Biphasic Medium Using Nicotinamide for Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis
Abstract
1. Introduction
2. Results
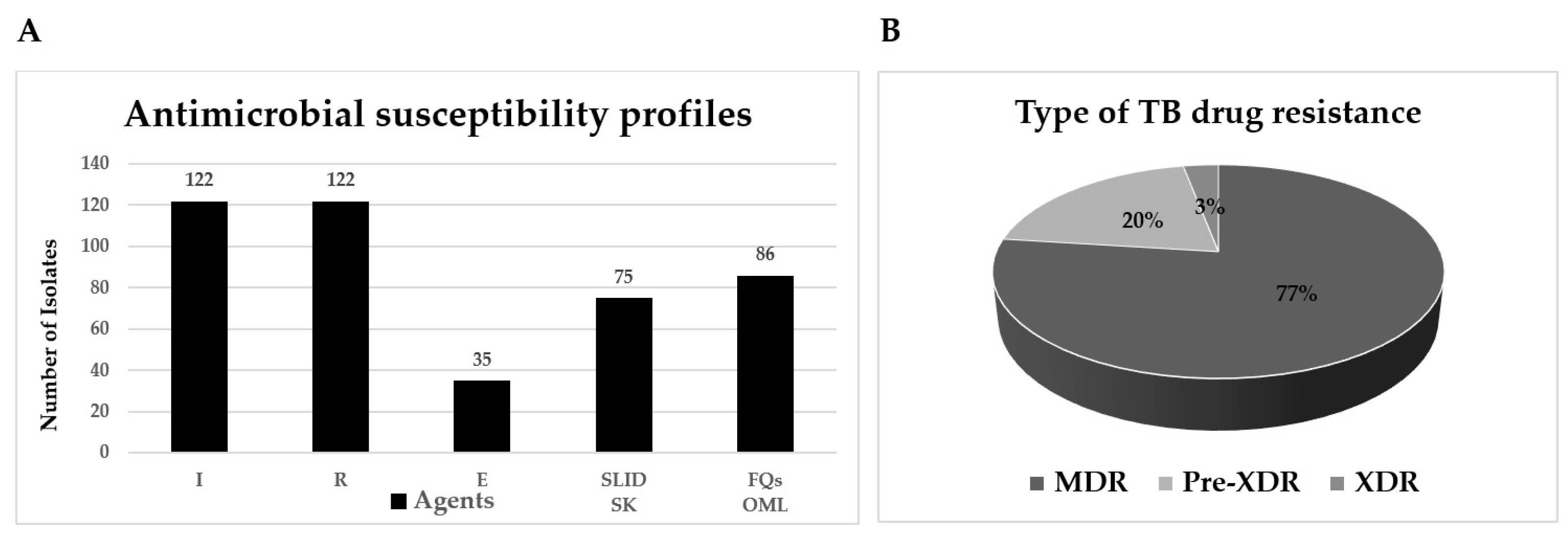
2.1. Antimicrobial Susceptibility Testing
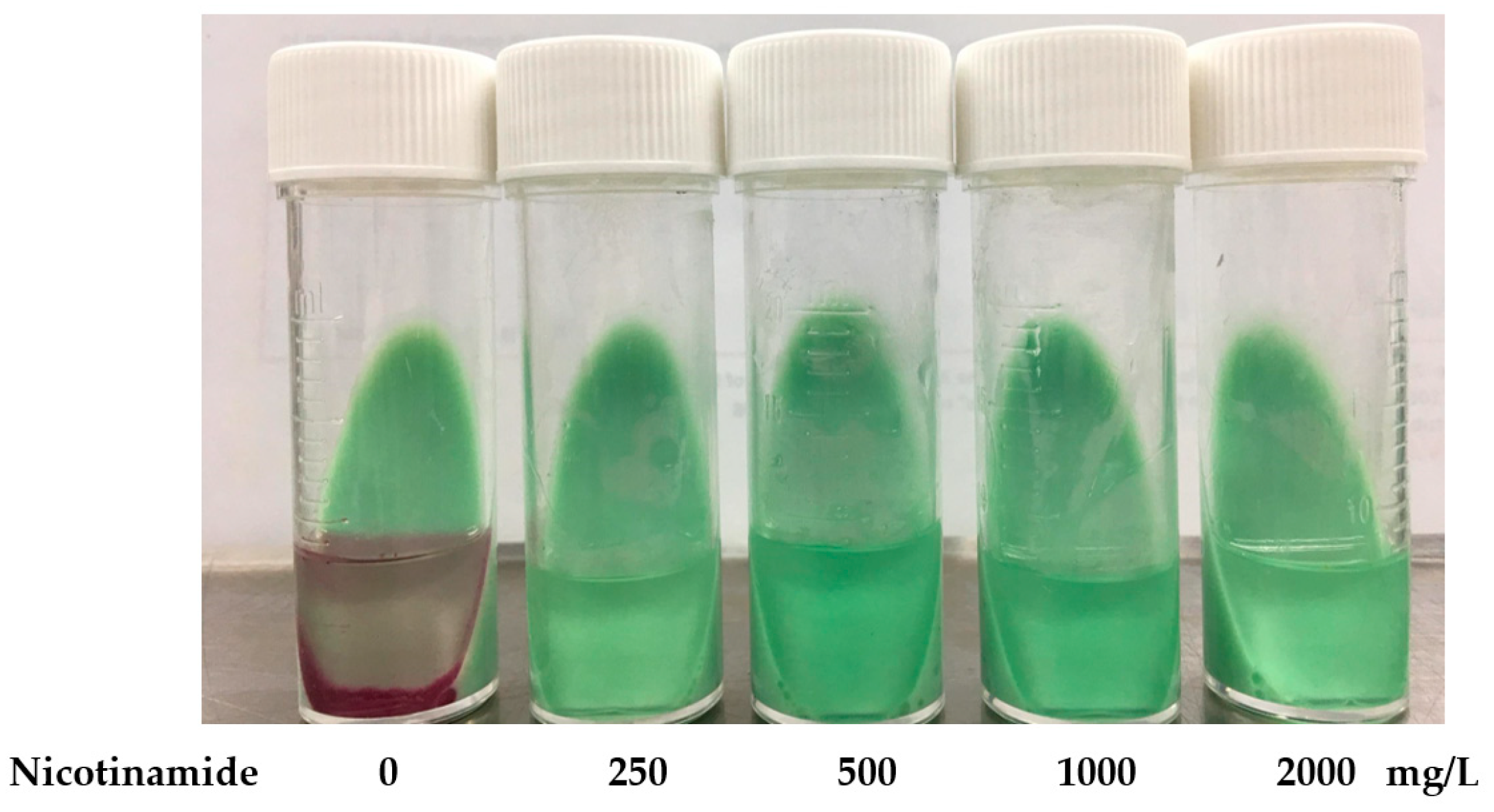
2.2. Setting Up the Cut-Off Point of NIC-BMA for PZA Susceptibility Testing
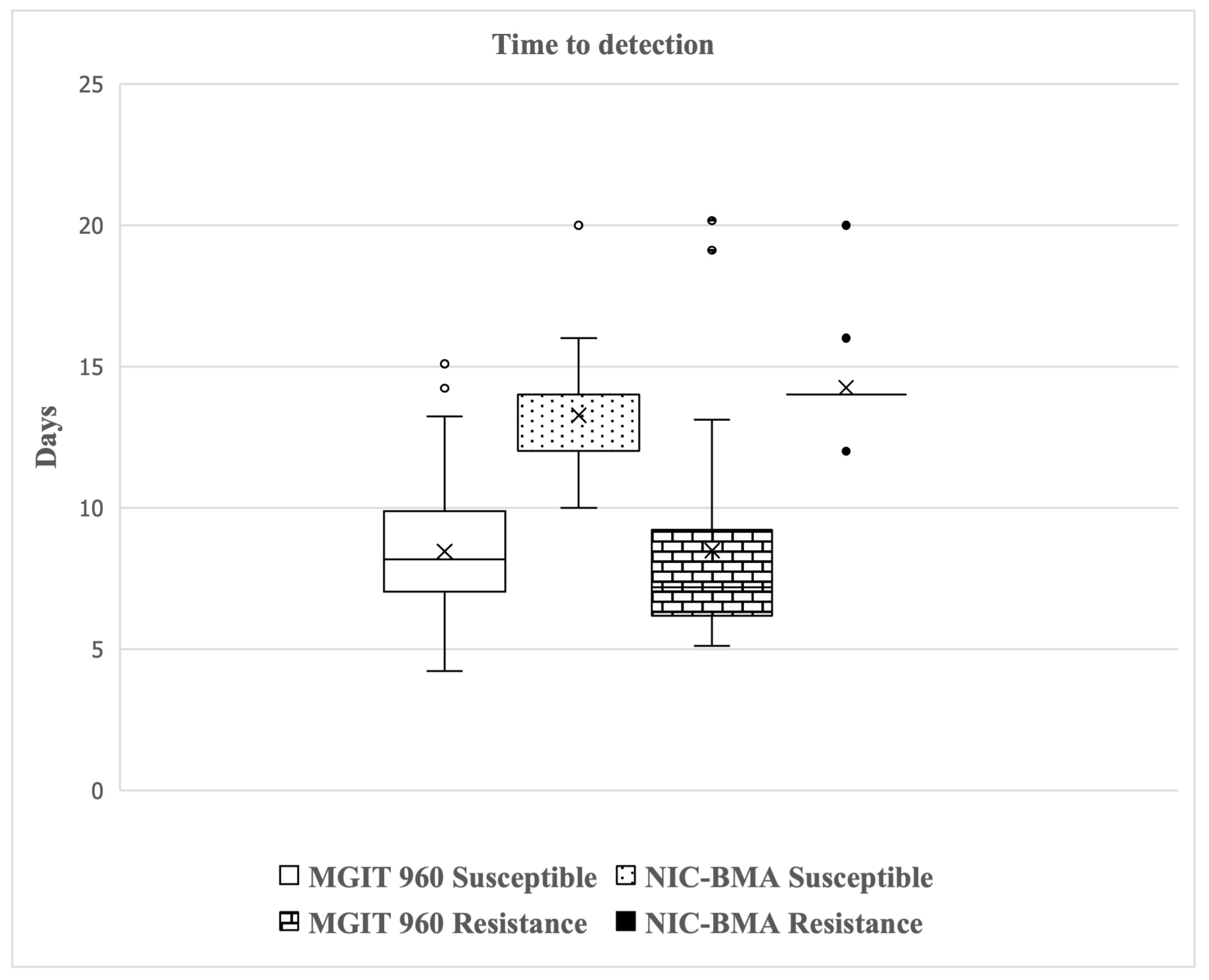
2.3. Validation of NIC-BMA and MGIT 960 System for PZA-Susceptibility Testing
2.4. pncA Gene Mutation
3. Discussion
4. Materials and Methods
4.1. M. tuberculosis Isolates
4.2. PZA Susceptibility Testing by BACTEC MGIT 960
4.3. Biphasic Medium Assay (BMA)
4.4. pncA Amplification and Sequencing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Treatment Guidelines for Drug-Resistant Tuberculosis; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Zhang, Y.; Shi, W.; Zhang, W.; Mitchison, D. Mechanisms of Pyrazinamide Action and Resistance. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chiu Chang, K.; Leung, C.C.; Wai Yew, W.; Gicquel, B.; Fallows, D.; Kaplan, G.; Chaisson, R.E.; Zhang, W. ZS-MDR-TB’versus ‘ZR-MDR-TB’: Improving Treatment of MDR-TB by Identifying Pyrazinamide Susceptibility. Emerg. Microbes Infect. 2012, 1, e5. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, M.G.; Soeters, H.M.; Warren, R.M.; York, T.; Sampson, S.L.; Streicher, E.M.; Van Helden, P.D.; Van Rie, A. A Global Perspective on Pyrazinamide Resistance: Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0133869. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Yew, W.-W.; Zhang, Y. Pyrazinamide Is a Two-Edged Sword: Do WHO Guidelines Matter? Antimicrob. Agents Chemother. 2018, 62, e01907-17. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Busby, S.M.; Valafar, F. Systematic Review of Mutations in Pyrazinamidase Associated with Pyrazinamide Resistance in Mycobacterium tuberculosis Clinical Isolates. Antimicrob. Agents Chemother. 2015, 59, 5267–5277. [Google Scholar] [CrossRef] [PubMed]
- Miotto, P.; Cabibbe, A.M.; Feuerriegel, S.; Casali, N.; Drobniewski, F.; Rodionova, Y.; Bakonyte, D.; Stakenas, P.; Pimkina, E.; Augustynowicz-Kopeć, E. Mycobacterium tuberculosis Pyrazinamide Resistance Determinants: A Multicenter Study. MBio 2014, 5, e01819-14. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.P.; Tyrrell, F.C.; Baynham, D.; Escuyer, V.E.; Green, N.; Kim, Y.; Longley-Olson, P.A.; Parrish, N.; Pennington, C.; Tan, D. Using Reduced Inoculum Densities of Mycobacterium tuberculosis in MGIT Pyrazinamide Susceptibility Testing to Prevent False-Resistant Results and Improve Accuracy: A Multicenter Evaluation. Tuberc. Res. Treat. 2017, 2017, 3748163. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.; Malone, L.; Kushner, S.; Oleson, J.J.; Subbarow, Y. The Effect of Nicotinic Acid Amide on Experimental Tuberculosis of White Mice. J. Lab. Clin. Med. 1948, 33, 1249–1253. [Google Scholar] [PubMed]
- Mirabal, N.C.; Yzquierdo, S.L.; Lemus, D.; Madruga, M.; Milián, Y.; Echemendía, M.; Takiff, H.; Martin, A.; Van der Stuyf, P.; Palomino, J.C. Evaluation of Colorimetric Methods Using Nicotinamide for Rapid Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 2010, 48, 2729–2733. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Wu, X.; Luo, J.; Fu, Y.; Zhao, L.; Ma, Y.; Li, Y.; Liang, Q.; Shang, Y.; Huang, H. Detection of Pyrazinamide Resistance of Mycobacterium tuberculosis Using Nicotinamide as a Surrogate. Clin. Microbiol. Infect. 2017, 23, 835–838. [Google Scholar] [CrossRef]
- Martin, A.; Cubillos-Ruiz, A.; Von Groll, A.; Del Portillo, P.; Portaels, F.; Palomino, J.C. Nitrate Reductase Assay for the Rapid Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis Using Nicotinamide. J. Antimicrob. Chemother. 2008, 61, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Takiff, H.; Vandamme, P.; Swings, J.; Palomino, J.C.; Portaels, F. A New Rapid and Simple Colorimetric Method to Detect Pyrazinamide Resistance in Mycobacterium tuberculosis Using Nicotinamide. J. Antimicrob. Chemother. 2006, 58, 327–331. [Google Scholar] [CrossRef]
- Akbal, A.U.; Durupinar, B.; Coban, A.Y. Colorimetric Methods for Rapid Determination of Pyrazinamide Resistance. Int. J. Mycobacteriology 2020, 9, 274–280. [Google Scholar] [CrossRef]
- Li, H.; Zhou, L.P.; Luo, J.; Yu, J.P.; Yang, H.; Wei, H.P. Rapid Colorimetric Pyrazinamide Susceptibility Testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 462–467. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Farnia, P.; Ghazvini, K.; Behdani, M.; Rashed, T.; Ghanaat, J. Rapid and Low-Cost Colorimetric Method Using 2, 3, 5-Triphenyltetrazolium Chloride for Detection of Multidrug-Resistant Mycobacterium tuberculosis. J. Med. Microbiol. 2006, 55, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Coban, A.Y.; Akbal, A.U.; Uzun, M.; Durupinar, B. Evaluation of Four Colourimetric Susceptibility Tests for the Rapid Detection of Multidrug-Resistant Mycobacterium tuberculosis Isolates. Mem. Inst. Oswaldo Cruz 2015, 110, 649–654. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, J.; Zhu, C.; Huang, X.; Lu, J.; Wang, Q.; Chen, Z.; Wang, J.; Zhang, Y.; Gu, D. Evaluation of a Novel Biphasic Culture Medium for Recovery of Mycobacteria: A Multi-Center Study. PLoS ONE 2012, 7, e36331. [Google Scholar] [CrossRef][Green Version]
- Gonzalo, X.; Drobniewski, F.; Hoffner, S.; Werngren, J. Evaluation of a Biphasic Media Assay for Pyrazinamide Drug Susceptibility Testing of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2014, 69, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. In: Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational. 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 1 December 2019).
- Lee, S.M.; Kim, K.J.; Chang, C.L. Detection of Rifampicin Resistance in Mycobacterium tuberculosis by Using Middlebrook 7H9 Broth Medium with 2, 3-Diphenyl-5-Thienyl-(2)-Tetrazolium Chloride. Ann. Clin. Microbiol. 2018, 21, 47–50. [Google Scholar] [CrossRef]
- den Hertog, A.L.; Menting, S.; Pfeltz, R.; Warns, M.; Siddiqi, S.H.; Anthony, R.M. Pyrazinamide Is Active against Mycobacterium tuberculosis Cultures at Neutral PH and Low Temperature. Antimicrob. Agents Chemother. 2016, 60, 4956–4960. [Google Scholar] [CrossRef][Green Version]
- Piersimoni, C.; Mustazzolu, A.; Iacobino, A.; Giannoni, F.; Santoro, G.; Gherardi, G.; Del Giudice, A.; Perna, R.; Fattorini, L. Pyrazinamide Susceptibility Testing: Proposed New Standard with the BACTECTM MGITTM 960 System. Int. J. Tuberc. Lung Dis. 2016, 20, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Jonmalung, J.; Prammananan, T.; Leechawengwongs, M.; Chaiprasert, A. Surveillance of Pyrazinamide Susceptibility among Multidrug-Resistant Mycobacterium tuberculosis Isolates from Siriraj Hospital, Thailand. BMC Microbiol. 2010, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Cancino-Muñoz, I.; Moreno-Molina, M.; Furió, V.; Goig, G.A.; Torres-Puente, M.; Chiner-Oms, Á.; Villamayor, L.M.; Sanz, F.; Guna-Serrano, M.R.; Comas, I. Cryptic Resistance Mutations Associated with Misdiagnoses of Multidrug-Resistant Tuberculosis. J. Infect. Dis. 2019, 220, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Zignol, M.; Dean, A.S.; Alikhanova, N.; Andres, S.; Cabibbe, A.M.; Cirillo, D.M.; Dadu, A.; Dreyer, A.; Driesen, M.; Gilpin, C. Population-Based Resistance of Mycobacterium tuberculosis Isolates to Pyrazinamide and Fluoroquinolones: Results from a Multicountry Surveillance Project. Lancet Infect. Dis. 2016, 16, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Petrella, S.; Gelus-Ziental, N.; Maudry, A.; Laurans, C.; Boudjelloul, R.; Sougakoff, W. Crystal Structure of the Pyrazinamidase of Mycobacterium tuberculosis: Insights into Natural and Acquired Resistance to Pyrazinamide. PLoS ONE 2011, 6, e15785. [Google Scholar] [CrossRef] [PubMed]
- Juréen, P.; Werngren, J.; Toro, J.-C.; Hoffner, S. Pyrazinamide Resistance and PncA Gene Mutations in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 1852–1854. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mishra, A.K.; Malonia, S.K.; Chauhan, D.S.; Sharma, V.D.; Venkatesan, K.; Katoch, V.M. The Paradox of Pyrazinamide: An Update on the Molecular Mechanisms of Pyrazinamide Resistance in Mycobacteria. J. Commun. Dis. 2006, 38, 288. [Google Scholar] [PubMed]
- Streicher, E.M.; Maharaj, K.; York, T.; Van Heerden, C.; Barnard, M.; Diacon, A.; Mendel, C.M.; Bosman, M.E.; Hepple, J.A.; Pym, A.S. Rapid Sequencing of the Mycobacterium tuberculosis PncA Gene for Detection of Pyrazinamide Susceptibility. J. Clin. Microbiol. 2014, 52, 4056–4057. [Google Scholar] [CrossRef]
- Yadon, A.N.; Maharaj, K.; Adamson, J.H.; Lai, Y.-P.; Sacchettini, J.C.; Ioerger, T.R.; Rubin, E.J.; Pym, A.S. A Comprehensive Characterization of PncA Polymorphisms That Confer Resistance to Pyrazinamide. Nat. Commun. 2017, 8, 588. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Cui, P.; Zhang, Y.; Zhang, W. Identification of Novel Efflux Proteins Rv0191, Rv3756c, Rv3008, and Rv1667c Involved in Pyrazinamide Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00940-17. [Google Scholar] [CrossRef]
- Buderer, N.M.F. Statistical Methodology: I. Incorporating the Prevalence of Disease into the Sample Size Calculation for Sensitivity and Specificity. Acad. Emerg. Med. 1996, 3, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Bwalya, P.; Yamaguchi, T.; Mulundu, G.; Nakajima, C.; Mbulo, G.; Solo, E.S.; Fukushima, Y.; Kasakwa, K.; Suzuki, Y. Genotypic Characterization of Pyrazinamide Resistance in Mycobacterium tuberculosis Isolated from Lusaka, Zambia. Tuberculosis 2018, 109, 117–122. [Google Scholar] [CrossRef] [PubMed]
| Routine DST Absolute Concentration (N) | PZA BACTEC MGIT 960 (N) | Number of Isolates at MIC of NIC-BMA | ||||
|---|---|---|---|---|---|---|
| <250 mg/L | 500 mg/L | 1000 mg/L | 2000 mg/L | >2000 mg/L | ||
| MDR-TB (122) | Resistance (40) | 0 | 0 | 7 | 12 | 21 |
| Susceptible (82) | 37 | 41 | 2 | 2 | 0 | |
| Susceptible (39) | Susceptible (39) | 26 | 13 | 0 | 0 | 0 |
| BACTEC MGIT 960 | NIC-BMA MIC ≥ 1000 mg/L | ||
|---|---|---|---|
| PZA Resistance | PZA Susceptible | Total | |
| PZA resistance | 40 | 0 | 40 |
| PZA susceptible | 4 | 117 | 121 |
| Total | 44 | 117 | 161 |
| MGIT 960 (No. of Isolates) | pncA Characterization (No. of Isolates) | pncA and Promoter Region Sequencing Outcomes (No. of Isolates) | NIC-BMA MIC (mg/L) (No. of Isolates) |
|---|---|---|---|
| Resistant (40) | Mutant pncA (37)
| - | <250 (0) |
| - | 500 (0) | ||
| A-11C R (1), D12A R (1), Y41STOP R (1), P62L R (1), INS-97 N (1), Y103STOP R (1), INS-150 R (1) | 1000 (7) | ||
| A-11G R (1), D12A R (1), T47P R (1), I90S R (1), G105V R (1), INS-132 N (1), INS-136 R (2), V139L R (1), INS-177 R (1), wild-type(2) | 2000 (12) | ||
| A-11C R (2), A-11G R (1), G24D R (1), L35P R (1), S67P R (1), W68G R (1), C72Y R (1), I90S R (2), S104R R (2), W119R R (1), L120R R (1), D126H + D129Y R (1), INS-136 R (1), V139A R (1), V139G R (1), DEL165-166 N (1), L172P R (1), wild-type(1) | >2000 (21) | ||
| Susceptible (82) | Mutant pncA (15)
| I31T S (4), D63P N (1), wild-type (32) | <250 (37) |
| I31T S (3), T61P I (3), V163A S (1), wild-type (34) | 500 (41) | ||
| D136G R (1), wild-type (1) | 1000 (2) | ||
| T61P I (1), F106L I (1) | 2000 (2) | ||
| - | >2000 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuansuwan, W.; Chuchottaworn, C.; Nakajima, C.; Suzuki, Y.; Chaichanawongsaroj, N. Biphasic Medium Using Nicotinamide for Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis. Antibiotics 2024, 13, 563. https://doi.org/10.3390/antibiotics13060563
Thuansuwan W, Chuchottaworn C, Nakajima C, Suzuki Y, Chaichanawongsaroj N. Biphasic Medium Using Nicotinamide for Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis. Antibiotics. 2024; 13(6):563. https://doi.org/10.3390/antibiotics13060563
Chicago/Turabian StyleThuansuwan, Waraporn, Charoen Chuchottaworn, Chie Nakajima, Yasuhiko Suzuki, and Nuntaree Chaichanawongsaroj. 2024. "Biphasic Medium Using Nicotinamide for Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis" Antibiotics 13, no. 6: 563. https://doi.org/10.3390/antibiotics13060563
APA StyleThuansuwan, W., Chuchottaworn, C., Nakajima, C., Suzuki, Y., & Chaichanawongsaroj, N. (2024). Biphasic Medium Using Nicotinamide for Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis. Antibiotics, 13(6), 563. https://doi.org/10.3390/antibiotics13060563






