Isolation of Multidrug-Resistant Mycobacterium Avium Subsp. Avium from a Wild Eurasian Otter (Lutra Lutra)
Abstract
1. Introduction
2. Case Presentation
2.1. Case Description and Postmortem Examination
2.2. Bacteriological Examination
2.3. Molecular Identification
2.4. Antimicrobial Susceptibility Testing
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- van Ingen, J. Diagnosis of nontuberculous mycobacterial infections. Semin. Respir. Crit. Care Med. 2013, 34, 103–109. [Google Scholar] [CrossRef]
- van der Werf, M.J.; Ködmön, C.; Katalinić-Janković, V.; Kummik, T.; Soini, H.; Richter, E.; Papaventsis, D.; Tortoli, E.; Perrin, M.; van Soolingen, D.; et al. Inventory study of non-tuberculous mycobacteria in the European Union. BMC Infect. Dis. 2014, 14, 62. [Google Scholar] [CrossRef]
- Jeon, D. Infection Source and Epidemiology of Nontuberculous Mycobacterial Lung Disease. Tuberc. Respir. Dis. 2019, 82, 94–101. [Google Scholar] [CrossRef]
- Shin, J.I.; Shin, S.J.; Shin, M.K. Differential Genotyping of Mycobacterium avium Complex and Its Implications in Clinical and Environmental Epidemiology. Microorganisms 2020, 8, 98. [Google Scholar] [CrossRef]
- Biet, F.; Boschiroli, M.L.; Thorel, M.F.; Guilloteau, L.A. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC). Vet. Res. 2005, 36, 411–436. [Google Scholar] [CrossRef]
- Alvarez, J.; García, I.G.; Aranaz, A.; Bezos, J.; Romero, B.; de Juan, L.; Mateos, A.; Gómez-Mampaso, E.; Domínguez, L. Genetic diversity of Mycobacterium avium isolates recovered from clinical samples and from the environment: Molecular characterization for diagnostic purposes. J. Clin. Microbiol. 2008, 46, 1246–1251. [Google Scholar] [CrossRef]
- Tran, Q.T.; Han, X.Y. Subspecies identification and significance of 257 clinical strains of Mycobacterium avium. J. Clin. Microbiol. 2014, 52, 1201–1206. [Google Scholar] [CrossRef]
- Kaevska, M.; Sterba, J.; Svobodova, J.; Pavlik, I. Mycobacterium avium subsp. avium and Mycobacterium neoaurum detection in an immunocompromised patient. Epidemiol. Infect. 2014, 142, 882–885. [Google Scholar] [CrossRef]
- Aranaz, A.; Liibana, E.; Mateos, A.; Domínguez, L. Laboratory Diagnosis of Avian mycobacteriosis. Semin. Avian Exot. Pet Med. 1997, 6, 9–17. [Google Scholar] [CrossRef]
- Hoop, R.K. Public health implications of exotic pet avian tuberculosis. Semin. Avian Exot. Pet Med. 1997, 6, 3–8. [Google Scholar] [CrossRef]
- Turenne, C.Y.; Wallace, R., Jr.; Behr, M.A. Mycobacterium avium in the postgenomic era. Clin. Microbiol. Rev. 2007, 20, 205–229. [Google Scholar] [CrossRef]
- Sattar, A.; Zakaria, Z.; Abu, J.; Aziz, S.A.; Rojas-Ponce, G. Isolation of Mycobacterium avium and other nontuberculous mycobacteria in chickens and captive birds in peninsular Malaysia. BMC Vet. Res. 2021, 17, 13. [Google Scholar] [CrossRef]
- Pate, M.; Moravkova, M.; Krt, B.; Pavlik, I.; Ocepek, M. Genotyping of Mycobacterium avium subsp. avium isolates from domestic animals in Slovenia by IS901 RFLP. Vet. Med. 2009, 54, 270–279. [Google Scholar] [CrossRef]
- Pavlik, I.; Jahn, P.; Moravkova, M.; Matlova, L.; Treml, F.; Cizek, A.; Nesnalova, E.; Dvorska-Bartosova, L.; Halouzka, R. Lung tuberculosis in a horse caused by Mycobacterium avium subsp. avium of serotype 2: A case report. Vet. Med. 2008, 53, 111–116. [Google Scholar] [CrossRef]
- Dhama, K.; Mahendran, M.; Tiwari, R.; Dayal Singh, S.; Kumar, D.; Singh, S.; Sawant, P.M. Tuberculosis in Birds: Insights into the Mycobacterium avium Infections. Vet. Med. Int. 2011, 2011, 712369. [Google Scholar] [CrossRef]
- Cvetnić, Ž.; Špičić, S.; Benić, M.; Katalinić-Janković, V.; Pate, M.; Krt, B.; Ocepek, M. Mycobacterial infection of pigs in Croatia. Acta Vet. Hung. 2007, 55, 1–9. [Google Scholar] [CrossRef]
- Špičić, S.; Pate, M.; Katalinić-Janković, V.; Duvnjak, S.; Ocepek, M.; Zdelar-Tuk, M.; Krt, B.; Mitak, M.; Cvetnić, Ž. Molecular epizootiology and epidemiology of Mycobacterium avium subsp. hominissuis isolated from human, animals and environment in Croatia. Wien. Tierärztl. Monat. 2010, 97, 219–224. [Google Scholar]
- Špičić, S.; Cvetnić, Ž.; Duvnjak, S.; Zdelar-Turk, M.; Kušar, D.; Ocepek, M.; Krt, B.; Mitak, M.; Pate, M. Molecular characterization of Mycobacterium avium subsp. avium from animals in Croatia using IS901 RFLP and MIRU-VNTR typing. Slov. Vet. Res. 2010, 47, 21–28. [Google Scholar]
- Reil, I.; Barbić, L.; Kompes, G.; Zdelar-Tuk, M.; Duvnjak, S.; Cvetnić, Ž.; Habrun, B.; Arapović, J.; Špičić, S. Risk of zoonoses involving slow-growing non-tuberculous mycobacteria: Survey of antimicrobial resistance among strains from domestic and wild animals. J. Glob. Antimicrob. Resist. 2023, 35, 6–10. [Google Scholar] [CrossRef]
- Varela-Castro, L.; Barral, M.; Arnal, M.C.; Fernández de Luco, D.; Gortázar, C.; Garrido, J.M.; Sevilla, I.A. Beyond tuberculosis: Diversity and implications of non-tuberculous mycobacteria at the wildlife-livestock interface. Transbound Emerg. Dis. 2022, 69, 2978–2993. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hanna, R.; Hill, R.; McCormick, C.M.; Skuce, R.A. Bovine tuberculosis in an Eurasian otter. Vet. Rec. 2009, 164, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; de Cruz, K.; Zanella, G.; Aaziz, R.; Bulach, T.; Karoui, C.; Hénault, S.; Joncour, G.; Boschiroli, M.L. Infection with Mycobacterium microti in animals in France. J. Clin. Microbiol. 2015, 53, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.; Figueira, L.; Martins, M.H.; Matos, M.; Alvares, S.; Pinto, M.L.; Coelho, A.C. Disseminated Mycobacterium avium subsp. paratuberculosis infection in two wild Eurasian otters (Lutra lutra L.) from Portugal. J. Zoo Wildl. Med. 2013, 44, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.M.; Attia, F.N. Mycobacterium Avium Complex. [Updated 25 February 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431110/ (accessed on 17 June 2024).
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. American Thoracic Society Statement. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardia spp., and other Aerobic Acti-Nomycetes (M24), 3rd ed.; CLSI: Wayne, PA, USA, 2018; pp. 37–44. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and other Aerobic Actinomycetes (M62), 1st ed.; CLSI: Wayne, PA, USA, 2018; pp. 4–11. [Google Scholar]
- Gunn-Moore, D.A. Mycobacterial infections in cats and dogs. In Textbook of Veterinary Internal Medicine, 7th ed.; Ettinger, S., Feldman, E., Eds.; W. B. Saunders: Philadelphia, PA, USA, 2010; pp. 875–881. [Google Scholar]
- Baral, R.M.; Metcalfe, S.S.; Krockenberger, M.B.; Catt, M.J.; Barrs, V.R.; McWhirter, C.; Hutson, C.A.; Wigney, D.I.; Martin, P.; Chen, S.C.; et al. Disseminated Mycobacterium avium infection in young cats: Overrepresentation of Abyssinian cats. J. Feline Med. Surg. 2006, 8, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Ghielmetti, G.; Giger, U. Mycobacterium avium: An Emerging Pathogen for Dog Breeds with Hereditary Immunodeficiencies. Curr. Clin. Microbiol. Rep. 2020, 7, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Armas, F.; Furlanello, T.; Camperio, C.; Trotta, M.; Novari, G.; Marianelli, C. Molecular characterization and drug susceptibility profile of a Mycobacterium avium subspecies avium isolate from a dog with disseminated infection. J. Med. Microbiol. 2016, 65, 78–285. [Google Scholar] [CrossRef]
- Van Der Heyden, N. Mycobacterial infections: New strategies in the treatment of avian tuberculosis. Semin. Avian Exot. Pet Med. 1997, 6, 25–33. [Google Scholar] [CrossRef]
- Kent, P.T.; Kubica, G.P. Public Health Mycobacteriology: A Guide for the Level III; USDHHS, Centers for Disease Control: Atlanta, GA, USA, 1985. [Google Scholar]
- Hance, A.J.; Grandchamp, B.; Lévy-Frébault, V.; Lecossier, D.; Rauzier, J.; Bocart, D.; Gicquel, B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol. Microbiol. 1989, 3, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Kunze, Z.M.; Portael, F.; McFadden, J.J. Biologically distinct subtypes of Mycobacterium avium differ in possession of insertion sequence IS901. J. Clin. Microbiol. 1992, 30, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hua, W.; Wang, S.; Zhang, Y.; Chen, X.; Liu, H.; Shao, L.; Chen, J.; Zhang, W. In vitro assessment of 17 antimicrobial agents against clinical Mycobacterium avium complex isolates. BMC Microbiol. 2022, 22, 175. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.W.H.; Chanin, P.R.F. The status of the Eurasian otter (Lutra lutra) in Europe. A review. J. Intern. Otter Survival Fund. 2000, 1, 7–28. [Google Scholar]
- Clinical and Laboratory Standards Institute. Laboratory Detection and Identification of Mycobacteria; Approved Guideline (M48-A); CLSI: Wayne, PA, USA, 2018; Volume 28/17, pp. 6–8. [Google Scholar]
- Hung, N.; Law, C. Lutra Lutra (Carnivora: Mustelidae). Mamm. Species 2016, 48, 109–122. [Google Scholar] [CrossRef]
- Stepień-Pyśniak, D.; Puk, K.; Guz, L.; Wawrzyniak, A.; Marek, A.; Kosikowska, U. Avian mycobacteriosis caused by Mycobacterium avium subspecies avium in four ornamental birds and in vitro drug sensitivity testing of isolates. Berl. Munch. Tierarztl. Wochenschr. 2016, 129, 65–71. [Google Scholar] [PubMed]
- Nugent, G.; Whitford, G.J.; Young, N. Use of released pigs as sentinels for Mycobacterium bovis. J. Wildl. Dis. 2002, 38, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Hibiya, K.; Higa, F.; Tateyama, M.; Fujita, J. Mycobacteriosis as zoonotic disease—Comparative pathological study on Mycobacterium avium complex infection. Kekkaku 2007, 82, 539–550. (In Japanese) [Google Scholar]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest. Med. 2015, 36, 13–34. [Google Scholar] [CrossRef]
- Stout, J.E.; Koh, W.J.; Yew, W.W. Update on pulmonary disease due to non-tuberculous mycobacteria. Int. J. Infect. Dis. 2016, 45, 123–134. [Google Scholar] [CrossRef]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. Nontuberculous Mycobacteria Network European Trials Group. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef]
- van Ingen, J.; Boeree, M.J.; van Soolingen, D.; Mouton, J.W. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updat. 2012, 15, 149–161. [Google Scholar] [CrossRef]
- Ledwoń, A.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Szeleszczuk, P. Drug Susceptibility of Non-tuberculous Strains of Mycobacterium Isolated from Birds from Poland. Pol. J. Microbiol. 2018, 67, 487–492. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hashem, H.R.; Al-Otaibi, A.S.; Alfifi, K.J.; El-Dawody, E.M.; Mahrous, E.; Hetta, H.F.; El-Kholy, A.W.; Ramadan, H.; El-Tarabili, R.M. Emerging MDR-Mycobacterium avium subsp. avium in house-reared domestic birds as the first report in Egypt. BMC Microbiol. 2021, 21, 237. [Google Scholar] [CrossRef]

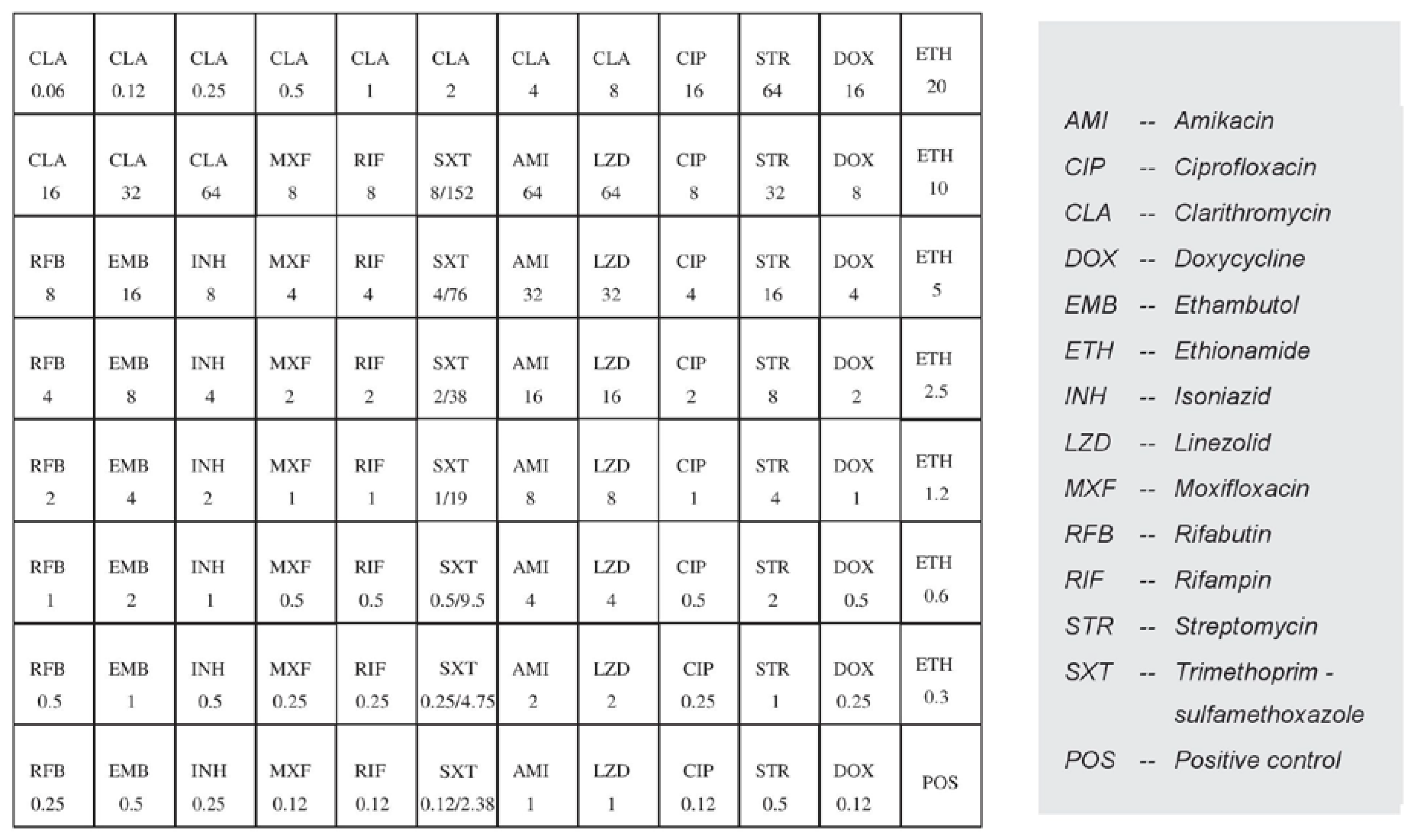
| Antimicrobial Agent | MIC (µg/mL) Criteria | MIC Results | Interpretation | ||
|---|---|---|---|---|---|
| S | I | R | |||
| Clarithromycin 1 | ≤8 | 16 | ≥32 | 2 | S |
| Linezolid 1 | ≤8 | 16 | ≥32 | 32 | R |
| Moxifloxacin 1 | ≤1 | 2 | ≥4 | >8 | R |
| Amikacin 1 | ≤16 | 32 | ≥64 | 16 | S |
| Streptomycin 3 | ≤16 | 32 | ≥64 | >64 | R |
| Isoniazid 3 | - | - | >0.2 | >8 | R |
| Trimethoprim/sulfamethoxazole 2 | ≤ 2/38 | - | ≥ 4/76 | 8/152 | R |
| Ciprofloxacin 2 | ≤ 1 | 2 | ≥4 | >16 | R |
| Doxycycline 2 | ≤ 1 | 2-4 | ≥8 | >16 | R |
| Ethionamide 3 | - | - | >5 | >20 | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reil, I.; Duvnjak, S.; Špičić, S.; Kompes, G.; Bagarić, A.; Đuras, M.; Gudan Kurilj, A.; Lukač, M.; Jelić, M.; Zdelar-Tuk, M. Isolation of Multidrug-Resistant Mycobacterium Avium Subsp. Avium from a Wild Eurasian Otter (Lutra Lutra). Antibiotics 2024, 13, 591. https://doi.org/10.3390/antibiotics13070591
Reil I, Duvnjak S, Špičić S, Kompes G, Bagarić A, Đuras M, Gudan Kurilj A, Lukač M, Jelić M, Zdelar-Tuk M. Isolation of Multidrug-Resistant Mycobacterium Avium Subsp. Avium from a Wild Eurasian Otter (Lutra Lutra). Antibiotics. 2024; 13(7):591. https://doi.org/10.3390/antibiotics13070591
Chicago/Turabian StyleReil, Irena, Sanja Duvnjak, Silvio Špičić, Gordan Kompes, Antonela Bagarić, Martina Đuras, Andrea Gudan Kurilj, Maja Lukač, Mišel Jelić, and Maja Zdelar-Tuk. 2024. "Isolation of Multidrug-Resistant Mycobacterium Avium Subsp. Avium from a Wild Eurasian Otter (Lutra Lutra)" Antibiotics 13, no. 7: 591. https://doi.org/10.3390/antibiotics13070591
APA StyleReil, I., Duvnjak, S., Špičić, S., Kompes, G., Bagarić, A., Đuras, M., Gudan Kurilj, A., Lukač, M., Jelić, M., & Zdelar-Tuk, M. (2024). Isolation of Multidrug-Resistant Mycobacterium Avium Subsp. Avium from a Wild Eurasian Otter (Lutra Lutra). Antibiotics, 13(7), 591. https://doi.org/10.3390/antibiotics13070591






