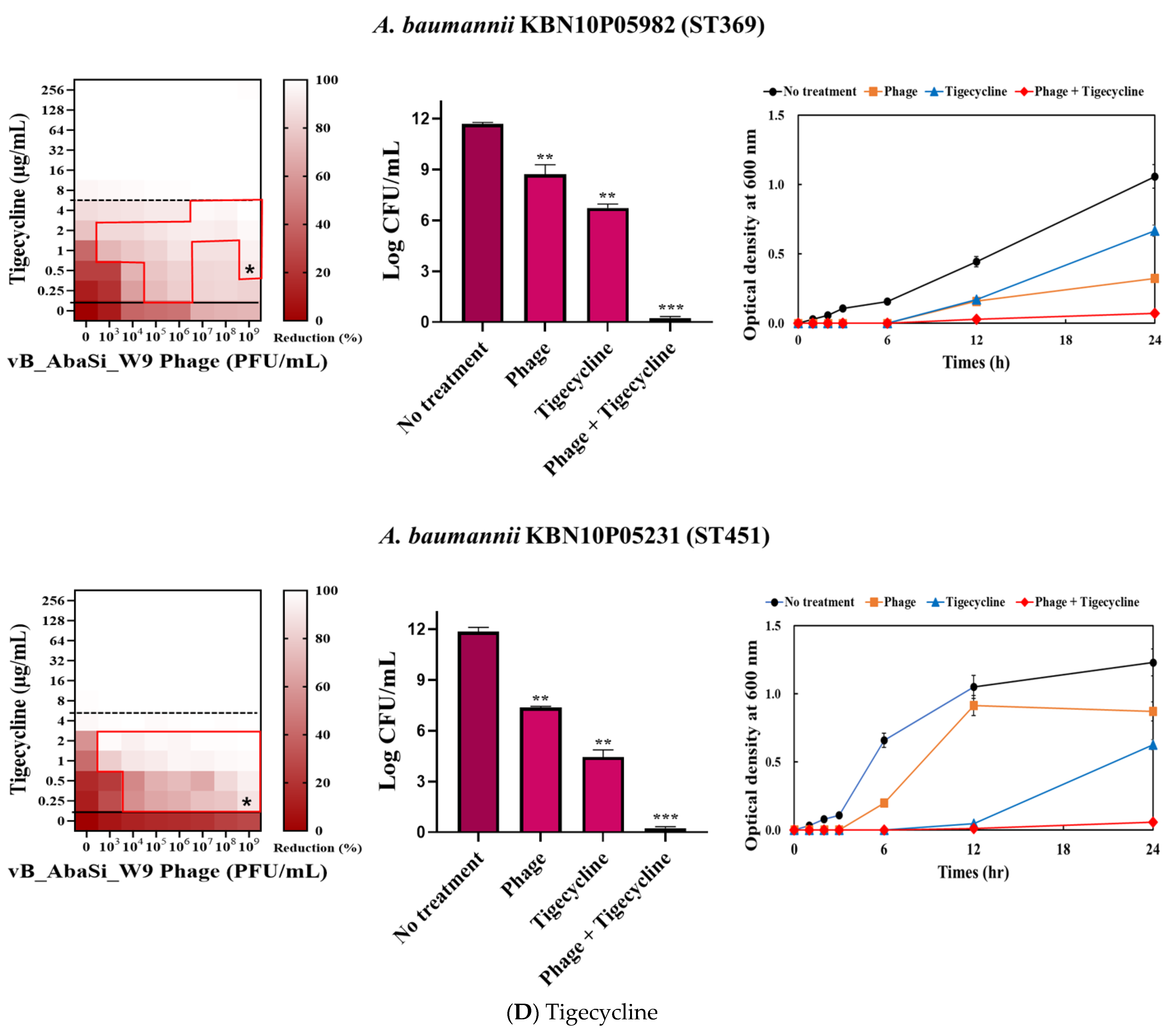
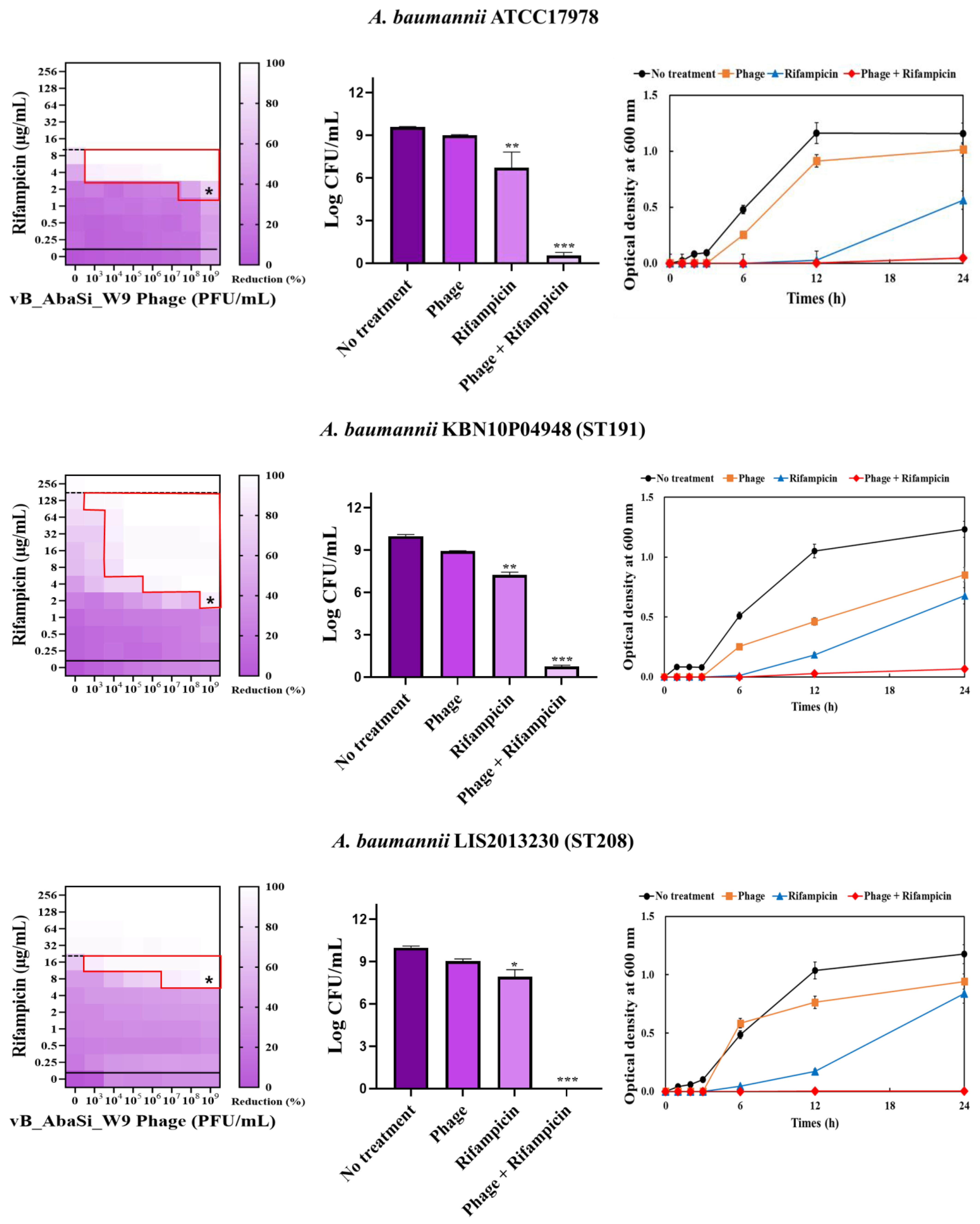
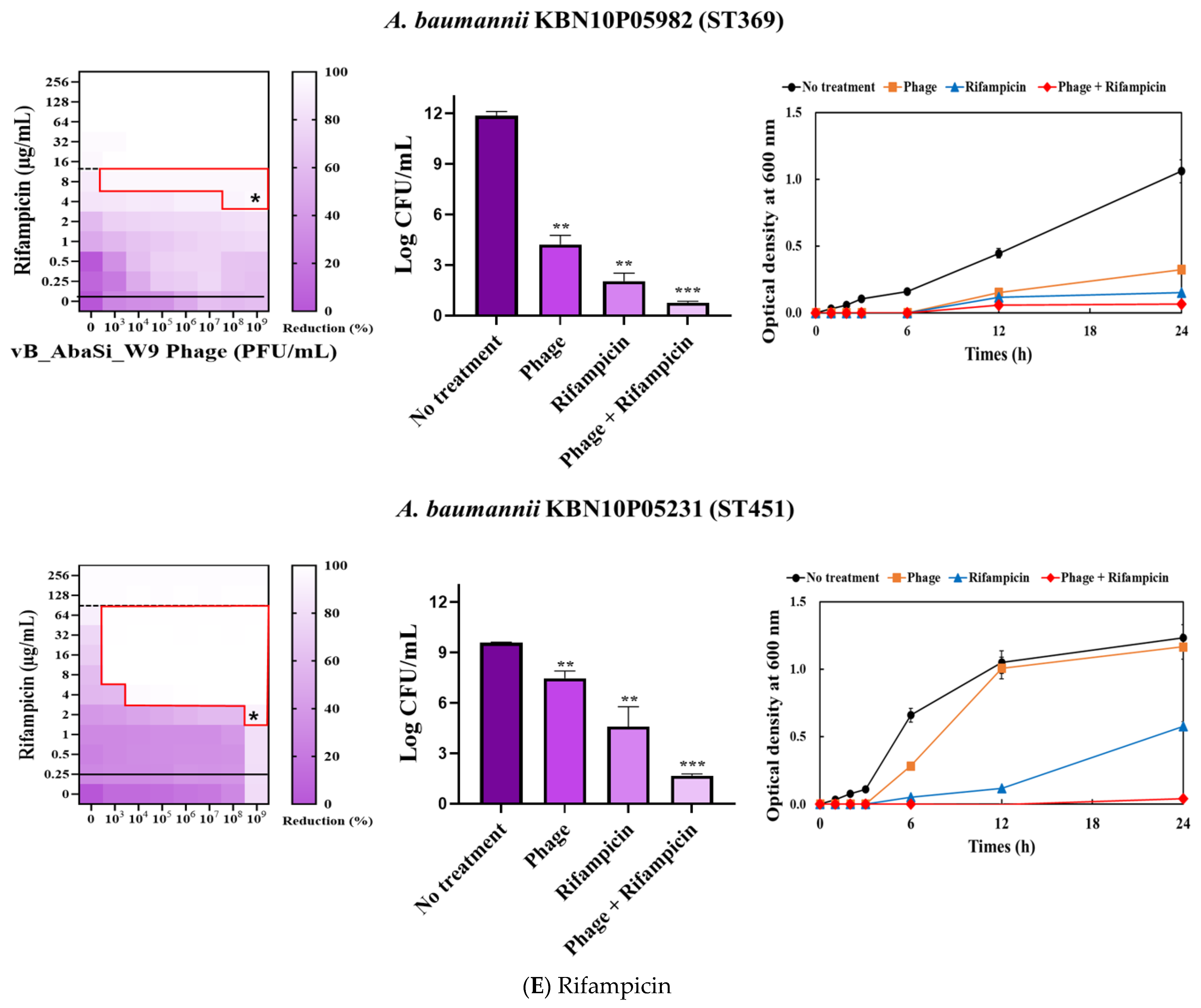
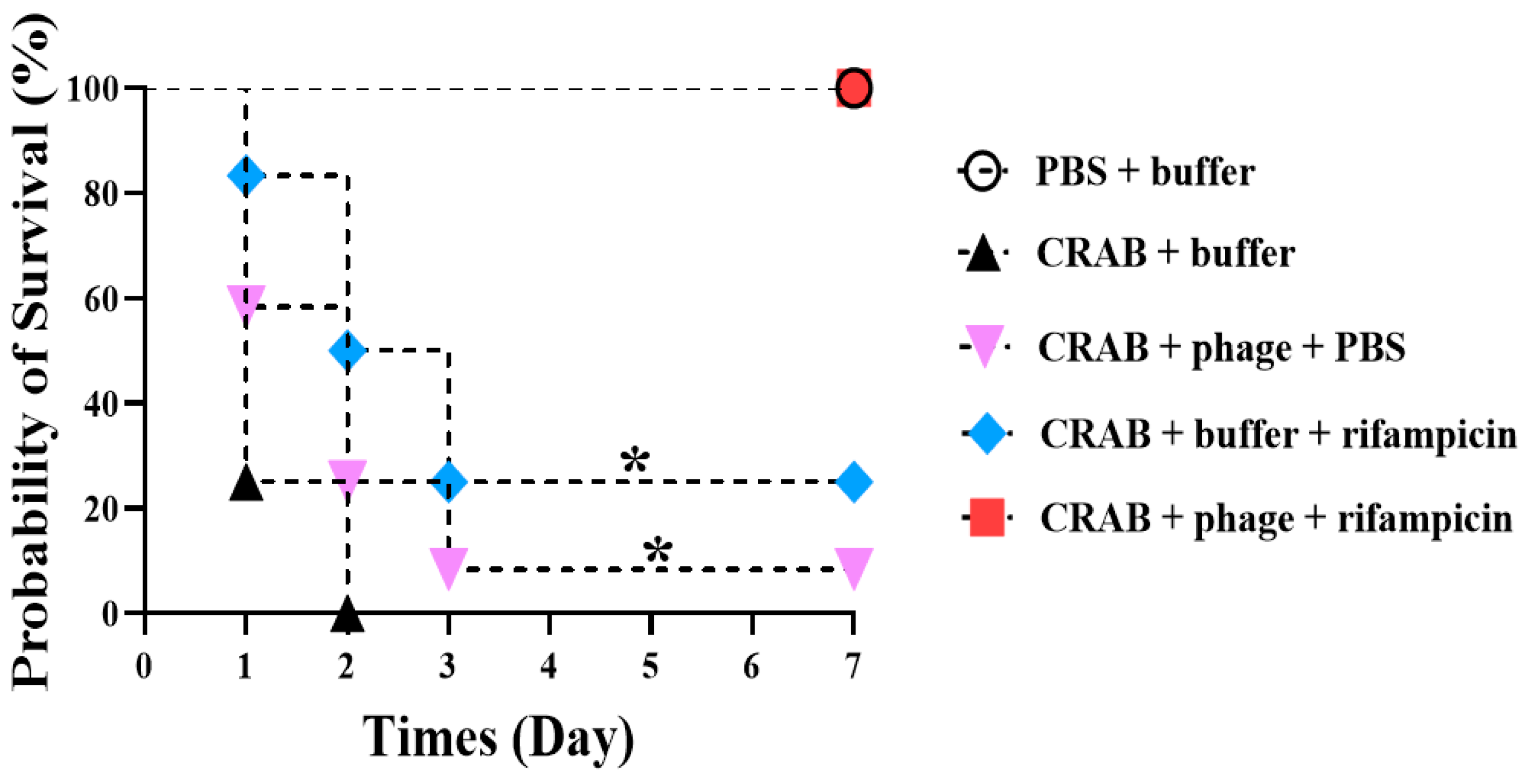
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacteria
4.2. Amplification and Purification of Bacteriophage vB_AbaSi_W9
4.3. Determination of Optimal Multiplicity of Infection (MOI), Phage Adsorption Assay, and Phage One-Step Growth Curve Assay
4.4. Stability of Phage at Different Temperature and pH
4.5. Synergy Testing of Phage and Antibiotics
4.6. In Vivo Experiment Using Mouse Infection Model
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.S.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a Global Pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Mann, T.; Barber, K.E.; Kaye, K.S. Carbapenem-Resistant Acinetobacter baumannii: Epidemiology, Surveillance and Management. Expert Rev. Anti-Infect. Ther. 2013, 11, 383–393. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.J.; Actis, L.; Pachón, J. Acinetobacter baumannii: Human Infections, Factors Contributing to Pathogenesis and Animal Models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.; Putra, V.; Lodise, T.P. Treatment of Patients with Serious Infections Due to Carbapenem-Resistant Acinetobacter baumannii: How Viable Are the Current Options? Pharmacotherapy 2021, 41, 762–780. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Timsit, J.F. Managing Acinetobacter baumannii Infections. Curr. Opin. Infect. Dis. 2019, 32, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An Increasing Threat in Hospitals: Multidrug-Resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Chaturongakul, S.; Ounjai, P. Phage-Host Interplay: Examples from Tailed Phages and Gram-Negative Bacterial Pathogens. Front. Microbiol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Look Who’s Talking: T-Even Phage Lysis Inhibition, the Granddaddy of Virus-Virus Intercellular Communication Research. Viruses 2019, 11, 951. [Google Scholar] [CrossRef]
- Sisakhtpour, B.; Mirzaei, A.; Karbasizadeh, V.; Hosseini, N.; Shabani, M.; Moghim, S. The Characteristic and Potential Therapeutic Effect of Isolated Multidrug-Resistant Acinetobacter baumannii Lytic Phage. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 1. [Google Scholar] [CrossRef]
- Jin, J.; Li, Z.J.; Wang, S.W.; Wang, S.M.; Huang, D.H.; Li, Y.H.; Ma, Y.Y.; Wang, J.; Liu, F.; Chen, X.D.; et al. Isolation and Characterization of ZZ1, a Novel Lytic Phage That Infects Acinetobacter baumannii Clinical Isolates. BMC Microbiol. 2012, 12, 56–156. [Google Scholar] [CrossRef]
- Liu, M.; Hernandez-Morales, A.; Clark, J.; Le, T.; Biswas, B.; Bishop-Lilly, K.A.; Henry, M.; Quinones, J.; Voegtly, L.J.; Cer, R.Z.; et al. Comparative Genomics of Acinetobacter baumannii and Therapeutic Bacteriophages from a Patient Undergoing Phage Therapy. Nat. Commun. 2022, 13, 3776. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Kang, G.; Bai, L.; Wang, P.; Huang, H. Isolation and Characterization of AbTJ, an Acinetobacter baumannii Phage, and Functional Identification of Its Receptor-Binding Modules. Viruses 2020, 12, 205. [Google Scholar] [CrossRef]
- Mardiana, M.; Teh, S.H.; Lin, L.C.; Lin, N.T. Isolation and Characterization of a Novel Siphoviridae Phage, VB_AbaS_TCUP2199, Infecting Multidrug-Resistant Acinetobacter baumannii. Viruses 2022, 14, 1240. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Pu, M.; Li, Y.; Wang, Y.; Li, M.; Song, L.; Li, M.; An, X.; Fan, H.; Tong, Y. Acinetobacter baumannii Phages: Past, Present and Future. Viruses 2023, 15, 673. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage Treatment of Human Infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Stone, E.; Campbell, K.; Grant, I.; McAuliffe, O. Understanding and Exploiting Phage–Host Interactions. Viruses 2019, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Housby, J.N.; Mann, N.H. Phage Therapy. Drug Discov. Today 2009, 14, 536–540. [Google Scholar] [CrossRef]
- Hassan, A.Y.; Lin, J.T.; Ricker, N.; Anany, H. The Age of Phage: Friend or Foe in the New Dawn of Therapeutic and Biocontrol Applications? Pharmaceuticals 2021, 14, 199. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage Therapy: An Alternative to Antibiotics in the Age of Multi-Drug Resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Mandal, S.M. Gut-Antimicrobial Peptides: Synergistic Co-Evolution with Antibiotics to Combat Multi-Antibiotic Resistance. Antibiotics 2023, 12, 1732. [Google Scholar] [CrossRef]
- Nikolic, I.; Vukovic, D.; Gavric, D.; Cvetanovic, J.; Aleksic Sabo, V.; Gostimirovic, S.; Narancic, J.; Knezevic, P. An Optimized Checkerboard Method for Phage-Antibiotic Synergy Detection. Viruses 2022, 14, 1542. [Google Scholar] [CrossRef]
- Liu, C.G.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar] [CrossRef]
- Zalts, R.; Neuberger, A.; Hussein, K.; Raz-Pasteur, A.; Geffen, Y.; Mashiach, T.; Finkelstein, R. Treatment of Carbapenem-Resistant Acinetobacter baumannii Ventilator-Associated Pneumonia: Retrospective Comparison between Intravenous Colistin and Intravenous Ampicillin–Sulbactam. Am. J. Ther. 2016, 23, e78–e85. [Google Scholar] [CrossRef] [PubMed]
- Comeau, A.M.; Tétart, F.; Trojet, S.N.; Prère, M.F.; Krisch, H.M. Phage-Antibiotic Synergy (PAS): β-Lactam and Quinolone Antibiotics Stimulate Virulent Phage Growth. PLoS ONE 2007, 2, e799. [Google Scholar] [CrossRef]
- Vashisth, M.; Yashveer, S.; Jaglan, A.B.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Anand, T. Synergy of a Virulent Phage (ΦAB182) with Antibiotics Leading to Successful Elimination of Biofilms Formed by MDR Acinetobacter baumannii. Can. J. Microbiol. 2022, 68, 731–746. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Roszak, M.; Golec, P.; Śleboda-Taront, D.; Łubowska, N.; Górska, M.; Jursa-Kulesza, J.; Rakoczy, R.; Wojciuk, B.; Dołęgowska, B. Antibiotics Act with VB_AbaP_AGC01 Phage against Acinetobacter baumannii in Human Heat-Inactivated Plasma Blood and Galleria Mellonella Models. Int. J. Mol. Sci. 2020, 21, 4390. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef]
- Blasco, L.; Bleriot, I.; de Aledo, M.G.; Fernández-García, L.; Pacios, O.; Oliveira, H.; López, M.; Ortiz-Cartagena, C.; Fernández-Cuenca, F.; Pascual, Á.; et al. Development of an Anti-Acinetobacter baumannii Biofilm Phage Cocktail: Genomic Adaptation to the Host. Antimicrob. Agents Chemother. 2022, 66, e0192321. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liang, L.; Lin, S.; Jia, S. Isolation and Characterization of a Virulent Bacteriophage AB1 of Acinetobacter baumannii. BMC Microbiol. 2010, 10, 131. [Google Scholar] [CrossRef]
- He, P.; Cao, F.; Qu, Q.; Geng, H.; Yang, X.; Xu, T.; Wang, R.; Jia, X.; Lu, M.; Zeng, P.; et al. Host Range Expansion of Acinetobacter Phage VB_Ab4_Hep4 Driven by a Spontaneous Tail Tubular Mutation. Front. Cell. Infect. Microbiol. 2024, 14, 1301089. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Kim, S.; Shin, M.; Kim, J. Isolation and Characterization of Novel Bacteriophages to Target Carbapenem-Resistant Acinetobacter Baumannii. Antibiotics 2024, 13, 610. [Google Scholar] [CrossRef]
- Letarov, A.V.; Kulikov, E.E. Adsorption of Bacteriophages on Bacterial Cells. Biochemistry 2017, 82, 1632–1658. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E. Phage Host Range and Efficiency of Plating. Methods Mol. Biol. 2009, 501, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Manohar, P.; Madurantakam Royam, M.; Loh, B.; Bozdogan, B.; Nachimuthu, R.; Leptihn, S. Synergistic Effects of Phage–Antibiotic Combinations against Citrobacter amalonaticus. ACS Infect. Dis. 2022, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Ribeiro, H.G.; Silva, A.C.; Silva, M.D.; Sousa, J.C.; Rodrigues, C.F.; Melo, L.D.R.; Henriques, A.F.; Sillankorva, S. Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms. Front. Microbiol. 2017, 8, 2407. [Google Scholar] [CrossRef] [PubMed]
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.P.; Costechareyre, D.; Molle, V. Bacteriophage Therapy for Staphylococcus aureus Infections: A Review of Animal Models, Treatments, and Clinical Trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314. [Google Scholar] [CrossRef]
- Sarker, S.A.; Berger, B.; Deng, Y.; Kieser, S.; Foata, F.; Moine, D.; Descombes, P.; Sultana, S.; Huq, S.; Bardhan, P.K.; et al. Oral Application of Escherichia coli Bacteriophage: Safety Tests in Healthy and Diarrheal Children from Bangladesh. Environ. Microbiol. 2017, 19, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, N.; Barr, J.J. Phage on Tap: A Quick and Efficient Protocol for the Preparation of Bacteriophage Laboratory Stocks. Methods Mol. Biol. 2018, 1838, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Joshi, H. Simple Two-Step, High Yield Protocol for Isolation and Amplification of Bacteriophages Against Methicillin-Resistant Staphylococcus aureus (MRSA). Curr. Protoc. 2022, 2, e395. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host Receptors for Bacteriophage Adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Multiple Comparison Analysis Testing in ANOVA. Biochem. Med. 2011, 21, 203–209. [Google Scholar] [CrossRef]
- Hecke, T. Van Power Study of ANOVA versus Kruskal-Wallis Test. J. Stat. Manag. Syst. 2013, 15, 241–247. [Google Scholar] [CrossRef]




| Antimicrobial against | Bacteria | Antimicrobial Susceptibility with Phage | |||
|---|---|---|---|---|---|
| Independent 1 MIC (µg/mL) | Combined MIC (µg/mL) | 2 FIC | Effects of 3 PAS | ||
| Meropenem | A. baumannii ATCC17978 | 256 | 0.5 | 0.002 | Synergy |
| A. baumannii KBN10P04948 (ST191) | 256 | 256 | 1.000 | Indifferent | |
| A. baumannii LIS2013230 (ST208) | 512 | 256 | 0.500 | Indifferent | |
| A. baumannii KBN10P05982 (ST369) | 256 | 0.5 | 0.002 | Synergy | |
| A. baumannii KBN10P05231 (ST451) | 256 | 128 | 0.500 | Indifferent | |
| Colistin | A. baumannii ATCC17978 | 4 | 2 | 0.500 | Indifferent |
| A. baumannii KBN10P04948 (ST191) | 8 | 2 | 0.250 | Indifferent | |
| A. baumannii LIS2013230 (ST208) | 16 | 8 | 0.500 | Indifferent | |
| A. baumannii KBN10P05982 (ST369) | 4 | 1 | 0.250 | Synergy | |
| A. baumannii KBN10P05231 (ST451) | 128 | 64 | 0.500 | Indifferent | |
| Ampicillin/Sulbactam | A. baumannii ATCC17978 | 128/64 | 2/1 | 0.016 | Synergy |
| A. baumannii KBN10P04948 (ST191) | 256/128 | 128/64 | 0.500 | Indifferent | |
| A. baumannii LIS2013230 (ST208) | 256/128 | 128/64 | 0.500 | Indifferent | |
| A. baumannii KBN10P05982 (ST369) | 256/128 | 64/32 | 0.250 | Synergy | |
| A. baumannii KBN10P05231 (ST451) | 512/256 | 64/32 | 0.004 | Synergy | |
| Tigecycline | A. baumannii ATCC17978 | 4 | 0.25 | 0.063 | Synergy |
| A. baumannii KBN10P04948 (ST191) | 16 | 2 | 0.125 | Synergy | |
| A. baumannii LIS2013230 (ST208) | 8 | 1 | 0.125 | Synergy | |
| A. baumannii KBN10P05982 (ST369) | 16 | 0.5 | 0.031 | Synergy | |
| A. baumannii KBN10P05231 (ST451) | 8 | 0.25 | 0.031 | Synergy | |
| Rifampicin | A. baumannii ATCC17978 | 16 | 2 | 0.125 | Synergy |
| A. baumannii KBN10P04948 (ST191) | 256 | 2 | 0.008 | Synergy | |
| A. baumannii LIS2013230 (ST208) | 32 | 8 | 0.250 | Synergy | |
| A. baumannii KBN10P05982 (ST369) | 32 | 4 | 0.125 | Synergy | |
| A. baumannii KBN10P05231 (ST451) | 128 | 2 | 0.016 | Synergy | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-J.; Kim, S.; Shin, M.; Kim, J. Synergistic Antimicrobial Effects of Phage vB_AbaSi_W9 and Antibiotics against Acinetobacter baumannii Infection. Antibiotics 2024, 13, 680. https://doi.org/10.3390/antibiotics13070680
Choi Y-J, Kim S, Shin M, Kim J. Synergistic Antimicrobial Effects of Phage vB_AbaSi_W9 and Antibiotics against Acinetobacter baumannii Infection. Antibiotics. 2024; 13(7):680. https://doi.org/10.3390/antibiotics13070680
Chicago/Turabian StyleChoi, Yoon-Jung, Shukho Kim, Minsang Shin, and Jungmin Kim. 2024. "Synergistic Antimicrobial Effects of Phage vB_AbaSi_W9 and Antibiotics against Acinetobacter baumannii Infection" Antibiotics 13, no. 7: 680. https://doi.org/10.3390/antibiotics13070680
APA StyleChoi, Y.-J., Kim, S., Shin, M., & Kim, J. (2024). Synergistic Antimicrobial Effects of Phage vB_AbaSi_W9 and Antibiotics against Acinetobacter baumannii Infection. Antibiotics, 13(7), 680. https://doi.org/10.3390/antibiotics13070680








