Essential Oils from Southern Italian Aromatic Plants Synergize with Antibiotics against Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis Cell Growth and Biofilm Formation
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity of the Essential Oils
2.2. Effects on the MIC Values of Antibiotics Induced by Their Combination with EOs
2.3. Effects on Biofilm Formation Induced by Combinations of EOs with Antibiotics
2.4. Effects of EOs in Combination with Antibiotics on Bacterial Methylation Profiles
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. EOs and Antibiotics
4.3. Determination of Minimum Inhibitory Concentration (MIC) of the EOs and Antibiotics
4.4. Synergy between Essential Oils and Antibiotics Evaluation
4.5. Biofilm Formation Assay
4.6. DNA Extraction
4.7. Quantification of Global N6-Methyladenosine and 5-Methylcytosine Levels
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Aelenei, P.; Miron, A.; Trifan, A.; Bujor, A.; Gille, E.; Aprotosoaie, A.C. Essential Oils and Their Components as Modulators of Antibiotic Activity against Gram-Negative Bacteria. Medicines 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Elmaidomy, A.H.; Shady, N.H.; Abdeljawad, K.M.; Elzamkan, M.B.; Helmy, H.H.; Tarshan, E.A.; Adly, A.N.; Hussien, Y.H.; Sayed, N.G.; Zayed, A.; et al. Antimicrobial Potentials of Natural Products against Multidrug Resistance Pathogens: A Comprehensive Review. RSC Adv. 2022, 12, 29078–29102. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 134723. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Cheikh Ismail, L.; Hasan, H.; Hashim, M.; Obaid, R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules 2023, 28, 1809. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, P.; Sena, G.; Crudo, M.; Passarino, G.; Bellizzi, D. Effect of Essential Oils of Apiaceae, Lamiaceae, Lauraceae, Myrtaceae, and Rutaceae family plants on growth, biofilm formation, and Quorum Sensing in Chromobacterium violaceum, Pseudomonas aeruginosa, and Enterococcus faecalis. Microorganisms 2023, 11, 1150. [Google Scholar] [CrossRef]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Godena, S. The antibacterial effect of selected essential oils and their bioactive constituents on Pseudomonas savastanoi pv. savastanoi: Phytotoxic properties and potential for future olive disease control. Microorganisms 2023, 11, 2735. [Google Scholar]
- ALrashidi, A.A.; Noumi, E.; Snoussi, M.; Feo, V. Chemical composition, antibacterial and anti-Quorum Sensing activities of Pimenta dioica L. essential oil and its major compound (Eugenol) against foodborne pathogenic bacteria. Plants 2022, 11, 540. [Google Scholar] [CrossRef]
- D’Aquila, P.; Paparazzo, E.; Crudo, M.; Bonacci, S.; Procopio, A.; Passarino, G.; Bellizzi, D. Antibacterial activity and epigenetic remodeling of Essential Oils from calabrian aromatic plants. Nutrients 2022, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-quorum Sensing and antimicrobial effect of Mediterranean plant essential oils against phytopathogenic bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef]
- Drioiche, A.; Baammi, S.; Zibouh, K.; Al Kamaly, O.; Alnakhli, A.M.; Remok, F.; Saidi, S.; Amaiach, R.; El Makhoukhi, F.; Elomri, A.; et al. A Study of the Synergistic Effects of Essential Oils from Origanum compactum and Origanum elongatum with Commercial Antibiotics against Highly Prioritized Multidrug-Resistant Bacteria for the World Health Organization. Metabolites 2024, 14, 210. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Cheyadmi, S.; Ouzakar, S.; Senhaji, N.S.; Abrini, J. Evaluation of the combined effect of antibiotics and essential oils against Campylobacter multidrug resistant strains and their biofilm formation. S. Afr. J. Bot. 2022, 150, 451–465. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic interactions of plant essential oils with antimicrobial agents: A new antimicrobial therapy. Crit. Rev. Food Sci. Nutr. 2022, 62, 1740–1751. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Romo-Castillo, M.; Flores-Bautista, V.A.; Guzmán-Gutiérrez, S.L.; Reyes-Chilpa, R.; León-Santiago, M.; Luna-Pineda, V.M. Synergy of plant essential oils in antibiotic therapy to combat Klebsiella pneumoniae infections. Pharmaceuticals 2023, 16, 839. [Google Scholar] [CrossRef]
- de Melo, A.L.F.; Rossato, L.; Veloso, T.C.; Cardoso, C.A.L.; Velasques, J.; Simionatto, S. Synergy between amikacin and Protium heptaphyllum essential oil against polymyxin resistance Klebsiella pneumoniae. J. Appl. Microbiol. 2023, 134, lxad195. [Google Scholar] [CrossRef]
- Karadağ, A.E.; Çaşkurlu, A.; Demirci, B.; Demirci, F. Binary synergistic combinations of lavender and fennel essential oils with amoxicillin. Planta Med. 2023, 89, 800–807. [Google Scholar] [CrossRef]
- Septama, A.W.; Yuandani, Y.; Khairunnisa, N.A.; Nasution, H.R.; Utami, D.S.; Kristiana, R.; Jantan, I. Antibacterial, bacteriolytic, antibiofilm, and synergistic effects of the peel oils of Citrus microcarpa and Citrus x amblycarpa with tetracycline against foodborne Escherichia coli. Lett. Appl. Microbiol. 2023, 6, ovad126. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Hu, J.; Liu, Z.; Zeng, Z.L. Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum beta-lactamase-producing Escherichia coli. FEMS Immunol. Med. Microbiol. 2008, 53, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Mahomoodally, M.F. Antibiotic potentiation of natural products: A promising target to fight pathogenic bacteria. Curr. Drug Targets 2021, 22, 555–572. [Google Scholar] [CrossRef]
- Panda, S.K.; Buroni, S.; Swain, S.S.; Bonacorsi, A.; da Fonseca Amorim, E.A.; Kulshrestha, M.; da Silva, L.C.N.; Tiwari, V. Recent advances to combat ESKAPE pathogens with special reference to essential oils. Front. Microbiol. 2022, 13, 1029098. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Laird, K. Synchronous application of antibiotics and essential oils: Dual mechanisms of action as a potential solution to antibiotic resistance. Crit. Rev. Microbiol. 2018, 44, 414–435. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 338–357. [Google Scholar] [CrossRef]
- Saeed, S.; Tariq, P. Antibacterial activity of oregano (Origanum vulgare Linn.) against gram positive bacteria. Pak. J. Pharm. Sci. 2009, 22, 421. [Google Scholar]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; Leite, A.L.; de Pontes, L.G.; Dos Santos, L.D.; et al. Proteomic analysis and antibacterial resistance mechanisms of Salmonella Enteritidis submitted to the inhibitory effect of Origanum vulgare essential oil, thymol and carvacrol. J. Proteomics. 2020, 214, 103625. [Google Scholar] [CrossRef] [PubMed]
- Kolypetri, S.; Kostoglou, D.; Nikolaou, A.; Kourkoutas, Y.; Giaouris, E. Chemical Composition, Antibacterial and Antibiofilm Actions of Oregano (Origanum vulgare subsp. hirtum) Essential Oil against Salmonella Typhimurium and Listeria monocytogenes. Foods 2023, 12, 2893. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, J.; Song, Y.; Raka, R.N.; Xiang, J.; Wu, H.; Xiao, J.; Jin, J.; Hui, X. Antibacterial activity of oregano essential oils against Streptococcus mutans in vitro and analysis of active components. BMC Complement. Med. Ther. 2023, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. and Subspecies. Iran. J. Pharm. Res. 2021, 20, 268–285. [Google Scholar] [PubMed]
- Maggini, V.; Pesavento, G.; Maida, I.; Lo Nostro, A.; Calonico, C.; Sassoli, C.; Perrin, E.; Fondi, M.; Mengoni, A.; Chiellini, C.; et al. Exploring the Effect of the Composition of Three Different Oregano Essential Oils on the Growth of Multidrug-Resistant Cystic Fibrosis Pseudomonas aeruginosa Strains. Nat. Prod. Commun. 2017, 12, 1949–1952. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- de Sá Filho, J.C.F.; de Castro Nizio, D.A.; de Oliveira, A.M.S.; Alves, M.F.; Camargos de Oliveira, R.; Luz, J.M.Q.; de Lima Nogueira, P.C.; Arrigoni-Blank, M.D.F.; Blank, A.F. Geographic location and seasonality affect the chemical composition of essential oils of Lippia alba accessions. Ind. Crops Prod. 2022, 188, 115602. [Google Scholar] [CrossRef]
- Hazrati, S.; Beidaghi, P.; Beyraghdar Kashkooli, A.; Hosseini, S.J.; Nicola, S. Effect of Harvesting Time Variations on Essential Oil Yield and Composition of Sage (Salvia officinalis). Horticulturae 2022, 8, 149. [Google Scholar] [CrossRef]
- Uzair, B.; Niaz, N.; Bano, A.; Khan, B.A.; Zafar, N.; Iqbal, M.; Tahira, R.; Fasim, F. Essential oils showing in vitro anti MRSA and synergistic activity with penicillin group of antibiotics. Pak. J. Pharm. Sci. 2017, 30, 1997–2002. [Google Scholar] [PubMed]
- Aslam, S. Effect of antibacterials on biofilms. Am. J. Infect. Control. 2008, 36, S175.e9–11. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Sblano, S.; Salvagno, L.; Carocci, A.; Clodoveo, M.L.; Corbo, F.; Fracchiolla, G. Anti-biofilm inhibitory synergistic effects of combinations of Essential Oils and antibiotics. Antibiotics 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, J.; Zhang, L.; Zhang, R.; Zhang, S.; Ye, S.; Zhao, Z.; Yang, D. Exploring the antibacterial mechanism of essential oils by membrane permeability, apoptosis and biofilm formation combination with proteomics analysis against methicillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 2020, 310, 151435. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Ndezo Bisso, B.; Tokam Kuaté, C.R.; Boulens, N.; Allémann, E.; Delie, F.; Dzoyem, J.P. Antibiofilm synergistic activity of streptomycin in combination with thymol-loaded poly (Lactic-co-glycolic Acid) nanoparticles against Klebsiella pneumoniae isolates. Evid. Based Complement. Altern. Med. 2022, 2022, 1936165. [Google Scholar] [CrossRef]
- Vanegas, D.; Abril-Novillo, A.; Khachatryan, A.; Jerves-Andrade, L.; Peñaherrera, E.; Cuzco, N.; Wilches, I.; Calle, J.; León-Tamariz, F. Validation of a method of broth microdilution for the determination of antibacterial activity of essential oils. BMC Res. Notes. 2021, 14, 439. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Gan, C.; Langa, E.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M.R. Synergistic Activity of Thymol with Commercial Antibiotics against Critical and High WHO Priority Pathogenic Bacteria. Plants 2023, 12, 1868. [Google Scholar] [CrossRef]
- Gómara, M.; Ramón-García, S. The FICI paradigm: Correcting flaws in antimicrobial in vitro synergy screens at their inception. Biochem. Pharmacol. 2019, 163, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Subba Rao, T. An Improved Crystal Violet Assay for Biofilm Quantification in 96-Well Microtitre Plate. bioRxiv 2017. bioRxiv:100214. [Google Scholar]

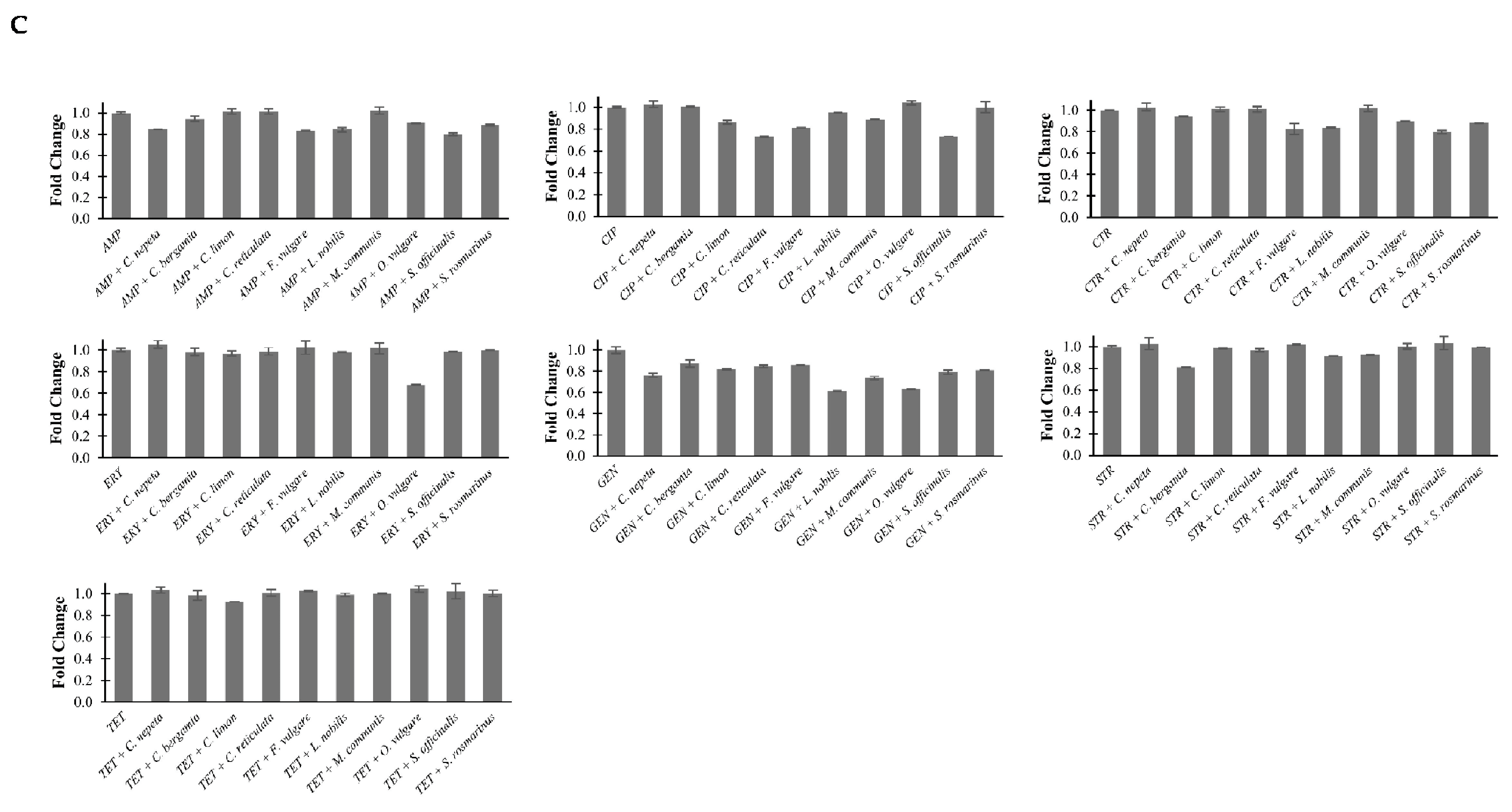
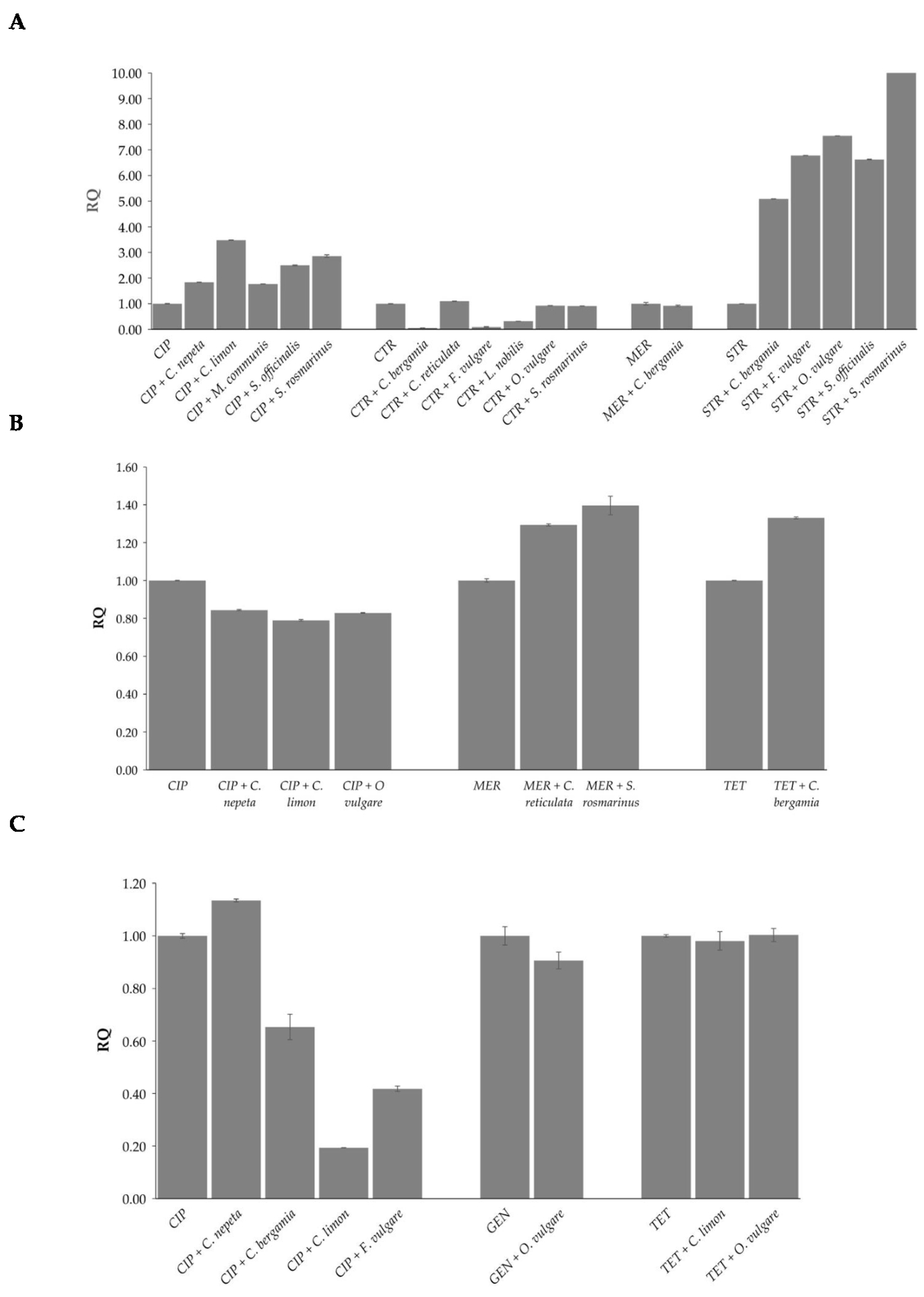
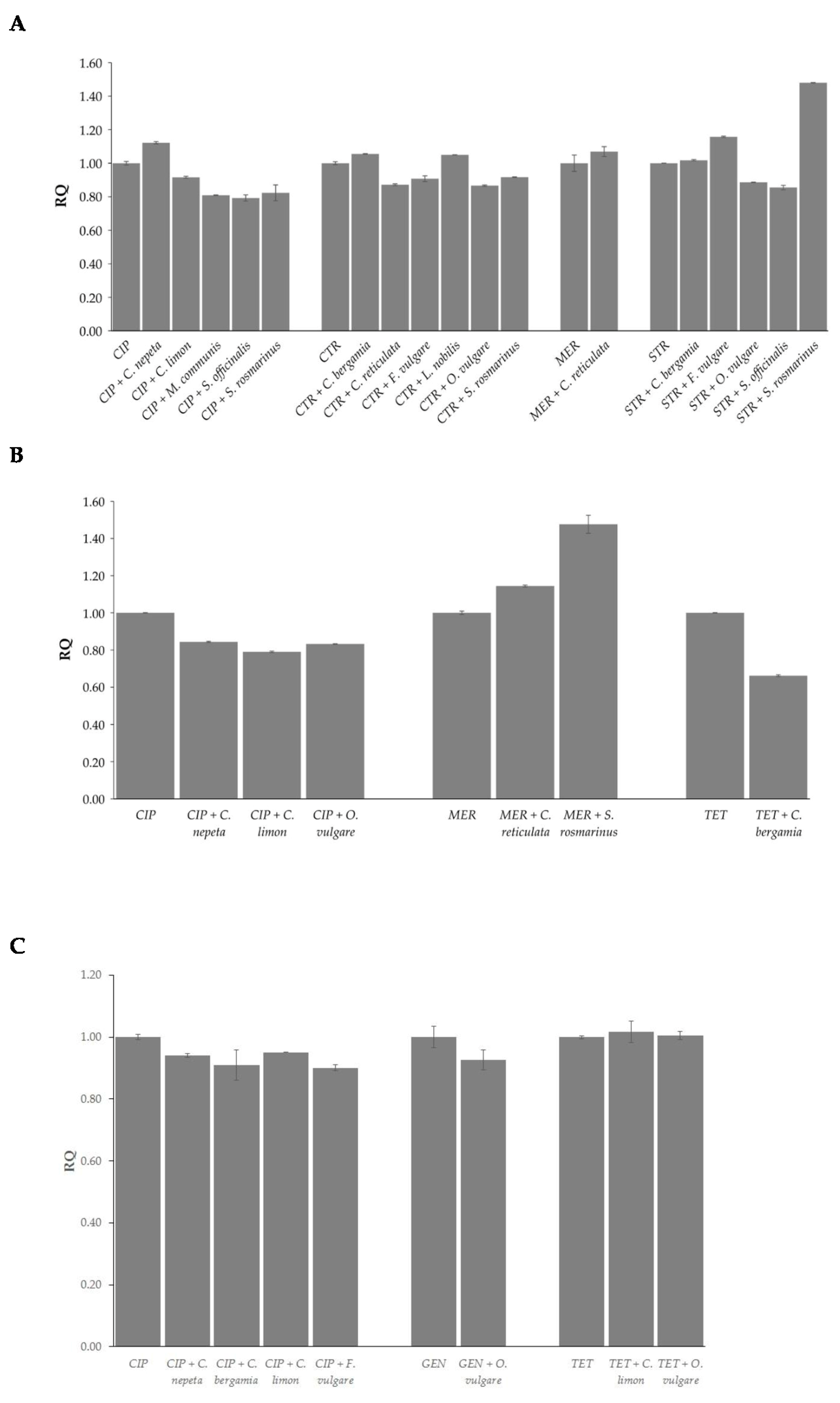
| E. coli | P. aeruginosa | E. faecalis | ||
|---|---|---|---|---|
| Essential Oils | C. nepeta | 5 | >5 | >5 |
| C. bergamia | 2.5 | >5 | >5 | |
| C. limon | 5 | >5 | >5 | |
| C. reticulata | 5 | >5 | >5 | |
| F. vulgare | 2.5 | >5 | >5 | |
| L. nobilis | 5 | >5 | >5 | |
| M. communis | 5 | >5 | >5 | |
| O. vulgare | 1.25 | 5 | 1.25 | |
| S. officinalis | 5 | >5 | >5 | |
| S. rosmarinus | 2.5 | >5 | 5 | |
| Antibiotics | AMP | 50 | 1000 | 5 |
| AZT | 0.09 | 20 | >4000 | |
| CIP | 0.6 | 0.20 | 4 | |
| CTR | 0.15 | 50 | 6.25 | |
| ERY | 300 | 315 | 500 | |
| GEN | 12.5 | 0.80 | 25 | |
| MER | 0.05 | 0.06 | 50 | |
| STR | 12.5 | 2 | 250 | |
| TET | 5 | 200 | 3 |
| E. coli | P. aeruginosa | E. faecalis | ||||||
|---|---|---|---|---|---|---|---|---|
| AMP | C. reticulata | 4 | AMP | C. nepeta | 2 | AMP | C. nepeta | 2 |
| L. nobilis | 2 | C. bergamia | 2 | C. limon | 2 | |||
| O. vulgare | 4 | C. limon | 2 | C. reticulata | 2 | |||
| S. rosmarinus | 2 | L. nobilis | 2 | F. vulgare | 2 | |||
| CIP | C. nepeta | 8 | O. vulgare | 2 | L. nobilis | 2 | ||
| C. bergamia | 2 | S. officinalis | 2 | M. communis | 2 | |||
| C. limon | 8 | S. rosmarinus | 2 | O. vulgare | 2 | |||
| C. reticulata | 8 | AZT | C. nepeta | 2 | S. officinalis | 2 | ||
| F. vulgare | 8 | C. bergamia | 2 | S. rosmarinus | 2 | |||
| L. nobilis | 2 | C. limon | 2 | CIP | C. nepeta | 8 | ||
| M. communis | 8 | C. reticulata | 2 | C. bergamia | 4 | |||
| O. vulgare | 4 | M. communis | 2 | C. limon | 16 | |||
| S. officinalis | 8 | O. vulgare | 2 | C. reticulata | 4 | |||
| S. rosmarinus | 8 | S. officinalis | 2 | F. vulgare | 4 | |||
| CTR | C. bergamia | 8 | S. rosmarinus | 2 | L. nobilis | 2 | ||
| C. limon | 4 | CIP | C. nepeta | 2 | M. communis | 2 | ||
| C. reticulata | 8 | C. limon | 2 | O. vulgare | 2 | |||
| F. vulgare | 4 | F. vulgare | 2 | S. officinalis | 2 | |||
| L. nobilis | 8 | L. nobilis | 2 | S. rosmarinus | 2 | |||
| O. vulgare | 8 | M. communis | 2 | CTR | C. reticulata | 2 | ||
| S. rosmarinus | 8 | O. vulgare | 2 | F. vulgare | 2 | |||
| ERY | C. nepeta | 2 | CTR | O. vulgare | 2 | L. nobilis | 2 | |
| C. bergamia | 2 | GEN | C. bergamia | 2 | M. communis | 2 | ||
| C. reticulata | 2 | C. limon | 2 | O. vulgare | 2 | |||
| O. vulgare | 2 | F. vulgare | 2 | S. officinalis | 2 | |||
| S. officinalis | 2 | M. communis | 2 | S. rosmarinus | 2 | |||
| S. rosmarinus | 2 | O. vulgare | 2 | ERY | O. vulgare | 2 | ||
| GEN | C. nepeta | 2 | S. rosmarinus | 2 | GEN | C. nepeta | 2 | |
| C. bergamia | 2 | MER | C. reticulata | 2 | F. vulgare | 2 | ||
| C. limon | 2 | S. rosmarinus | 2 | L. nobilis | 2 | |||
| C. reticulata | 8 | TET | C. nepeta | 2 | M. communis | 2 | ||
| F. vulgare | 4 | C. bergamia | 4 | O. vulgare | 4 | |||
| M. communis | 2 | C. limon | 2 | S. rosmarinus | 2 | |||
| O. vulgare | 4 | C. reticulata | 2 | STR | C. nepeta | 2 | ||
| S. officinalis | 2 | F. vulgare | 2 | F. vulgare | 2 | |||
| S. rosmarinus | 2 | S. officinalis | 2 | S. rosmarinus | 2 | |||
| STR | C. nepeta | 4 | S. rosmarinus | 2 | TET | C. limon | 16 | |
| C. bergamia | 8 | F. vulgare | 2 | |||||
| C. limon | 4 | O. vulgare | 4 | |||||
| C. reticulata | 16 | S. rosmarinus | 2 | |||||
| F. vulgare | 8 | |||||||
| M. communis | 4 | |||||||
| O. vulgare | 16 | |||||||
| S. officinalis | 8 | |||||||
| S. rosmarinus | 8 | |||||||
| E. coli | P. aeruginosa | E. faecalis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FICI | Effect | FICI | Effect | FICI | Effect | ||||||
| AMP | C. reticulata | 0.75 | Additive | AMP | C. nepeta | 0.6 | Additive | AMP | C. nepeta | 0.92 | Additive |
| L. nobilis | 0.63 | Additive | C. bergamia | 0.6 | Additive | C. limon | 0.92 | Additive | |||
| O. vulgare | 0.75 | Additive | C. limon | 0.6 | Additive | C. reticulata | 0.92 | Additive | |||
| S. rosmarinus | 1 | Additive | L. nobilis | 0.6 | Additive | F. vulgare | 0.92 | Additive | |||
| CIP | C. nepeta | 0.25 | Synergistic | O. vulgare | 0.6 | Additive | L. nobilis | 0.92 | Additive | ||
| C. limon | 0.25 | Synergistic | S. officinalis | 0.6 | Additive | M. communis | 0.92 | Additive | |||
| C. reticulata | 0.25 | Synergistic | S. rosmarinus | 0.6 | Additive | S. officinalis | 0.92 | Additive | |||
| F. vulgare | 0.38 | Synergistic | AZT | C. nepeta | 0.69 | Additive | S. rosmarinus | 1 | Additive | ||
| L. nobilis | 1 | Additive | C. bergamia | 0.69 | Additive | CIP | C. nepeta | 0.23 | Synergistic | ||
| M. communis | 0.25 | Synergistic | C. limon | 0.69 | Additive | C. bergamia | 0.44 | Synergistic | |||
| S. officinalis | 0.25 | Synergistic | C. reticulata | 0.69 | Additive | C. limon | 0.11 | Synergistic | |||
| S. rosmarinus | 0.38 | Synergistic | M. communis | 0.69 | Additive | C. reticulata | 0.44 | Synergistic | |||
| CTR | C. nepeta | 0.33 | Synergistic | O. vulgare | 0.69 | Additive | F. vulgare | 0.44 | Synergistic | ||
| C. bergamia | 0.07 | Synergistic | S. officinalis | 0.69 | Additive | L. nobilis | 0.92 | Additive | |||
| C. limon | 0.09 | Synergistic | S. rosmarinus | 0.69 | Additive | M. communis | 0.92 | Additive | |||
| C. reticulata | 0.04 | Synergistic | CIP | C. nepeta | 0.31 | Synergistic | S. officinalis | 0.92 | Additive | ||
| F. vulgare | 0.15 | Synergistic | C. bergamia | 0.61 | Additive | S. rosmarinus | 1 | Additive | |||
| L. nobilis | 0.04 | Synergistic | C. limon | 0.31 | Synergistic | CTR | C. limon | 0.92 | Additive | ||
| M. communis | 0.33 | Synergistic | C. reticulata | 0.61 | Additive | C. reticulata | 0.92 | Additive | |||
| O. vulgare | 0.14 | Synergistic | F. vulgare | 0.31 | Synergistic | F. vulgare | 0.92 | Additive | |||
| S. officinalis | 0.33 | Synergistic | L. nobilis | 0.31 | Synergistic | L. nobilis | 0.92 | Additive | |||
| S. rosmarinus | 0.07 | Synergistic | M. communis | 0.31 | Synergistic | M. communis | 0.92 | Additive | |||
| ERY | C. nepeta | 0.73 | Additive | O. vulgare | 0.31 | Synergistic | S. rosmarinus | 1 | Additive | ||
| C. bergamia | 0.95 | Additive | S. officinalis | 0.61 | Additive | ERY | O. vulgare | 0.6 | Additive | ||
| C. reticulata | 0.73 | Additive | S. rosmarinus | 0.61 | Additive | GEN | C. nepeta | 0.92 | Additive | ||
| S. officinalis | 0.73 | Additive | GEN | C. bergamia | 0.71 | Additive | F. vulgare | 0.92 | Additive | ||
| S. rosmarinus | 0.95 | Additive | C. limon | 0.71 | Additive | L. nobilis | 0.92 | Additive | |||
| GEN | C. nepeta | 0.75 | Additive | F. vulgare | 0.71 | Additive | M. communis | 0.92 | Additive | ||
| C. bergamia | 1 | Additive | M. communis | 0.71 | Additive | O. vulgare | 0.44 | Synergistic | |||
| C. limon | 0.75 | Additive | O. vulgare | 0.71 | Additive | S. rosmarinus | 0.92 | Additive | |||
| C. reticulata | 0.19 | Synergistic | S. rosmarinus | 0.71 | Additive | STR | C. nepeta | 0.57 | Additive | ||
| F. vulgare | 0.5 | Synergistic | MER | C. reticulata | 0.5 | Synergistic | F. vulgare | 0.57 | Additive | ||
| M. communis | 0.75 | Additive | S. rosmarinus | 0.5 | Synergistic | S. rosmarinus | 0.5 | Synergistic | |||
| O. vulgare | 0.75 | Additive | TET | C. nepeta | 0.92 | Additive | TET | C. limon | 0.09 | Synergistic | |
| S. officinalis | 0.75 | Additive | C. bergamia | 0.46 | Synergistic | F. vulgare | 0.69 | Additive | |||
| S. rosmarinus | 1 | Additive | C. limon | 0.92 | Additive | O. vulgare | 0.35 | Synergistic | |||
| STR | C. nepeta | 0.88 | Additive | C. reticulata | 0.92 | Additive | S. rosmarinus | 0.69 | Additive | ||
| C. bergamia | 0.37 | Synergistic | F. vulgare | 0.92 | Additive | ||||||
| C. limon | 0.5 | Synergistic | S. officinalis | 0.92 | Additive | ||||||
| C. reticulata | 0.12 | Synergistic | S. rosmarinus | 0.92 | Additive | ||||||
| F. vulgare | 0.37 | Synergistic | |||||||||
| M. communis | 0.88 | Additive | |||||||||
| O. vulgare | 0.31 | Synergistic | |||||||||
| S. officinalis | 0.25 | Synergistic | |||||||||
| S. rosmarinus | 0.37 | Synergistic | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sena, G.; De Rose, E.; Crudo, M.; Filippelli, G.; Passarino, G.; Bellizzi, D.; D’Aquila, P. Essential Oils from Southern Italian Aromatic Plants Synergize with Antibiotics against Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis Cell Growth and Biofilm Formation. Antibiotics 2024, 13, 605. https://doi.org/10.3390/antibiotics13070605
Sena G, De Rose E, Crudo M, Filippelli G, Passarino G, Bellizzi D, D’Aquila P. Essential Oils from Southern Italian Aromatic Plants Synergize with Antibiotics against Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis Cell Growth and Biofilm Formation. Antibiotics. 2024; 13(7):605. https://doi.org/10.3390/antibiotics13070605
Chicago/Turabian StyleSena, Giada, Elisabetta De Rose, Michele Crudo, Gianfranco Filippelli, Giuseppe Passarino, Dina Bellizzi, and Patrizia D’Aquila. 2024. "Essential Oils from Southern Italian Aromatic Plants Synergize with Antibiotics against Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis Cell Growth and Biofilm Formation" Antibiotics 13, no. 7: 605. https://doi.org/10.3390/antibiotics13070605
APA StyleSena, G., De Rose, E., Crudo, M., Filippelli, G., Passarino, G., Bellizzi, D., & D’Aquila, P. (2024). Essential Oils from Southern Italian Aromatic Plants Synergize with Antibiotics against Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis Cell Growth and Biofilm Formation. Antibiotics, 13(7), 605. https://doi.org/10.3390/antibiotics13070605






