Abstract
Both obstructive sleep apnea (OSA) and acute lower respiratory tract infections (LRTIs) are important global health issues. The pathophysiological links between OSA and LRTIs include altered immune responses due to chronic intermittent hypoxia and sleep fragmentation, increased aspiration risk, and a high burden of comorbidities. In this narrative review, we evaluated the current evidence on the association between OSA and the incidence and outcomes of acute LRTIs in adults, specifically community-acquired pneumonia and viral pneumonia caused by influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Studies have demonstrated that OSA patients are more likely to develop bacterial pneumonia and exhibit a higher risk of invasive pneumococcal disease. The risk intensifies with the severity of OSA, influencing hospitalization rates and the need for intensive care. OSA is also associated with an increased risk of contracting influenza and suffering more severe disease, potentially necessitating hospitalization. Similarly, OSA contributes to increased COVID-19 disease severity, reflected by higher rates of hospitalization, longer hospital stays, and a higher incidence of acute respiratory failure. The effect of OSA on mortality rates from these infections is, however, somewhat ambiguous. Finally, we explored antibiotic therapy for OSA patients with LRTIs, addressing care settings, empirical regimens, risks, and pharmacokinetic considerations. Given the substantial burden of OSA and its significant interplay with acute LRTIs, enhanced screening, targeted vaccinations, and optimized management strategies for OSA patients should be prioritized.
1. Introduction
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder, affecting nearly one billion people worldwide [1]. The prevalence of OSA has increased over the past decades and exceeds 50% in some countries; still, the majority of OSA patients remain unrecognized and undertreated [1,2]. OSA is characterized by repeated periods of complete or partial collapse of the upper airway that lead to oxygen desaturation or arousal from sleep [3]. This induces chronic intermittent hypoxia (IH), sleep fragmentation, and increased sympathetic nervous system activity, all of which have been shown to increase the risk of chronic organ damage [4,5]. Numerous reports confirm that OSA is associated with an increased risk and worse outcomes of chronic cardiovascular, pulmonary, metabolic, neurological, and mood disorders [6,7].
Acute lower respiratory tract infections (LRTIs) are infections within the respiratory tract below the larynx. In the adult population, LRTIs commonly refer to bacterial and viral pneumonia and bronchitis [8]. LRTIs continue to be a major health issue, causing substantial mortality, morbidity, and economic burden. In 2019, LRTIs claimed 2.6 million lives and were ranked as the fourth leading cause of death globally [9,10]. The recent Coronavirus Infectious Disease 2019 (COVID-19) pandemic has dramatically increased morbidity and mortality due to LRTIs and has raised awareness of viral LRTIs, especially in pandemic settings [11].
The susceptibility to respiratory infections and outcomes of patients with LRTIs are determined by the virulence of the organism and the host immune response [12]. Although many studies have proven that OSA induces a pro-inflammatory state [13], the relationship between OSA and respiratory infections has only recently been called to attention. The coexistence of OSA and chronic respiratory infections such as bronchiectasis and cystic fibrosis is common and may worsen patient outcomes by enhancement of pro-inflammatory stimuli [14]. This relationship goes beyond the association between OSA and chronic respiratory infections, as the increasing body of evidence suggests that OSA may also have an important role in the susceptibility to, pathogenesis of, and outcomes of acute respiratory infections. Given the high burden of both OSA and acute LRTIs in the general population, their potential link is critical to explore.
This review aims to evaluate the current evidence on the association of obstructive sleep apnea with the incidence and outcomes of acute lower respiratory tract infections in adults. Specifically, we focus on the relationship between OSA and community-acquired pneumonia (CAP), as well as viral pneumonia caused by influenza viruses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
2. Literature Search Strategy
The search strategy was structured to evaluate the relationship between OSA and bacterial, influenza, and COVID-19 pneumonia. The search was conducted using the PubMed database on 10 January 2024, without any restrictions on the date of publication. The literature search was repeated on 15 April 2024, to screen for any newly published papers that might have been missed by the initial search. The search was conducted using a set of keywords detailed in Table 1. Original research articles that focused on OSA as a potential risk factor for acquiring acute LRTIs or experiencing higher severity or adverse outcomes of these infections were included. Authors excluded publications not available in English, narrative reviews, letters to the editor, and abstracts for which the full text was not available. An overview of the study selection is presented in Figure S1, an online data supplement.

Table 1.
Keywords used for the search strategy of PubMed database.
The initial screening process involved evaluating the titles and abstracts of the retrieved articles, which was independently conducted by both authors (MN, MV). In instances where discrepancies arose regarding the inclusion or exclusion of specific studies, the authors had an in-depth discussion to resolve conflicts. During these discussions, authors performed a full-text review of the articles in question to make an optimal informed decision. Additional studies that were not captured through the primary database search were included after manually examining the reference lists of the selected articles. The data extraction process for the selected studies included the authors and date of publication, the title of the study, its aim, design, study groups, inclusion and exclusion criteria, outcomes, an overview of the key findings, and any identified study limitations. This extraction process was divided between the two authors (MN, MV). To guarantee consistency and reliability in the data extraction process, both authors conducted oversight of each other’s data extraction. The summary of the most relevant literature on the association of OSA and LRTIs is presented in Table 2.

Table 2.
The summary of the most relevant literature on the association between OSA and LRTIs.
3. Obstructive Sleep Apnea and Community-Acquired Pneumonia
Our review identified six studies evaluating the risk of contracting and severity of community-acquired pneumonia in OSA patients (Table 2). Out of those six, five studies supported that OSA increases the risk of bacterial pneumonia [15,16,17,18,19]. Patients with OSA are found to have up to a 3-fold increased risk of pneumonia and a 5-fold increased risk of invasive pneumococcal disease [15,16,18]. The risk of pneumonia increases with OSA severity in a dose–response manner, with the highest risk observed in patients with severe OSA [17,18,19].
Hospitalized patients with pneumonia who have OSA tend to be about ten years younger and have a higher burden of comorbidities, such as obesity, chronic respiratory diseases, and heart failure, compared to patients without OSA [20]. Comorbidities, especially asthma and chronic obstructive pulmonary disease (COPD), as well as older age, increase the risk of LRTIs and LRTI recurrence in OSA patients [15]. While the relationship between OSA and pneumonia seems to be independent of baseline demographics and smoking, the confounding effects of obesity and multimorbidity are less clear. Most studies did not account for these [16] or have yielded mixed results [17,19]. In the Atherosclerosis Risk in Communities (ARIC) prospective cohort study with a median follow-up of 20.4 years, severe OSA increased the risk of pneumonia hospitalization by 87% after adjustment for baseline demographics and lifestyle, but this association was attenuated and became non-significant in models that included body mass index (BMI) or chronic respiratory conditions such as COPD and asthma [17]. Conversely, in a large retrospective cohort study from Taiwan, OSA was associated with a 20% increased risk of incident pneumonia independent of comorbidities including pre-existing diabetes mellitus, hypertension, coronary heart disease, heart failure, cerebrovascular disease, dementia, epilepsy, Parkinson’s disease, chronic kidney disease, liver cirrhosis, gastroesophageal reflux disease, cancer, asthma, COPD, and tuberculosis [19]. Notably, the ARIC study found that a hypoxic burden, represented by the time with oxygen saturation below 90% during total sleep time (T90) of more than 5% versus less than 1%, was associated with a 50% increased risk of pneumonia. This association was observed in models that accounted for BMI, suggesting that the hypoxic burden may capture the physiological consequences of OSA on LRTIs that extend beyond the effects of obesity alone [17].
Two out of six studies evaluated the association between OSA and severity of LRTIs [18,20] (Table 2). A significant moderate positive correlation between the apnea–hypopnea index (AHI) and the pneumonia severity index (PSI) levels was shown in a study by Chiner et al. [18]. A retrospective cohort study of patients with pneumonia at 347 United States hospitals showed that OSA is associated with the risk of more severe CAP, albeit with the same or lesser mortality. Patients with OSA were twice as likely to require invasive mechanical ventilation (IMV) at the time of admission and had more than a 50% increased likelihood of clinical deterioration requiring intensive care unit (ICU) admission and IMV later in the hospital stay [20]. The observed same or lower mortality compared to non-OSA patients may be related to the high prevalence of obesity in OSA and the so-called “obesity paradox,” by which obese patients may have a survival benefit likely due to increased metabolic reserve and more intense monitoring [43,44].
As OSA is associated with pneumonia severity, identifying OSA in pneumonia patients is important. However, the lack of somnolence, measured by the Epworth Sleepiness Scale in patients with OSA who have pneumonia [18], suggests that the level of daytime sleepiness is not a reliable way to screen for OSA in these patients. While the appropriateness of other questionnaires that, in addition to subjective symptoms, include anthropometric measurements such as STOP-Bang remains to be determined, performing a sleep study continues to be the preferred method to diagnose OSA in patients at risk for pneumonia.
4. Obstructive Sleep Apnea and Influenza Pneumonia
The influenza virus is highly transmissible. It can evade the immune system through antigenic drifts and shifts, leading to recurring seasonal epidemics and occasional pandemics [45,46]. Certain risk factors increase the severity of influenza infections. Respiratory diseases like asthma heighten the risk of hospitalization [47], and COPD is linked with increased morbidity and mortality [48]. Therefore, identifying patients at high risk for severe influenza is critical for promoting vaccination and adequate preventive measures.
The susceptibility to influenza infection among patients with OSA appears to be heightened, although the evidence is of poorer quality. Nonetheless, the current body of literature suggests that individuals with OSA are at a higher risk for severe manifestations of influenza. Two retrospective studies, investigating the comprehensive Taiwanese national database, indicated that OSA patients, identified by International Classification of Diseases (ICD) codes, have an 18% higher risk of contracting influenza and almost two times higher risk of experiencing severe acute respiratory infection defined as influenza infection necessitating hospitalization [24,25]. In both studies, OSA was associated with outcomes independent of obesity and other relevant comorbid diseases. However, it must be noted that these studies are limited by the lack of data on BMI and OSA severity.
Larger, multicentric studies focusing on risk factors for severe influenza infections and patient outcomes have identified OSA, along with central sleep apnea syndromes, as significant contributors. These conditions have been linked to a nearly tenfold increase in the likelihood of ICU admission [21] and a four times higher risk of non-invasive ventilation (NIV) failure [22] in patients with influenza infection. However, these studies did not find a statistically significant association between OSA and influenza mortality rates, suggesting that the presence of OSA exacerbates the course of influenza without necessarily affecting the outcome of the infection.
A retrospective cohort study by Mok et al. revealed that OSA patients non-adherent to continuous positive airway pressure (CPAP) therapy show an increased likelihood of hospitalization due to influenza, compared to those adherent to CPAP [23]. Further, the CPAP-compliant cohort displayed higher AHI values than their non-compliant counterparts, although not to a statistically significant extent. This suggests that CPAP adherence could protect against severe influenza despite more severe OSA. Other patient outcomes, such as length of stay and disease complications, did not significantly differ between groups. However, due to a limited sample size, this study might be underpowered to detect all relevant differences.
5. Obstructive Sleep Apnea and COVID-19 Pneumonia
Several risk factors exacerbate COVID-19-related disease severity and outcomes. Among the well-documented risk factors are obesity, diabetes, cardiovascular diseases, and metabolic syndrome [49,50]. OSA shares many common risk factors with COVID-19, and many studies have explored a possible association between the two. Our search identified five systematic reviews and meta-analyses that aimed to clarify this link.
The initial systematic review, published in February 2021, included only six papers with original data, five of which were case series and reports and suggested an increased risk of severe outcomes from COVID-19 in OSA patients [51]. A subsequent meta-analysis that evaluated 428 studies on poor prognostic factors for COVID-19 patients, highlighted OSA as a potential contributor. This analysis included seven studies presenting data on OSA with over 3800 subjects, suggesting that OSA more than doubles the risk for hospitalization [52]. Additionally, a meta-analysis evaluating European studies pointed out that OSA increases the risk for COVID-19-related hospitalization almost three times, but is not a predictor of intrahospital mortality [53]. These conclusions seem to be based on two studies included in the analysis, one examining OSA and the risk for COVID-19-related hospitalization, and one investigating COVID-19 hospital mortality. Two meta-analyses from 2021 and 2022 presented more conclusive evidence regarding the link between OSA and COVID-19 [54,55]. A meta-analysis from 2021 including 21 studies and over 50,000 COVID-19 patients concluded that OSA increases the risk not only for severe COVID-19 disease, need for ICU admission and mechanical ventilation, but also mortality [54]. A meta-analysis published in 2022 by Hu et al. confirmed OSA as a predictor of fatal outcomes in COVID-19 patients, increasing the risk by 50% [55].
Given the established link between OSA and progressive COVID-19 disease and adverse outcomes, our current research seeks to expand on these findings by focusing further on multicentric observational studies or those using large national or regional databases to provide a broader understanding of how OSA impacts the risk of contracting the SARS-CoV-2 virus, severity of COVID-19 disease, and associated mortality risks. Individual studies are presented in Table 2.
The evidence concerning the impact of OSA on the severity of COVID-19 is relatively conclusive. Of the 17 studies reviewed, 15 examined various indicators of COVID-19 severity, including hospitalization, ICU admission, length of stay, acute respiratory failure, and the need for intubation. Notably, 13 of these studies reported OSA as an independent predictor of these severe disease indicators (Table 2). The study by Cade et al. [30] linked OSA to hospitalization and severe outcomes, and the study by Mashaqi et al. [26] associated it with ICU admissions and longer hospital stays. In both studies, however, these relationships weakened after adjustments for BMI and comorbidities. Only two studies found no correlation between OSA and COVID-19 severity even before making such adjustments [38,40]. Regardless of whether OSA is an independent risk factor, it is evident that this patient population is at an increased risk for COVID-19 disease progression requiring inpatient or ICU admission, longer hospital stays, and complications.
A study by Pena Orbea et al. [31] evaluated sleep-related hypoxia measures and identified higher values of T90 and lower oxygen saturation, but not AHI, as independent predictors of severe COVID-19 and mortality. These findings suggest that hypoxemia, rather than sleep interruptions found in OSA patients, more significantly affects COVID-19 progression.
Data on COVID-19-related mortality remain inconsistent: six studies concluded that OSA does not increase mortality risk and another five reported OSA as an independent predictor of mortality (Table 2). Challenges in determining contributors to mortality include the widespread underdiagnosis of OSA, reliance on ICD codes for detecting OSA, “obesity paradox”, and already very high mortality in critically ill patients.
In addition to classical indicators of disease severity, such as hospitalization and mortality rates, there is evidence that patients with OSA are also at increased risk of post-acute sequelae of SARS-CoV-2, also known as long COVID syndrome [56,57]. Patients with OSA may face significant challenges if they develop long COVID. Persistent fatigue, lack of energy, cognitive disturbances, and mental disorders are common in both conditions [58,59,60,61,62,63,64]. While it is unclear to what extent OSA contributes to the symptoms of long COVID, data suggest that treating previously undiagnosed OSA in patients with long COVID may lead to the resolution of symptoms in some patients [65]. Cardiovascular complications are also a concern, as long COVID’s association with symptoms like palpitations and chest pain [59] could heighten the already increased risk of cardiac complications among OSA patients [66].
6. Obstructive Sleep Apnea and Lower Respiratory Tract Infections: Pathophysiology
The etiopathogenic link between OSA and an increased susceptibility to LRTIs is likely complex and influenced by several factors. For clarity, the mechanisms that may lead to acute LRTIs in OSA patients can be grouped into three main categories: altered immunity, risk of aspiration, and the role of comorbidities common in this population as depicted in Figure 1. These mechanisms likely act synergistically rather than in isolation.
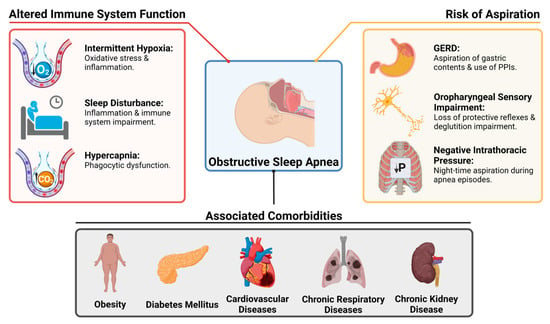
Figure 1.
Pathophysiological Interactions and Risk Factors for Lower Respiratory Tract Infections in Patients with Obstructive Sleep Apnea. GERD: Gastroesophageal reflux disease, PPIs: Proton pump inhibitors.
6.1. Altered Immunity
OSA is considered a pro-inflammatory condition [67], primarily due to intermittent hypoxia [68] and sleep fragmentation [69]. IH induces oxidative stress, leading to increased tumor necrosis factor-alpha (TNF-α) transcription [67,70] and higher interleukin 6 (IL-6) levels, which correlate with OSA severity [71]. Furthermore, IL-6 elevates C-reactive protein (CRP) levels, which, alongside TNF-α, is linked to sleep fragmentation and deprivation [67,72]. The resulting chronic low-grade inflammation leads to continuous immune system activation and gradual deterioration, known as immune senescence, increasing vulnerability to infections [73,74].
IH and resultant inflammation in OSA could affect pulmonary tissue directly. Overexpressed hypoxia-inducible factor (HIF)-1α, observed in COPD patients [75], might also be present in OSA [76], increasing infection risks through upregulated platelet-activating factor receptors that some bacteria use as receptors [75]. Additionally, sleep disruption independently impacts immune function by altering NK cells, T cell redistribution [77], immune cell proportions, and cytokine levels [78]. This could impact the response to vaccination [77], boosting humoral but impairing cellular immunity, which leads to higher infection risks despite adequate antibody titers [79,80,81,82]. Moreover, there is a well-established link between disrupted sleep patterns and an increased risk of pneumonia [83]. Next, hypercapnia in OSA patients [84], particularly those with comorbid COPD [85] or obesity hypoventilation syndrome (OHS) [86], reduces neutrophil phagocytic capabilities and pro-phagocytic cytokines [87]. Finally, IH could worsen outcomes by increasing the hypoxemic burden in patients with LRTI-related hypoxic respiratory failure.
6.2. Risk of Aspiration
Aspiration of oropharyngeal contents in OSA patients represents a significant potential mechanism contributing to LRTIs, particularly from bacterial agents. Several factors link OSA to an increased risk of aspiration, including an association with gastroesophageal reflux disease (GERD), impaired swallowing, oropharyngeal sensory deficits, and generation of negative intrathoracic pressure during sleep apnea episodes.
The relationship between OSA and GERD is well-established and appears to be bidirectional [88,89,90,91]. Pneumonia risk in these patients might be elevated due to GERD-related microaspiration [92]. Further, proton pump inhibitors create a less acidic gastric environment, delay gastric emptying, and may enhance bacterial colonization, increasing infection risk with aspiration [93].
OSA patients often have dysfunctional swallowing mechanisms, oropharyngeal sensory impairment, and alterations in deglutition muscle fibers [94,95,96]. Persistent, low-grade upper airway trauma causes sensory nerve damage, impairing the adductor reflex that prevents aspiration [94]. This sensory-motor impairment leads to swallowing–respiration discoordination, evidenced by increased latency in triggering the swallowing reflex [97].
Aspiration risk in OSA patients is elevated during apnea episodes due to negative intrathoracic pressure [3,98], which facilitates oropharyngeal content suction into the lungs. Studies have shown that OSA patients aspirate larger volumes at night compared to healthy individuals [99,100], potentially increasing bacterial load to the lower respiratory tract. CPAP therapy has been shown to reduce deglutition disturbances and prevent aspiration episodes in OSA patients [101].
6.3. The Role of Obesity and Other Comorbidities
Although OSA might be identified as a potential independent risk factor for severe LRTIs and adverse outcomes, it is crucial to consider the broader spectrum of risks faced by the OSA patient population.
Obesity is a significant risk factor for OSA, with BMI as a key predictor [102,103]. In critically ill patients, obesity increases the risks of complications like difficult airway management, acute respiratory distress syndrome, acute kidney injury, thromboembolic events, and infections [104]. Conversely, the “obesity paradox” suggests a protective effect against mortality in obese patients with LRTIs, possibly due to earlier presentation, higher hospital admission likelihood, better nutritional reserves, reduced lung injury, and altered immune response with less inflammation [105,106].
OSA is linked to numerous diseases, either causally or through common risk factors. Conditions such as diabetes mellitus [107], hypertension [108], coronary artery disease [109], heart failure [110], asthma [111], COPD [85], and end-stage kidney disease [112] are prevalent among OSA patients. These conditions increase the risk of severe disease and complications from LRTIs [113,114]. Cardiac events are significant complications for LRTI patients, and comorbid chronic heart disease may predispose OSA patients to these events [115]. Similarly, comorbid COPD is important as these patients often receive corticosteroids, increasing pneumonia risk [116]. Many studies confirmed OSA as an independent risk factor, often adjusted for chronic diseases as covariates. However, OSA patients who also suffer from these conditions might be at an even greater risk.
7. Obstructive Sleep Apnea and Lower Respiratory Tract Infections: Treatment
7.1. Settings of Care and Empiric Antibiotics
In community-acquired acute LRTIs, determining the causal agent clinically or radiologically is challenging. Thus, the American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) 2019 guidelines recommend empirical antibiotic therapy [117]. The choice of regimen depends on the care setting, complication risk, and illness severity, with clinical decision support tools like the pneumonia severity index (PSI) aiding hospitalization decisions [117,118]. OSA can affect PSI scores due to comorbid conditions like heart failure and kidney disease. Further, diabetes elevates glucose levels, while COPD and/or OHS contribute to hypercapnia, worsening acidosis, hypoventilation, and hypoxemia. All these factors may increase PSI scores, resulting in hospital admission and necessitating the use of broader antimicrobial coverage.
For outpatient management, ATS/IDSA recommends regimens based on complication risk [117]. Patients with chronic conditions like heart, lung, liver, or renal disease, or diabetes should receive dual therapy with an anti-pneumococcal beta-lactam (e.g., amoxicillin-clavulanate or cefuroxime) and a macrolide (azithromycin or clarithromycin) or doxycycline. Alternatively, respiratory fluoroquinolones (gemifloxacin, levofloxacin, or moxifloxacin) can be used as monotherapy. Given their higher risk of streptococcal pneumonia and invasive pneumococcal disease [16], OSA patients may benefit from this more aggressive approach compared to beta-lactam-, macrolide-, or doxycycline-monotherapy.
7.2. Specific Risks Guiding Empiric Antibiotic Therapy
Guideline-recommended antibiotic regimens for CAP cover common typical (Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis) and atypical pathogens (Mycoplasma pneumoniae, Legionella species, Chlamydia pneumoniae) [117]. A study identified Pseudomonas aeruginosa in over 10% of OSA patients with bacterial pneumonia [18]. Conditions like chronic aspiration, COPD, coronary artery disease, heart failure, and PPI use [119], common in OSA patients, increase Pseudomonas risk. Frequent hospitalizations, antibiotic use, and ICU admissions also heighten this risk [119]. Thus, in severe LRTI cases, early coverage for Pseudomonas with an antipseudomonal beta-lactam or respiratory fluoroquinolone is advisable [117].
Case reports indicate OSA patients may develop Legionnaires’ disease from CPAP machines [120,121]. If Legionella is suspected, characterized by relative bradycardia, diarrhea, neurological disturbances, and/or hyponatremia [122], early testing for Legionella urine antigen and treatment with a respiratory fluoroquinolone over macrolides should be considered [123].
Historically, anaerobic coverage was recommended for patients prone to aspiration, like those with OSA [124]. However, current guidelines advise against routine anaerobic coverage, such as clindamycin or metronidazole, unless there is a lung abscess or empyema, as aspiration pneumonia bacteria are similar to CAP pathogens [117].
For influenza or COVID-19, the CDC recommends specific antiviral therapies for patients with chronic diseases such as asthma, COPD, and pulmonary fibrosis [125,126]. Given the increased risk and severity of infections in OSA patients, antivirals should also be considered in this population.
7.3. Antibiotic Pharmacokinetics, Side Effects, and Resistance
The pharmacokinetics of medications, including antibiotics, can be altered in OSA patients. IH inhibits cytochrome P450 expression in animal models [127,128], leading to impaired metabolism and higher drug concentrations, potentially increasing toxicity risk. This affects antimicrobials undergoing hepatic metabolism, such as macrolides [129]. Additionally, OSA-associated non-alcoholic fatty liver disease [130] can further slow drug metabolism. Further, antibiotics like vancomycin [131] may require dose adjustments in OSA patients with higher BMI. Finally, renal impairment necessitates careful dosage adjustments for many antibiotics due to altered renal clearance [132].
Attention should also be given to the side effects of pneumonia antibiotics in OSA patients with comorbidities. The cardiotoxic effects of fluoroquinolones [133] and macrolides [134], and the renal toxicity of vancomycin [135], pose significant risks to OSA patients with cardiac or renal diseases. OSA patients’ increased exposure to antibiotics due to a higher pneumonia risk can lead to antibiotic resistance [136], posing additional risks.
8. Discussion
The existing evidence indicates that in adults, OSA increases the risk and severity of both bacterial and viral pneumonia. However, the data on mortality are less consistent, showing either the same or decreased risk compared to non-OSA patients for CAP, while presenting an increased mortality risk in some, but not all, studies involving COVID-19 patients. OSA seems to have no significant effects on mortality in influenza patients, but this has not been extensively studied. It is unclear to what extent obesity and a high comorbidity burden mediate the observed association. Indeed, in some studies, the risk of pneumonia hospitalization in OSA patients was attenuated after adjustment for BMI and comorbidities.
Most of the available evidence comes from studies that used ICD codes to evaluate both OSA and the presence of comorbidities. While administrative data are commonly used for large datasets as it is easy to obtain and non-costly, they may introduce potential biases due to coding variability and missing data [137]. Consequently, the results of these studies should be interpreted with caution. The use of administrative data also lacks granularity; thus, OSA severity was commonly not reported, and OSA features such as sleep duration, sleep fragmentation, and hypoxic burden were not evaluated. This is important as both sleep fragmentation and hypoxemic burden are associated with oxidative stress and inflammation, but their relative contribution to pneumonia risk remains unknown. Only a few studies evaluated T90, showing that it was a better predictor of pneumonia severity when compared to AHI [17,31]. Other sleep disorders that result in sleep disruption such as insomnia or shift work-related circadian rhythm sleep disorder may also increase susceptibility to respiratory infections [83]. In addition, the clinical presentation of OSA is heterogeneous with a variety of clinical phenotypes that differ in symptoms and comorbidities, which cannot be captured solely by AHI severity.
While CPAP remains the first-line therapy for OSA, there have been some concerns about whether CPAP increases the risk of respiratory infections further. Infrequent cleaning, improper handling, and the use of humidification with CPAP can create an environment that allows for the growth of microorganisms and subsequently increases the risk of infections [138,139]. Some studies, as well as several case reports, have suggested that CPAP therapy may increase the risk of respiratory infections in CPAP users [120,121,140,141,142]. Therefore, regular cleaning and maintenance of CPAP equipment are necessary to mitigate these potential risks [143]. Despite these concerns, data from recent studies are reassuring. A retrospective case-control study by Mercieca et al. found no difference in the prevalence of upper and lower respiratory tract infections or nasal swab results between CPAP and non-CPAP-treated patients, irrespective of humidifier use [144]. Additionally, in a retrospective cohort study of 482 adult patients with OSA, CPAP use was not associated longitudinally with the risk of respiratory infections [145].
At the start of the COVID-19 pandemic, the medical community was unfamiliar with the factors increasing the risk of contracting the novel SARS-CoV-2 virus. Given the shared comorbidities between OSA and COVID-19 [146], OSA patients were considered at higher risk. These patients were inadvertently placed at even greater risk due to measures taken to “flatten the curve” to prevent the spread of this airborne infection [147,148]. Specifically, there were recommendations to cease the use of CPAP machines because of concerns about increased virus transmission through aerosols and droplets [149,150]. Despite these concerns, subsequent studies indicated that the benefits of adhering to CPAP therapy not only outweigh the potential risks but also protect against severe COVID-19 disease and adverse outcomes in compliant patients [30,151]. Moreover, findings from influenza studies further confirm the protective effects of CPAP in OSA patients [23]. Furthermore, the use of novel technologies can be used to detect early changes in the respiratory health of CPAP users. CPAP telemonitoring data from COVID-19 patients showed reduced adherence or complete cessation of CPAP use in days preceding a COVID-19 diagnosis [152]. This offers new possibilities for early detection of respiratory infections in patients with OSA.
Current CDC guidelines advocate for pneumococcal vaccination for individuals over 65 and those under 65 with conditions such as COPD, emphysema, and asthma [153], along with annual influenza vaccinations for everyone aged six months and older [154], emphasizing the importance of those with respiratory conditions like asthma, COPD, and cystic fibrosis [155]. Heightened vigilance against COVID-19 is also recommended, particularly in light of new variants, which exhibit significant evasion of immunity provided by vaccines [156,157,158]. Given the findings discussed in this review study, indicating that patients with OSA are at an increased risk of severe outcomes from these infections, it would be advisable to include OSA patients in those prioritized for vaccination. Serologic studies for OSA patients concerning influenza and COVID-19 vaccinations [79,80,81], offer reassuring data, suggesting a positive response to vaccination. However, there might be a possibility of an immune system imbalance favoring humoral over cellular responses, potentially compromising the effectiveness of the protection from vaccination.
The association between LRTIs and OSA should also be considered in the context of global health. Low-income and middle-income countries (LMICs) have the highest LRTI morbidity and mortality burden [159] but are also facing increasing rates of obesity [160]. Still, the data on the prevalence and natural history of OSA in LMICs are scarce [161]. Although epidemiological data are lacking, the estimated prevalence of OSA in LMICs seems comparable or higher than in developed countries [1]. Given the evidence on the association between OSA and LRTIs, improving access to diagnosis and treatment for patients with suspected sleep apnea in LMICs could offer additional benefits in preventing LRTIs and improving outcomes in resource-limited settings.
This paper has several limitations inherent to narrative review methodology such as potential incomplete coverage, lack of quantitative analysis, and positive results bias. Heterogeneity in study designs and the lack of explicit criteria for study selection may have also introduced some bias. Nevertheless, to our knowledge, no previous study has comprehensively evaluated evidence on the association between OSA and various acute respiratory infections.
9. Conclusions
OSA increases the risk and severity of both bacterial and viral acute LRTIs, with no clear evidence of increasing pneumonia mortality. However, the relative contribution of chronic comorbidities to the risk and prognosis of LRTIs in OSA patients is not fully understood. Future research should focus on identifying OSA indices beyond AHI that could help predict pneumonia risk as well as potential risks and benefits of CPAP therapy. Strategies to minimize the risk of infection such as vaccination should be considered, especially for patients with severe OSA. Finally, OSA should be considered an important risk factor for LRTIs in national and international guidelines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13060532/s1, Figure S1: Flowchart of Study Selection.
Author Contributions
Conceptualization, M.V.; methodology, M.V. and M.N.; data curation, M.V. and M.N.; writing—original draft preparation, M.V. and M.N.; writing—review and editing, M.V. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Acknowledgments
The authors express gratitude to Aleksandar Rašković, for his invaluable contributions and guidance on the section concerning antibiotic treatment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W.; Bassetti, C.L.; Bonsignore, M.R.; Farre, R.; Ferini-Strambi, L.; Grote, L.; Hedner, J.; Kohler, M.; Martinez-Garcia, M.A.; Mihaicuta, S.; et al. Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur. Respir. J. 2018, 52, 1702616. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ko, I.; Kim, D.K. Association of Obstructive Sleep Apnea with the Risk of Affective Disorders. JAMA Otolaryngol. Head. Neck Surg. 2019, 145, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Shaddock, E. Epidemiology of lower respiratory tract infections in adults. Expert. Rev. Respir. Med. 2019, 13, 63–77. [Google Scholar] [CrossRef] [PubMed]
- International Respiratory Coalition (IRC). Lower Respiratory Tract Infections. Available online: https://international-respiratory-coalition.org/diseases/lower-respiratory-tract-infections/ (accessed on 10 April 2024).
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 April 2024).
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Mizgerd, J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 2008, 358, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Huppertz, T.; Radsak, M.; Gouveris, H. Cellular Immune Dysfunction in Obstructive Sleep Apnea. Front. Surg. 2022, 9, 890377. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Zanini, U.; Monzani, A.; Parati, G.; Luppi, F.; Lombardi, C.; Perger, E. Sleep-Disordered Breathing and Chronic Respiratory Infections: A Narrative Review in Adult and Pediatric Population. Int. J. Mol. Sci. 2023, 24, 5504. [Google Scholar] [CrossRef] [PubMed]
- Keto, J.; Feuth, T.; Linna, M.; Saaresranta, T. Lower respiratory tract infections among newly diagnosed sleep apnea patients. BMC Pulm. Med. 2023, 23, 332. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.R.; Meche, A.; McGrath, L.; Miles, A.; Alfred, T.; Yan, Q.; Chilson, E. Risk of Pneumococcal Disease in US Adults by Age and Risk Profile. Open Forum Infect. Dis. 2023, 10, ofad192. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Zineldin, I.; Misialek, J.R.; Full, K.M.; Lakshminarayan, K.; Ishigami, J.; Cowan, L.T.; Matsushita, K.; Demmer, R.T. OSA and Subsequent Risk of Hospitalization with Pneumonia, Respiratory Infection, and Total Infection: The Atherosclerosis Risk in Communities Study. Chest 2023, 163, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Chiner, E.; Llombart, M.; Valls, J.; Pastor, E.; Sancho-Chust, J.N.; Andreu, A.L.; Sánchez-de-la-Torre, M.; Barbé, F. Association between Obstructive Sleep Apnea and Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0152749. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Liu, C.J.; Wang, H.K.; Wu, L.A.; Chang, S.C.; Perng, D.W.; Su, W.J.; Chen, Y.M.; Lin, E.Y.; Chen, T.J.; et al. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ 2014, 186, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lindenauer, P.K.; Stefan, M.S.; Johnson, K.G.; Priya, A.; Pekow, P.S.; Rothberg, M.B. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest 2014, 145, 1032–1038. [Google Scholar] [CrossRef]
- Beumer, M.C.; Koch, R.M.; van Beuningen, D.; OudeLashof, A.M.; van de Veerdonk, F.L.; Kolwijck, E.; van der Hoeven, J.G.; Bergmans, D.C.; Hoedemaekers, C.W.E. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J. Crit. Care 2019, 50, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Charrier, L.; Almeida, A.; Christaki, E.; Moreira Marques, T.; Tosatto, V.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Karagiannis, C.; et al. Burden of primary influenza and respiratory syncytial virus pneumonia in hospitalised adults: Insights from a 2-year multi-centre cohort study (2017–2018). Intern. Med. J. 2023, 53, 404–408. [Google Scholar] [CrossRef]
- Mok, E.M.; Greenough, G.; Pollack, C.C. Untreated obstructive sleep apnea is associated with increased hospitalization from influenza infection. J. Clin. Sleep Med. 2020, 16, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Chen, H.C.; Li, H.Y.; Tsai, Y.T.; Yang, Y.H.; Liu, C.Y.; Lee, Y.C.; Hsu, C.M.; Lee, L.A. Sleep Apnea and Risk of Influenza-Associated Severe Acute Respiratory Infection: Real-World Evidence. Nat. Sci. Sleep 2022, 14, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Chang, R.; Chiu, L.T.; Hung, Y.M.; Wei, J.C. Obstructive sleep apnea and influenza infection: A nationwide population-based cohort study. Sleep Med. 2021, 81, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mashaqi, S.; Lee-Iannotti, J.; Rangan, P.; Celaya, M.P.; Gozal, D.; Quan, S.F.; Parthasarathy, S. Obstructive sleep apnea and COVID-19 clinical outcomes during hospitalization: A cohort study. J. Clin. Sleep Med. 2021, 17, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.B.; Kim, M.; Malkani, R.G.; Abbott, S.M.; Zee, P.C. Obstructive Sleep Apnea and Risk of COVID-19 Infection, Hospitalization and Respiratory Failure. Sleep Breath 2021, 25, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Strausz, S.; Kiiskinen, T.; Broberg, M.; Ruotsalainen, S.; Koskela, J.; Bachour, A.; Palotie, A.; Palotie, T.; Ripatti, S.; Ollila, H.M. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir. Res. 2021, 8, e000845. [Google Scholar] [CrossRef] [PubMed]
- Rögnvaldsson, K.G.; Eyþórsson, E.S.; Emilsson, Ö.I.; Eysteinsdóttir, B.; Pálsson, R.; Gottfreðsson, M.; Guðmundsson, G.; Steingrímsson, V. Obstructive sleep apnea is an independent risk factor for severe COVID-19: A population-based study. Sleep 2022, 45, zsab272. [Google Scholar] [CrossRef] [PubMed]
- Cade, B.E.; Dashti, H.S.; Hassan, S.M.; Redline, S.; Karlson, E.W. Sleep Apnea and COVID-19 Mortality and Hospitalization. Am. J. Respir. Crit. Care Med. 2020, 202, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Pena Orbea, C.; Wang, L.; Shah, V.; Jehi, L.; Milinovich, A.; Foldvary-Schaefer, N.; Chung, M.K.; Mashaqi, S.; Aboussouan, L.; Seidel, K.; et al. Association of Sleep-Related Hypoxia with Risk of COVID-19 Hospitalizations and Mortality in a Large Integrated Health System. JAMA Netw. Open 2021, 4, e2134241. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A. Impact of coronavirus disease-2019 on chronic respiratory disease in South Korea: An NHIS COVID-19 database cohort study. BMC Pulm. Med. 2021, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Sansom, S.; Frankenberger, C.; Ward, E.; Hota, B. Clinical Course and Factors Associated with Hospitalization and Critical Illness Among COVID-19 Patients in Chicago, Illinois. Acad. Emerg. Med. 2020, 27, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Povitz, M.; Gershon, A.S.; Ryan, C.M.; Talarico, R.; Franco Avecilla, D.A.; Robillard, R.; Ayas, N.T.; Pendharkar, S.R. Association of clinically significant obstructive sleep apnoea with risks of contracting COVID-19 and serious COVID-19 complications: A retrospective population-based study of health administrative data. Thorax 2023, 78, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Celik, Y.; Arbatli, S.; Isik, S.R.; Balcan, B.; Karataş, F.; Uzel, F.I.; Tabak, L.; Çetin, B.; Baygül, A.; et al. Effect of High-Risk Obstructive Sleep Apnea on Clinical Outcomes in Adults with Coronavirus Disease 2019: A Multicenter, Prospective, Observational Clinical Trial. Ann. Am. Thorac. Soc. 2021, 18, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Girardin, J.L.; Seixas, A.; Ramos Cejudo, J.; Osorio, R.S.; Avirappattu, G.; Reid, M.; Parthasarathy, S. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron. Respir. Dis. 2021, 18, 1479973120986806. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Miguel, A.; Bliek-Bueno, K.; Poblador-Plou, B.; Carmona-Pírez, J.; Poncel-Falcó, A.; González-Rubio, F.; Ioakeim-Skoufa, I.; Pico-Soler, V.; Aza-Pascual-Salcedo, M.; Prados-Torres, A.; et al. Chronic diseases associated with increased likelihood of hospitalization and mortality in 68,913 COVID-19 confirmed cases in Spain: A population-based cohort study. PLoS ONE 2021, 16, e0259822. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Ancochea, J.; Soriano, J.B. Clinical Characteristics and Prognostic Factors for Intensive Care Unit Admission of Patients with COVID-19: Retrospective Study Using Machine Learning and Natural Language Processing. J. Med. Internet Res. 2020, 22, e21801. [Google Scholar] [CrossRef] [PubMed]
- Lohia, P.; Sreeram, K.; Nguyen, P.; Choudhary, A.; Khicher, S.; Yarandi, H.; Kapur, S.; Badr, M.S. Preexisting respiratory diseases and clinical outcomes in COVID-19: A multihospital cohort study on predominantly African American population. Respir. Res. 2021, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Mechineni, A.; Talugula, S.; Gardner, J.; Rubinstein, I.; Gordon, H.S. Impact of Obstructive Sleep Apnea on Health Outcomes in Veterans Hospitalized with COVID-19 Infection. Ann. Am. Thorac. Soc. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Bailly, S.; Galerneau, L.M.; Ruckly, S.; Seiller, A.; Terzi, N.; Schwebel, C.; Dupuis, C.; Tamisier, R.; Mourvillier, B.; Pepin, J.L.; et al. Impact of obstructive sleep apnea on the obesity paradox in critically ill patients. J. Crit. Care 2020, 56, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Bolona, E.; Hahn, P.Y.; Afessa, B. Intensive care unit and hospital mortality in patients with obstructive sleep apnea. J. Crit. Care 2015, 30, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, C.S.; Allen, J.L.Y.; Gkrania-Klotsas, E. Influenza: Epidemiology and hospital management. Medicine 2021, 49, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, R.; Snyder, J.D.; Samarasinghe, A.E. Influenza in Asthmatics: For Better or for Worse? Front. Immunol. 2018, 9, 1843. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.; Decramer, M.; Calverley, P.M.; Pride, N.B.; Soriano, J.B.; Vermeire, P.A.; Vestbo, J. Impact of COPD in North America and Europe in 2000: Subjects’ perspective of Confronting COPD International Survey. Eur. Respir. J. 2002, 20, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Denson, J.L.; Gillet, A.S.; Zu, Y.; Brown, M.; Pham, T.; Yoshida, Y.; Mauvais-Jarvis, F.; Douglas, I.S.; Moore, M.; Tea, K.; et al. Metabolic Syndrome and Acute Respiratory Distress Syndrome in Hospitalized Patients With COVID-19. JAMA Netw. Open 2021, 4, e2140568. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Cappuccio, F.P. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med. Rev. 2021, 55, 101382. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Tzoulaki, I.; van Smeden, M.; Moons, K.G.M.; Evangelou, E.; Belbasis, L. Prognostic factors for adverse outcomes in patients with COVID-19: A field-wide systematic review and meta-analysis. Eur. Respir. J. 2022, 59, 2002964. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Mathioudakis, A.G.; Nikitara, K.; Stamatelopoulos, K.; Georgiopoulos, G.; Phalkey, R.; Leonardi-Bee, J.; Fernandez, E.; Carnicer-Pont, D.; Vestbo, J.; et al. Prognostic factors for mortality, intensive care unit and hospital admission due to SARS-CoV-2: A systematic review and meta-analysis of cohort studies in Europe. Eur. Respir. Rev. 2022, 31, 220098. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Kurniawan, A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis. Sleep Med. 2021, 82, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Han, X.; Ren, J.; Wang, Y.; Yang, H. Significant association of obstructive sleep apnoea with increased risk for fatal COVID-19: A quantitative meta-analysis based on adjusted effect estimates. Sleep Med. Rev. 2022, 63, 101624. [Google Scholar] [CrossRef] [PubMed]
- Mandel, L.H.; Colleen, G.; Abedian, S.; Ammar, N.; Charles Bailey, L.; Bennett, T.D.; Daniel Brannock, M.; Brosnahan, S.B.; Chen, Y.; Chute, C.G.; et al. Risk of post-acute sequelae of SARS-CoV-2 infection associated with pre-coronavirus disease obstructive sleep apnea diagnoses: An electronic health record-based analysis from the RECOVER initiative. Sleep 2023, 46, zsad126. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Henríquez-Beltrán, M.; Lamperti, L.; Nova-Lamperti, E.; Sanhueza, S.; Cabrera, C.; Quiroga, R.; Antilef, B.; Ormazábal, V.; Zúñiga, F.; et al. Impact of Obstructive Sleep Apnea (OSA) in COVID-19 Survivors, Symptoms Changes Between 4-Months and 1 Year After the COVID-19 Infection. Front. Med. 2022, 9, 884218. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 2000, 118, 372–379. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, A.G.; Perry, G.S.; Chapman, D.P.; Croft, J.B. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005–2008. Sleep 2012, 35, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J. Res. Med. Sci. 2014, 19, 205–210. [Google Scholar] [PubMed]
- Menzler, K.; Mayr, P.; Knake, S.; Cassel, W.; Viniol, C.; Reitz, L.; Tsalouchidou, P.E.; Janzen, A.; Anschuetz, K.; Mross, P.; et al. Undiagnosed obstructive sleep apnea syndrome as a treatable cause of new-onset sleepiness in some post-COVID patients. Eur. J. Neurol. 2024, 31, e16159. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kimoff, R.J. Sleep fragmentation in obstructive sleep apnea. Sleep 1996, 19, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Savransky, V.; Nanayakkara, A.; Bevans, S.; Li, J.; Smith, P.L.; Polotsky, V.Y. Intermittent hypoxia has organ-specific effects on oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1274–R1281. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Sadeghi Bahmani, D.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Van der Touw, T.; Andronicos, N.M.; Smart, N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019, 24, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.D.; Walters, E.H.; Simpson, J.L.; Keely, S.; Wark, P.A.B.; O’Toole, R.F.; Hansbro, P.M. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology 2020, 25, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Coronado, E.G.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Prospéro-García, O.; Méndez-Díaz, M.; Pérez-Tapia, M.; Pavón, L.; Morales-Montor, J. The Bidirectional Relationship between Sleep and Immunity against Infections. J. Immunol. Res. 2015, 2015, 678164. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.M.; Wiegert, N.A.; Moran, J.J.; Muller, D.; Weber, S.; Hayney, M.S. Humoral immune responses to influenza vaccination in patients with obstructive sleep apnea. Pharmacotherapy 2007, 27, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Tufik, S.; Andersen, M.L.; Rosa, D.S.; Tufik, S.B.; Pires, G.N. Effects of Obstructive Sleep Apnea on SARS-CoV-2 Antibody Response After Vaccination Against COVID-19 in Older Adults. Nat. Sci. Sleep 2022, 14, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.Q.; Warner, N.D.; Ovsyannikova, I.G.; Covassin, N.; Poland, G.A.; Somers, V.K.; Kennedy, R.B. Excessive daytime sleepiness is associated with impaired antibody response to influenza vaccination in older male adults. Front. Cell Infect. Microbiol. 2023, 13, 1229035. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Tieken, S.; Fehm, H.L.; Born, J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav. Immun. 2004, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Malhotra, A.; Gao, X.; Hu, F.B.; Neuman, M.I.; Fawzi, W.W. A prospective study of sleep duration and pneumonia risk in women. Sleep 2012, 35, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Resta, O.; Foschino Barbaro, M.P.; Bonfitto, P.; Talamo, S.; Mastrosimone, V.; Stefano, A.; Giliberti, T. Hypercapnia in obstructive sleep apnoea syndrome. Neth. J. Med. 2000, 56, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, R.; Siddiqi, T.A.; Quan, S.F. Sleep disorders in chronic obstructive pulmonary disease: Etiology, impact, and management. J. Clin. Sleep Med. 2015, 11, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Macavei, V.M.; Spurling, K.J.; Loft, J.; Makker, H.K. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J. Clin. Sleep Med. 2013, 9, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.L.; Howell, H.A.; Nair, A.; Vohwinkel, C.U.; Welch, L.C.; Beitel, G.J.; Hauser, A.R.; Sznajder, J.I.; Sporn, P.H. Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am. J. Respir. Cell Mol. Biol. 2013, 49, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hua, L.; Chen, L.; Mu, T.; Dong, D.; Xu, J.; Shen, C. Causal association between obstructive sleep apnea and gastroesophageal reflux disease: A bidirectional two-sample Mendelian randomization study. Front. Genet. 2023, 14, 1111144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Yang, X.P.; Niu, X.; Xiao, X.Y.; Chen, X. The relationship between obstructive sleep apnea hypopnea syndrome and gastroesophageal reflux disease: A meta-analysis. Sleep Breath 2019, 23, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, Ö.I.; Bengtsson, A.; Franklin, K.A.; Torén, K.; Benediktsdóttir, B.; Farkhooy, A.; Weyler, J.; Dom, S.; De Backer, W.; Gislason, T.; et al. Nocturnal gastro-oesophageal reflux, asthma and symptoms of OSA: A longitudinal, general population study. Eur. Respir. J. 2013, 41, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- You, C.R.; Oh, J.H.; Seo, M.; Lee, H.Y.; Joo, H.; Jung, S.H.; Lee, S.H.; Choi, M.G. Association Between Non-erosive Reflux Disease and High Risk of Obstructive Sleep Apnea in Korean Population. J. Neurogastroenterol. Motil. 2014, 20, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Lai, C.C.; Wang, Y.H.; Tseng, P.H.; Wang, K.; Wang, C.Y.; Chen, L. Risk of pneumonia in patients with gastroesophageal reflux disease: A population-based cohort study. PLoS ONE 2017, 12, e0183808. [Google Scholar] [CrossRef] [PubMed]
- Fohl, A.L.; Regal, R.E. Proton pump inhibitor-associated pneumonia: Not a breath of fresh air after all? World J. Gastrointest. Pharmacol. Ther. 2011, 2, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Jobin, V.; Payne, R.; Beauregard, J.; Naor, N.; Kimoff, R.J. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep 2005, 28, 585–593. [Google Scholar] [CrossRef]
- Ghannouchi, I.; Speyer, R.; Doma, K.; Cordier, R.; Verin, E. Swallowing function and chronic respiratory diseases: Systematic review. Respir. Med. 2016, 117, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Pizzorni, N.; Radovanovic, D.; Pecis, M.; Lorusso, R.; Annoni, F.; Bartorelli, A.; Rizzi, M.; Schindler, A.; Santus, P. Dysphagia symptoms in obstructive sleep apnea: Prevalence and clinical correlates. Respir. Res. 2021, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, S.; Sudo, E.; Matsuse, T.; Ohga, E.; Ishii, T.; Ouchi, Y.; Fukuchi, Y. Impaired swallowing reflex in patients with obstructive sleep apnea syndrome. Chest 1999, 116, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, K.; Eggli, D.F.; Maxwell, S.L. Quantitative aspiration during sleep in normal subjects. Chest 1997, 111, 1266–1272. [Google Scholar] [CrossRef]
- Beal, M.; Chesson, A.; Garcia, T.; Caldito, G.; Stucker, F.; Nathan, C.O. A pilot study of quantitative aspiration in patients with symptoms of obstructive sleep apnea: Comparison to a historic control group. Laryngoscope 2004, 114, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Chitose, S.I.; Sato, K.; Sato, F.; Ono, T.; Umeno, H. Recurrent aspiration pneumonia precipitated by obstructive sleep apnea. Auris Nasus Larynx 2021, 48, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017, 1, 00019. [Google Scholar] [PubMed]
- Anderson, M.R.; Shashaty, M.G.S. Impact of Obesity in Critical Illness. Chest 2021, 160, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Yang, T.; Wang, M.; Xi, X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS ONE 2018, 13, e0198669. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, S.; Eurich, D.T.; Padwal, R.S.; Malhotra, A.; Minhas-Sandhu, J.K.; Marrie, T.J.; Majumdar, S.R. Obesity and outcomes in patients hospitalized with pneumonia. Clin. Microbiol. Infect. 2013, 19, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Botros, N.; Concato, J.; Mohsenin, V.; Selim, B.; Doctor, K.; Yaggi, H.K. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am. J. Med. 2009, 122, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Broytman, O.; Curran-Everett, D.; Sorkness, R.L.; Crisafi, G.; Bleecker, E.R.; Erzurum, S.; Gaston, B.M.; Wenzel, S.E.; Jarjour, N.N. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J. Allergy Clin. Immunol. Pract. 2015, 3, 566–575.e561. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Miller, G.; Mendelson, W.B. Sleep apnea syndrome in chronic renal disease. Am. J. Med. 1989, 86, 308–314. [Google Scholar] [CrossRef]
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef]
- Naqvi, S.B.; Collins, A.J. Infectious complications in chronic kidney disease. Adv. Chronic Kidney Dis. 2006, 13, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Viasus, D.; Garcia-Vidal, C.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J. Infect. 2013, 66, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, R.H.; Heerfordt, C.K.; Boel, J.B.; Dessau, R.B.; Ostergaard, C.; Sivapalan, P.; Eklöf, J.; Jensen, J.S. Inhaled corticosteroids and risk of lower respiratory tract infection with Moraxella catarrhalis in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2023, 10, e001726. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Park, J.S.; Lee, C.W.; Choi, W.I. Pneumonia severity index in viral community acquired pneumonia in adults. PLoS ONE 2019, 14, e0210102. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Babu, B.L.; Reyes, L.F.; Chalmers, J.D.; Soni, N.J.; Sibila, O.; Faverio, P.; Cilloniz, C.; Rodriguez-Cintron, W.; Aliberti, S. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur. Respir. J. 2018, 52, 1701190. [Google Scholar] [CrossRef] [PubMed]
- Srivali, N.; Chongnarungsin, D.; Ungprasert, P.; Edmonds, L.C. Two cases of Legionnaires’ disease associated with continuous positive airway pressure therapy. Sleep Med. 2013, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- Schnirman, R.; Nur, N.; Bonitati, A.; Carino, G. A case of legionella pneumonia caused by home use of continuous positive airway pressure. SAGE Open Med. Case Rep. 2017, 5, 2050313x17744981. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.F.; Awosika, A.O.; Sundareshan, V. Legionnaires’ Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kato, H.; Hagihara, M.; Asai, N.; Shibata, Y.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. Meta-analysis of fluoroquinolones versus macrolides for treatment of legionella pneumonia. J. Infect. Chemother. 2021, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Cesar, L.; Gonzalez, C.; Calia, F.M. Bacteriologic flora of aspiration-induced pulmonary infections. Arch. Intern. Med. 1975, 135, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Influenza Antiviral Medications: Summary for Clinicians|CDC. Available online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm#highrisk (accessed on 4 May 2024).
- People with Certain Medical Conditions|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 4 May 2024).
- Zhang, X.B.; Chen, X.Y.; Chiu, K.Y.; He, X.Z.; Wang, J.M.; Zeng, H.Q.; Zeng, Y. Intermittent Hypoxia Inhibits Hepatic CYP1a2 Expression and Delays Aminophylline Metabolism. Evid. Based Complement. Alternat Med. 2022, 2022, 2782702. [Google Scholar] [CrossRef] [PubMed]
- Fradette, C.; Du Souich, P. Effect of hypoxia on cytochrome P450 activity and expression. Curr. Drug Metab. 2004, 5, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Fohner, A.E.; Sparreboom, A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genom. 2017, 27, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir. Crit. Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F. Vancomycin Dosing and Monitoring: Critical Evaluation of the Current Practice. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Munar, M.Y.; Singh, H. Drug dosing adjustments in patients with chronic kidney disease. Am. Fam. Physician 2007, 75, 1487–1496. [Google Scholar] [PubMed]
- Gorelik, E.; Masarwa, R.; Perlman, A.; Rotshild, V.; Abbasi, M.; Muszkat, M.; Matok, I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-analysis and Network Meta-analysis. Drug Saf. 2019, 42, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Cai, Y.; Chai, D.; Liang, B.; Bai, N.; Wang, R. The cardiotoxicity of macrolides: A systematic review. Pharmazie 2010, 65, 631–640. [Google Scholar] [PubMed]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Gershon, A.; Wijeysundera, D.; Bryson, G.L.; Badner, N.; van Walraven, C. Identifying Obstructive Sleep Apnea in Administrative Data: A Study of Diagnostic Accuracy. Anesthesiology 2015, 123, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, K.; Goroncy-Bermes, P. Investigation of the hygienic safety of continuous positive airways pressure devices after reprocessing. J. Hosp. Infect. 2005, 61, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ortolano, G.A.; Schaffer, J.; McAlister, M.B.; Stanchfield, I.; Hill, E.; Vandenburgh, L.; Lewis, M.; John, S.; Canonica, F.P.; Cervia, J.S. Filters reduce the risk of bacterial transmission from contaminated heated humidifiers used with CPAP for obstructive sleep apnea. J. Clin. Sleep Med. 2007, 3, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Sanner, B.M.; Fluerenbrock, N.; Kleiber-Imbeck, A.; Mueller, J.B.; Zidek, W. Effect of continuous positive airway pressure therapy on infectious complications in patients with obstructive sleep apnea syndrome. Respiration 2001, 68, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Jao, L.Y.; Su, W.L.; Chang, H.C.; Lan, C.C.; Wu, Y.K.; Yang, M.C. Pneumocystis jirovecii pneumonia presenting as a solitary pulmonary granuloma due to unclean continuous positive airway pressure equipment: A case report. J. Clin. Sleep Med. 2022, 18, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Caiano Gil, J.; Calisto, R.; Amado, J.; Barreto, V. Eikenella corrodens and Porphyromonas asaccharolytica pleural empyema in a diabetic patient with obstructive sleep apnea syndrome on noninvasive ventilation. Rev. Port. Pneumol. 2013, 19, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R. Providing Cleaning Recommendations for Positive Airway Pressure Devices. Ann. Am. Thorac. Soc. 2024, 21, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Mercieca, L.; Pullicino, R.; Camilleri, K.; Abela, R.; Mangion, S.A.; Cassar, J.; Zammit, M.; Gatt, C.; Deguara, C.; Barbara, C.; et al. Continuous Positive Airway Pressure: Is it a route for infection in those with Obstructive Sleep Apnoea? Sleep Sci. 2017, 10, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Gavidia, R.; Shieu, M.M.; Dunietz, G.L.; Braley, T.J. Respiratory infection risk in positive airway pressure therapy users: A retrospective cohort study. J. Clin. Sleep Med. 2023, 19, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Mutti, C.; Azzi, N.; Soglia, M.; Pollara, I.; Alessandrini, F.; Parrino, L. Obstructive sleep apnea, cpap and COVID-19: A brief review. Acta Biomed. 2020, 91, e2020196. [Google Scholar] [CrossRef]
- Feng, Z.; Glasser, J.W.; Hill, A.N. On the benefits of flattening the curve: A perspective. Math. Biosci. 2020, 326, 108389. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Baker, M.A.; Rhee, C. Airborne Transmission of SARS-CoV-2: Theoretical Considerations and Available Evidence. JAMA 2020, 324, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M. Sleep labs, lung function tests and COVID-19 pandemic—Only emergencies allowed! Pulmonology 2020, 26, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Oyefeso, O.; Koeckerling, D.; Mudalige, N.L.; Pan, D. COVID-19: Community CPAP and NIV should be stopped unless medically necessary to support life. Thorax 2020, 75, 367. [Google Scholar] [CrossRef] [PubMed]
- Sampol, J.; Sáez, M.; Martí, S.; Pallero, M.; Barrecheguren, M.; Ferrer, J.; Sampol, G. Impact of home CPAP-treated obstructive sleep apnea on COVID-19 outcomes in hospitalized patients. J. Clin. Sleep Med. 2022, 18, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.L.; Bailly, S.; Borel, J.C.; Logerot, S.; Sapène, M.; Martinot, J.B.; Lévy, P.; Tamisier, R. Detecting COVID-19 and other respiratory infections in obstructive sleep apnoea patients through CPAP device telemonitoring. Digit. Health 2021, 7, 20552076211002957. [Google Scholar] [CrossRef] [PubMed]
- Pneumococcal Vaccination: Who and When to Vaccinate|CDC. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html#adults-19-64 (accessed on 10 April 2024).
- Influenza Vaccination: A Summary for Clinicians|CDC. Available online: https://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm#vaccinated (accessed on 10 April 2024).
- People at Higher Risk of Flu Complications|CDC. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 10 April 2024).
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef] [PubMed]
- Song, X.D.; Yang, G.J.; Jiang, X.L.; Wang, X.J.; Zhang, Y.W.; Wu, J.; Wang, M.M.; Chen, R.R.; He, X.J.; Dong, G.; et al. Seroprevalence of SARS-CoV-2 neutralising antibodies and cross-reactivity to JN.1 one year after the BA.5/BF.7 wave in China. Lancet Reg. Health West. Pac. 2024, 44, 101040. [Google Scholar] [CrossRef] [PubMed]
- Jeworowski, L.M.; Mühlemann, B.; Walper, F.; Schmidt, M.L.; Jansen, J.; Krumbholz, A.; Simon-Lorière, E.; Jones, T.C.; Corman, V.M.; Drosten, C. Humoral immune escape by current SARS-CoV-2 variants BA.2.86 and JN.1, December 2023. Euro Surveill. 2024, 29, 2300740. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.D.; Patel, S.A.; Narayan, K.M. Obesity in Low- and Middle-Income Countries: Burden, Drivers, and Emerging Challenges. Annu. Rev. Public Health 2017, 38, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Rae, D.E.; Redman, K.N.; Knutson, K.L.; von Schantz, M.; Gómez-Olivé, F.X.; Scheuermaier, K. Sleep disorders in low- and middle-income countries: A call for action. J. Clin. Sleep Med. 2021, 17, 2341–2342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).