Activity of Aztreonam/Avibactam and Recently Approved β-Lactamase Inhibitor Combinations against Enterobacterales and Pseudomonas aeruginosa from Intensive Care Unit and Non-Intensive Care Unit Patients
Abstract
1. Introduction
2. Results
3. Discussion
4. Methods
4.1. Organism Collection
4.2. Susceptibility Testing
4.3. Screening for β-Lactamases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paramythiotou, E.; Routsi, C. Association between infections caused by multidrug-resistant gram-negative bacteria and mortality in critically ill patients. World J. Crit. Care Med. 2016, 5, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Kanj, S.S.; Kiratisin, P.; Rodrigues, C.; Van Duin, D.; Villegas, M.V.; Yu, Y. Early appropriate diagnostics and treatment of MDR Gram-negative infections. JAC Antimicrob. Resist. 2022, 4, dlac089. [Google Scholar] [CrossRef] [PubMed]
- Bonine, N.G.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Lodise, T. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status From Serious Gram-negative Bacterial Infections. Am. J. Med. Sci. 2019, 357, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Bonine, N.G. Antimicrobial Resistance or Delayed Appropriate Therapy-Does One Influence Outcomes More Than the Other Among Patients With Serious Infections Due to Carbapenem-Resistant Versus Carbapenem-Susceptible Enterobacteriaceae? Open Forum Infect. Dis. 2019, 6, ofz194. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Poulakou, G.; Ruppe, E.; Bouza, E.; Van Hal, S.J.; Brink, A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: A visionary approach. Intensive Care Med. 2017, 43, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Castanheira, M.; Mendes, R.E.; Flamm, R.K. Frequency and antimicrobial susceptibility of Gram-negative bacteria isolated from patients with pneumonia hospitalized in ICUs of US medical centres (2015–2017). J. Antimicrob. Chemother. 2018, 73, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Puzniak, L.A.; Dillon, R.J.; Shorr, A.F. The risk of inappropriate empiric treatment and its outcomes based on pathogens in non-ventilated (nvHABP), ventilated (vHABP) hospital-acquired and ventilator-associated (VABP) bacterial pneumonia in the US, 2012–2019. BMC Infect. Dis. 2022, 22, 775. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Sepulcri, C.; Giacobbe, D.R.; Peghin, M. Treatment of bloodstream infections due to Gram-negative bacteria with difficult-to-treat resistance. Antibiotics 2020, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum beta-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar]
- Sader, H.S.; Mendes, R.E.; Carvalhaes, C.G.; Kimbrough, J.H.; Castanheira, M. Changing Epidemiology of Carbapenemases Among Carbapenem-Resistant Enterobacterales From United States Hospitals and the Activity of Aztreonam-Avibactam Against Contemporary Enterobacterales (2019–2021). Open Forum Infect. Dis. 2023, 10, ofad046. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Cisneros, J.M.; Torre-Cisneros, J.; Rodriguez-Hernandez, M.J.; Tallon-Aguilar, L.; Calbo, E.; Horcajada, J.P.; Queckenberg, C.; Zettelmeyer, U.; Arenz, D.; et al. Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: Results from the REJUVENATE study. J. Antimicrob. Chemother. 2020, 75, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Duncan, L.R.; Arends, S.J.R.; Carvalhaes, C.G.; Castanheira, M. Antimicrobial activity of aztreonam-avibactam and comparator agents when tested against a large collection of contemporary Stenotrophomonas maltophilia isolates from medical centers worldwide. Antimicrob. Agents Chemother. 2020, 64, e01413–e01420. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M100Ed34. Performance Standards for Antimicrobial Susceptibility Testing: 34th Informational Supplement; Institute CaLS ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; Volume 100. [Google Scholar]
- FDA. Antibacterial Susceptibility Test Interpretive Criteria; Administration USFaD (ed): Washington, DC, USA, 2024. [Google Scholar]
- Ylipalosaari, P.; Ala-Kokko, T.I.; Laurila, J.; Ohtonen, P.; Syrjala, H. Intensive care acquired infection is an independent risk factor for hospital mortality: A prospective cohort study. Crit. Care 2006, 10, R66. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Gouel-Cheron, A.; Swihart, B.J.; Warner, S.; Mathew, L.; Strich, J.R.; Mancera, A.; Follmann, D.; Kadri, S.S. Epidemiology of ICU-Onset Bloodstream Infection: Prevalence, Pathogens, and Risk Factors among 150,948 ICU Patients at 85 U.S. Hospitals. Crit. Care Med. 2022, 50, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.; Zilberberg, M.D.; Shorr, A.F. Bloodstream Infection in the Intensive Care Unit: Evolving Epidemiology and Microbiology. Antibiotics 2024, 13, 123. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Duncan, L.; Kimbrough, J.H.; Carvalhaes, C.G.; Castanheira, M. Ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam activities against multidrug-resistant Enterobacterales from United States Medical Centers (2018–2022). Diagn. Microbiol. Infect. Dis. 2023, 106, 115945. [Google Scholar] [CrossRef]
- Boattini, M.; Comini, S.; Bianco, G.; Iannaccone, M.; Casale, R.; Cavallo, R.; Costa, C. Activity of cefiderocol and synergy of novel beta-lactam-beta-lactamase inhibitor-based combinations against metallo-beta-lactamase-producing gram-negative bacilli: Insights from a two-year study (2019–2020). J. Chemother. 2023, 35, 198–204. [Google Scholar] [CrossRef]
- Simner, P.J.; Mostafa, H.H.; Bergman, Y.; Ante, M.; Tekle, T.; Adebayo, A.; Beisken, S.; Dzintars, K.; Tamma, P.D. Progressive Development of Cefiderocol Resistance in Escherichia coli During Therapy is Associated With an Increase in blaNDM-5 Copy Number and Gene Expression. Clin. Infect. Dis. 2022, 75, 47–54. [Google Scholar] [CrossRef]
- Sader, H.S.; Huband, M.D.; Castanheira, M.; Flamm, R.K. Pseudomonas aeruginosa antimicrobial susceptibility results from four years (2012 to 2015) of the International Network for Optimal Resistance Monitoring program in the United States. Antimicrob. Agents Chemother. 2017, 61, e02252. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Eleventh Edition M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- EUCAST. Breakpoint Tables for Interpretation of MIC’s and Zone Diameters Version 14.0. The European Committee on Antimicrobial Susceptibility Testing; EUCAST: Basel, Switzerland, 2024. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
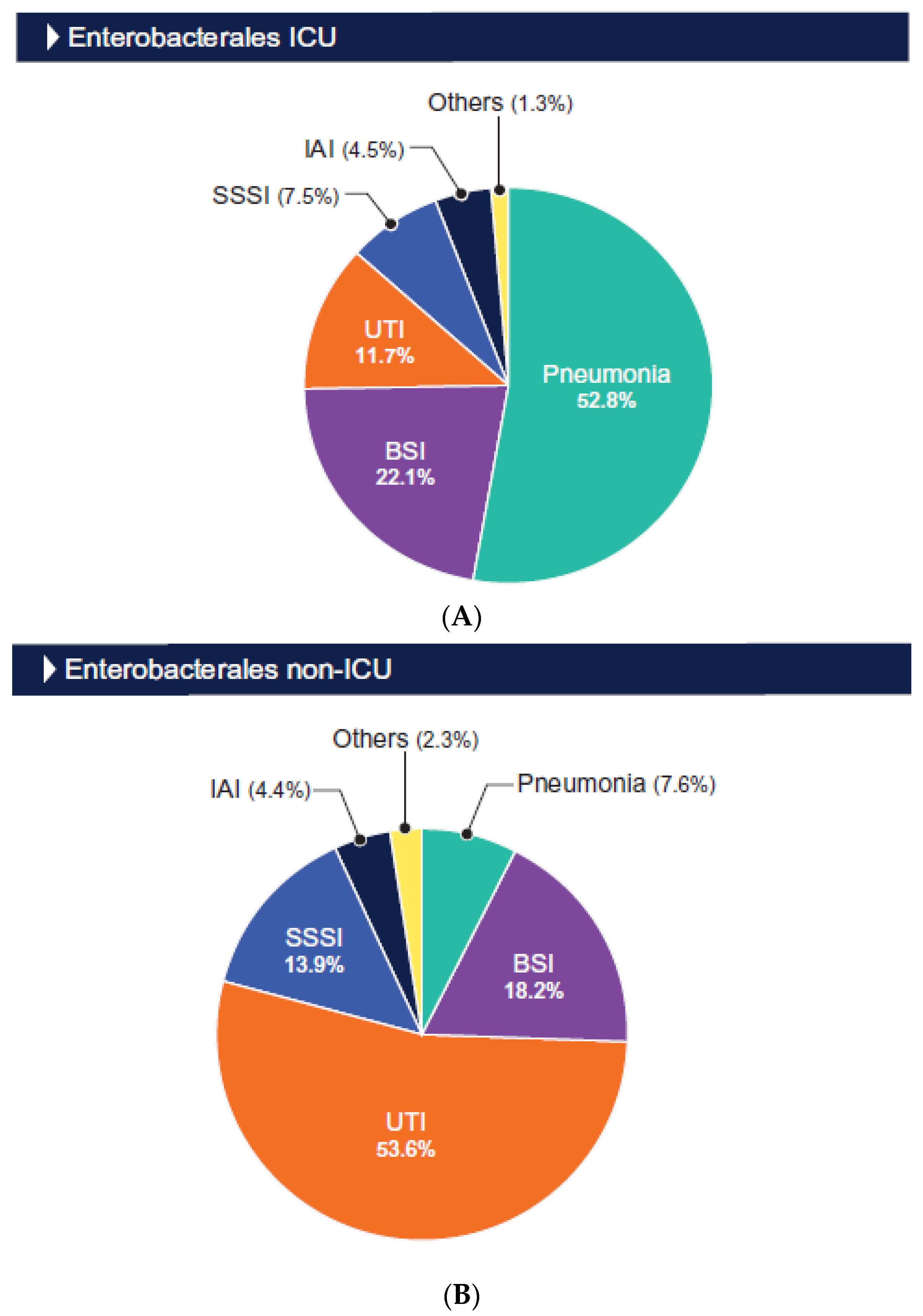


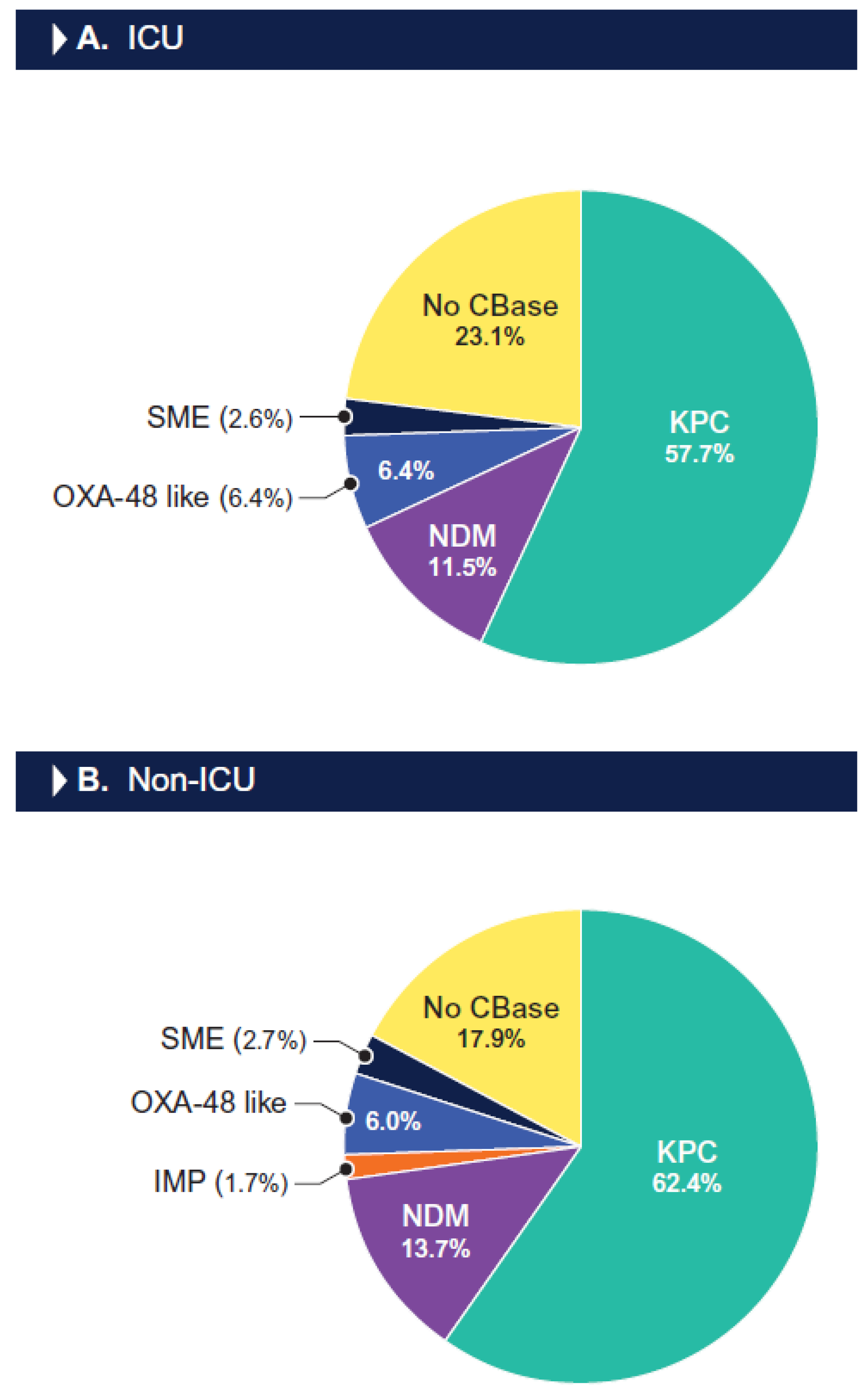
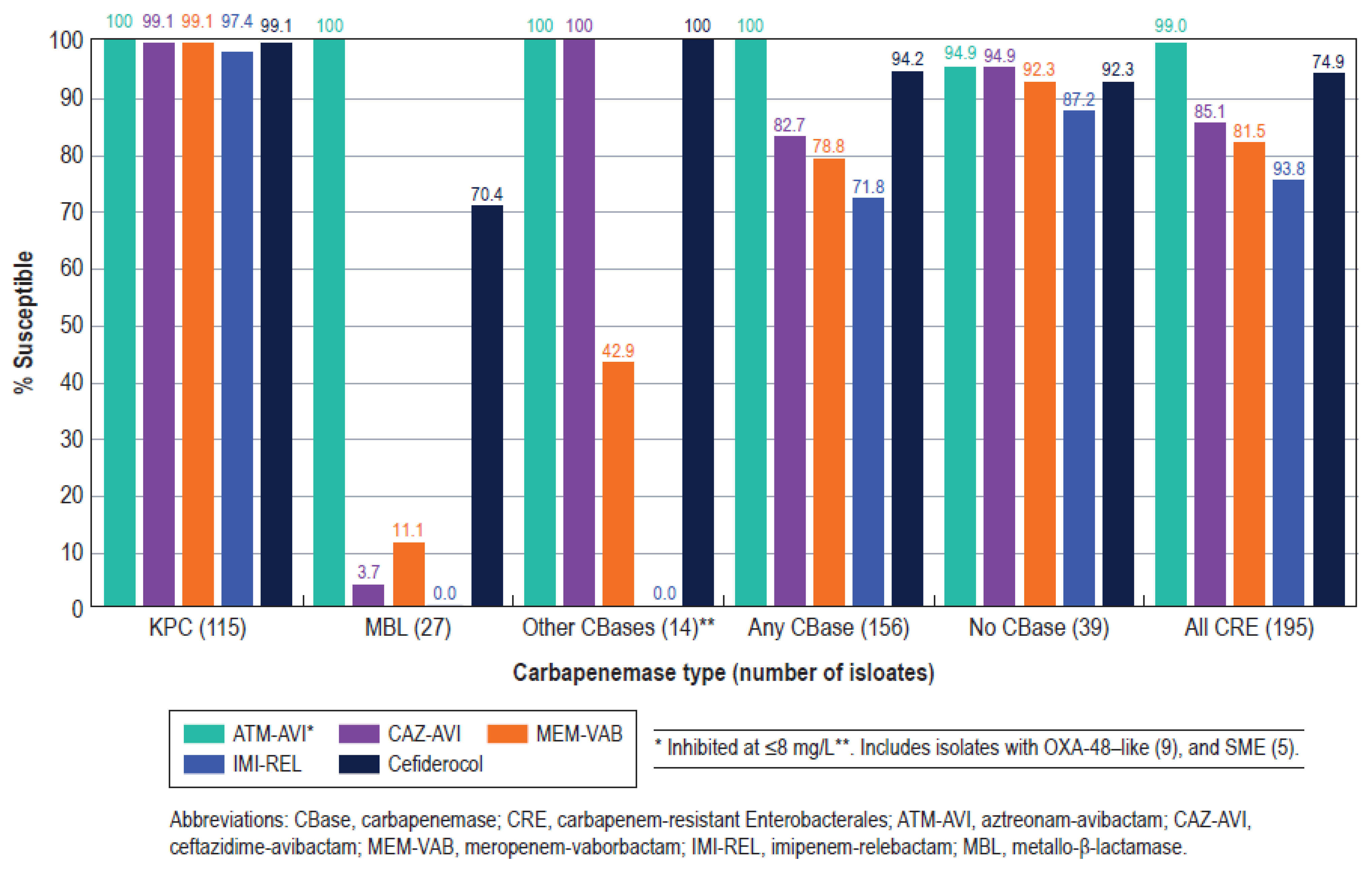
| Organism/ | % Susceptible (No. of Isolates Tested) a | ||
|---|---|---|---|
| Antimicrobial Agent | ICU | VAP | Non-ICU |
| Enterobacterales | (4117) | (630) | (17,879) |
| Aztreonam/avibactam | 100.0/>99.9 b | 100.0/99.8 b | >99.9/99.9 b |
| Ceftazidime/avibactam | 99.7 | 99.5 | 99.9 |
| Ceftolozane/tazobactam | 90.1 | 86.6 | 95.5 |
| Meropenem/vaborbactam | 99.7 | 99.8 | 99.9 |
| Imipenem/relebactam | 94.4 c | 96.7 c | 91.9 c |
| Piperacillin/tazobactam | 82.4 | 78.5 | 90.5 |
| Ceftazidime | 81.7 | 81.2 | 88.1 |
| Ceftriaxone | 77.2 | 76.2 | 84.6 |
| Meropenem | 98.0 | 97.9 | 99.3 |
| Imipenem | 92.1 | 94.1 | 89.8 |
| Levofloxacin | 84.8 | 89.3 | 83.5 |
| Gentamicin | 91.3 | 93.3 | 92.1 |
| Amikacin | 94.9 | 96.5 | 95.1 |
| Tigecycline | 96.6 | 95.5 | 95.5 |
| Colistin | 77.0 d | 77.1 d | 78.6 d |
| CRE | (78) | (15) | (117) |
| Aztreonam/avibactam | 100.0/98.7 b | 100.0/100.0 b | 98.3/96.6 b |
| Ceftazidime/avibactam | 88.5 | 93.3 | 82.9 |
| Meropenem/vaborbactam | 82.1 | 93.3 | 81.2 |
| Imipenem/relebactam | 78.2 c | 93.3 c | 72.6 c |
| Cefiderocol | 92.3 | 100.0 | 94.9 |
| Levofloxacin | 38.5 | 53.3 | 32.5 |
| Gentamicin | 60.3 | 60.0 | 64.1 |
| Amikacin | 69.2 | 73.3 | 65.8 |
| Tigecycline | 96.2 | 86.7 | 94.9 |
| Colistin | 83.3 d | 73.3 d | 82.1 d |
| MDR Enterobacterales | (979) | (149) | (3057) |
| Aztreonam/avibactam | 100.0/99.8 b | 100.0/100.0 b | 99.9/99.7 b |
| Ceftazidime/avibactam | 98.7 | 98.0 | 99.1 |
| Ceftolozane/tazobactam | 58.6 | 43.6 | 73.7 |
| Meropenem/vaborbactam | 98.6 | 99.3 | 99.3 |
| Imipenem/relebactam | 96.1 c | 97.2 c | 96.4 c |
| Piperacillin/tazobactam | 36.8 | 22.1 | 52.6 |
| Ceftazidime | 26.8 | 23.5 | 37.3 |
| Ceftriaxone | 15.4 | 8.7 | 27.5 |
| Meropenem | 91.7 | 91.3 | 96.0 |
| Imipenem | 90.0 | 89.3 | 93.2 |
| Levofloxacin | 56.6 | 68.5 | 45.0 |
| Gentamicin | 68.9 | 77.2 | 63.4 |
| Amikacin | 86.1 | 93.3 | 85.1 |
| Tigecycline | 96.3 | 92.6 | 96.4 |
| Colistin | 87.3 d | 88.6 d | 90.8 d |
| P. aeruginosa | (1304) | (308) | (2770) |
| Aztreonam/avibactam | 78.0 e | 75.6 e | 81.9 e |
| Ceftazidime/avibactam | 96.3 | 95.8 | 97.6 |
| Ceftolozane/tazobactam | 97.2 | 97.1 | 98.4 |
| Meropenem/vaborbactam | 90.0 f | 87.0 f | 94.3 f |
| Imipenem/relebactam | 97.1 | 95.5 | 98.0 |
| Piperacillin/tazobactam | 77.8 | 75.6 | 84.6 |
| Ceftazidime | 81.4 | 80.8 | 87.8 |
| Cefepime | 84.7 | 83.1 | 89.0 |
| Meropenem | 76.9 | 70.8 | 85.8 |
| Imipenem | 77.4 | 73.7 | 84.3 |
| Levofloxacin | 73.3 | 68.3 | 73.2 |
| Tobramycin | 92.1 | 90.9 | 92.1 |
| Colistin | 99.8 d | 100.0 d | 99.7 d |
| Organism/ | % Susceptible (No. of Isolates Tested) a | ||
|---|---|---|---|
| Antimicrobial Agent | ICU | VAP | Non-ICU |
| K. pneumoniae | (949) | (145) | (3581) |
| Aztreonam/avibactam | 100.0/100.0 b | 100.0/100.0 b | 100.0/100.0 b |
| Ceftazidime/avibactam | 99.7 | 100.0 | 99.7 |
| Ceftolozane/tazobactam | 92.8 | 93.1 | 96.0 |
| Meropenem/vaborbactam | 99.4 | 100.0 | 99.7 |
| Imipenem/relebactam | 98.8 c | 100.0 c | 99.1 c |
| Piperacillin/tazobactam | 79.6 | 80.0 | 87.8 |
| Ceftazidime | 80.2 | 84.8 | 86.1 |
| Ceftriaxone | 79.8 | 82.8 | 85.6 |
| Meropenem | 96.4 | 97.9 | 98.2 |
| Imipenem | 97.0 | 98.6 | 98.2 |
| Levofloxacin | 83.3 | 85.5 | 86.7 |
| Gentamicin | 89.9 | 92.4 | 92.2 |
| Amikacin | 96.1 | 97.2 | 98.3 |
| Tigecycline | 97.7 | 93.1 | 98.2 |
| Colistin | 97.5 d | 97.9 d | 98.0 d |
| E. coli | (898) | (103) | (6367) |
| Aztreonam/avibactam | 100.0/100.0 b | 100.0/100.0 b | >99.9/99.9 b |
| Ceftazidime/avibactam | 100.0 | 100.0 | 99.9 |
| Ceftolozane/tazobactam | 97.5 | 99.0 | 98.9 |
| Meropenem/vaborbactam | 100.0 | 100.0 | 99.9 |
| Imipenem/relebactam | 100.0 c | 100.0 c | 99.9 c |
| Piperacillin/tazobactam | 92.3 | 89.2 | 95.3 |
| Ceftazidime | 82.6 | 85.4 | 89.3 |
| Ceftriaxone | 79.1 | 82.5 | 87.0 |
| Meropenem | 99.9 | 100.0 | 99.9 |
| Imipenem | 100.0 | 100.0 | 99.8 |
| Levofloxacin | 71.7 | 79.6 | 76.1 |
| Gentamicin | 86.0 | 88.3 | 89.9 |
| Amikacin | 90.3 | 94.2 | 91.8 |
| Tigecycline | 100.0 | 100.0 | 100.0 |
| Colistin | 99.3 d | 100.0 d | 99.7 d |
| E. cloacae | (515) | (96) | (1498) |
| Aztreonam/avibactam | 100.0/99.8 b | 100.0/100.0 b | 100.0/99.9 b |
| Ceftazidime/avibactam | 98.6 | 99.0 | 99.7 |
| Ceftolozane/tazobactam | 69.3 | 57.3 | 80.5 |
| Meropenem/vaborbactam | 98.6 | 100.0 | 99.9 |
| Imipenem/relebactam | 97.7 c | 98.5 c | 99.9 c |
| Piperacillin/tazobactam | 63.8 | 54.2 | 74.9 |
| Ceftazidime | 62.7 | 53.1 | 73.6 |
| Ceftriaxone | 59.8 | 50.0 | 69.6 |
| Meropenem | 97.1 | 96.9 | 98.8 |
| Imipenem | 96.5 | 95.8 | 97.9 |
| Levofloxacin | 93.2 | 97.9 | 93.2 |
| Gentamicin | 95.7 | 99.0 | 96.7 |
| Amikacin | 98.4 | 99.0 | 99.1 |
| Tigecycline | 97.9 | 96.9 | 98.1 |
| Colistin | 79.7 d | 80.2 d | 80.4 d |
| Susceptibility by Resistance Phenotype (No. of Isolates) | |||
|---|---|---|---|
| Antimicrobial Agent | Ceftazidime/Avibactam-Resistant (39) a | Meropenem/Vaborbactam Non-Susceptible (36) a | Imipenem/Relebactam-Resistant (225) a |
| Aztreonam/avibactam | 97.4/87.2 b | 100.0/94.4 b | 100.0/99.6 b |
| Ceftazidime/avibactam | 0.0 | 33.3 | 88.9 |
| Meropenem/vaborbactam | 38.5 | 0.0 | 85.3 |
| Imipenem/relebactam | 20.0 | 8.3 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sader, H.S.; Mendes, R.E.; Kimbrough, J.H.; Hubler, C.M.; Castanheira, M. Activity of Aztreonam/Avibactam and Recently Approved β-Lactamase Inhibitor Combinations against Enterobacterales and Pseudomonas aeruginosa from Intensive Care Unit and Non-Intensive Care Unit Patients. Antibiotics 2024, 13, 564. https://doi.org/10.3390/antibiotics13060564
Sader HS, Mendes RE, Kimbrough JH, Hubler CM, Castanheira M. Activity of Aztreonam/Avibactam and Recently Approved β-Lactamase Inhibitor Combinations against Enterobacterales and Pseudomonas aeruginosa from Intensive Care Unit and Non-Intensive Care Unit Patients. Antibiotics. 2024; 13(6):564. https://doi.org/10.3390/antibiotics13060564
Chicago/Turabian StyleSader, Helio S., Rodrigo E. Mendes, John H. Kimbrough, Cory M. Hubler, and Mariana Castanheira. 2024. "Activity of Aztreonam/Avibactam and Recently Approved β-Lactamase Inhibitor Combinations against Enterobacterales and Pseudomonas aeruginosa from Intensive Care Unit and Non-Intensive Care Unit Patients" Antibiotics 13, no. 6: 564. https://doi.org/10.3390/antibiotics13060564
APA StyleSader, H. S., Mendes, R. E., Kimbrough, J. H., Hubler, C. M., & Castanheira, M. (2024). Activity of Aztreonam/Avibactam and Recently Approved β-Lactamase Inhibitor Combinations against Enterobacterales and Pseudomonas aeruginosa from Intensive Care Unit and Non-Intensive Care Unit Patients. Antibiotics, 13(6), 564. https://doi.org/10.3390/antibiotics13060564





