Comparison of the Impact of VRP-034 and Polymyxin B upon Markers of Kidney Injury in Human Proximal Tubule Monolayers In Vitro
Abstract
1. Introduction
2. Results
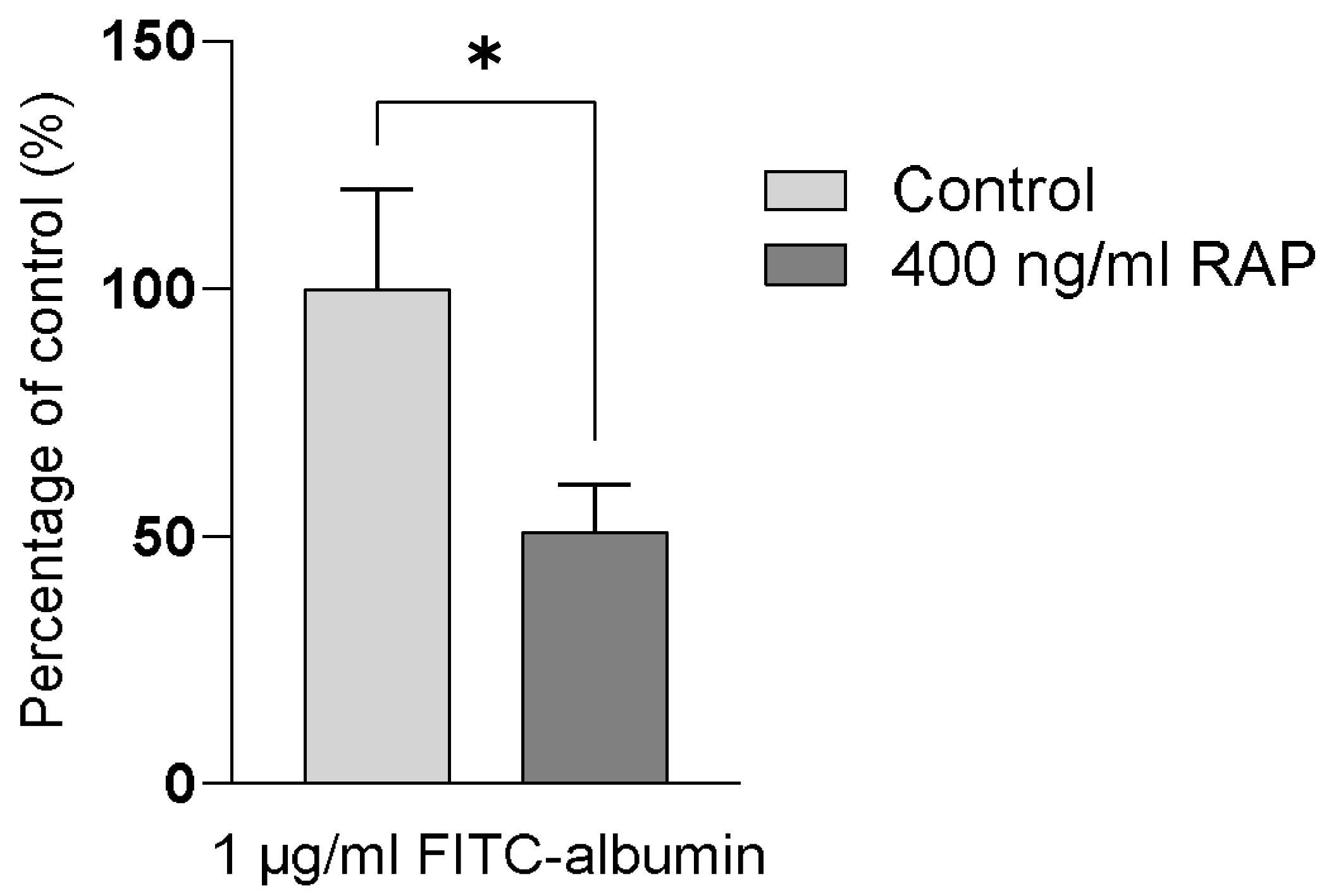
2.1. Intracellular Accumulation of Megalin/Cubilin-Dependent FITC-Albumin into hPTC Monolayers
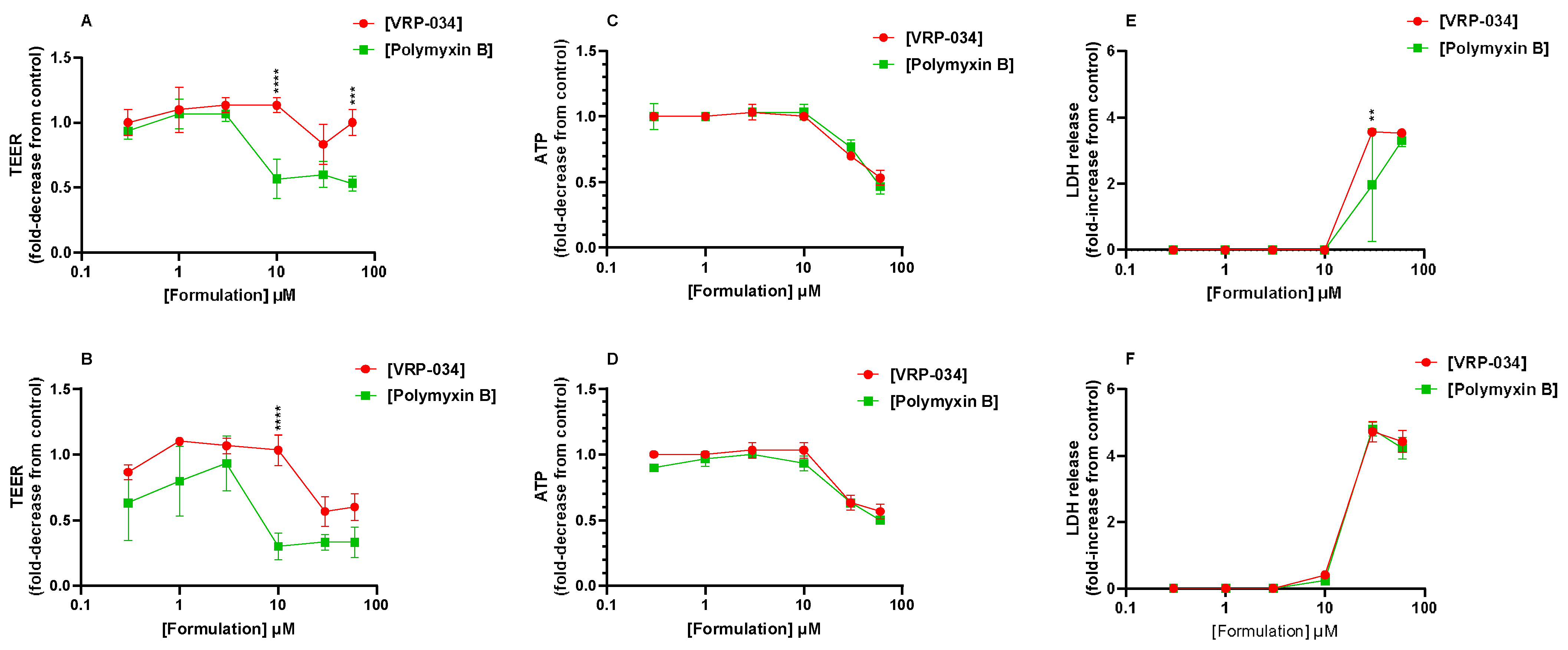
2.2. Comparison of VRP-034 and Polymyxin B Treatment on Gross Viability Markers from hPTC Monolayers
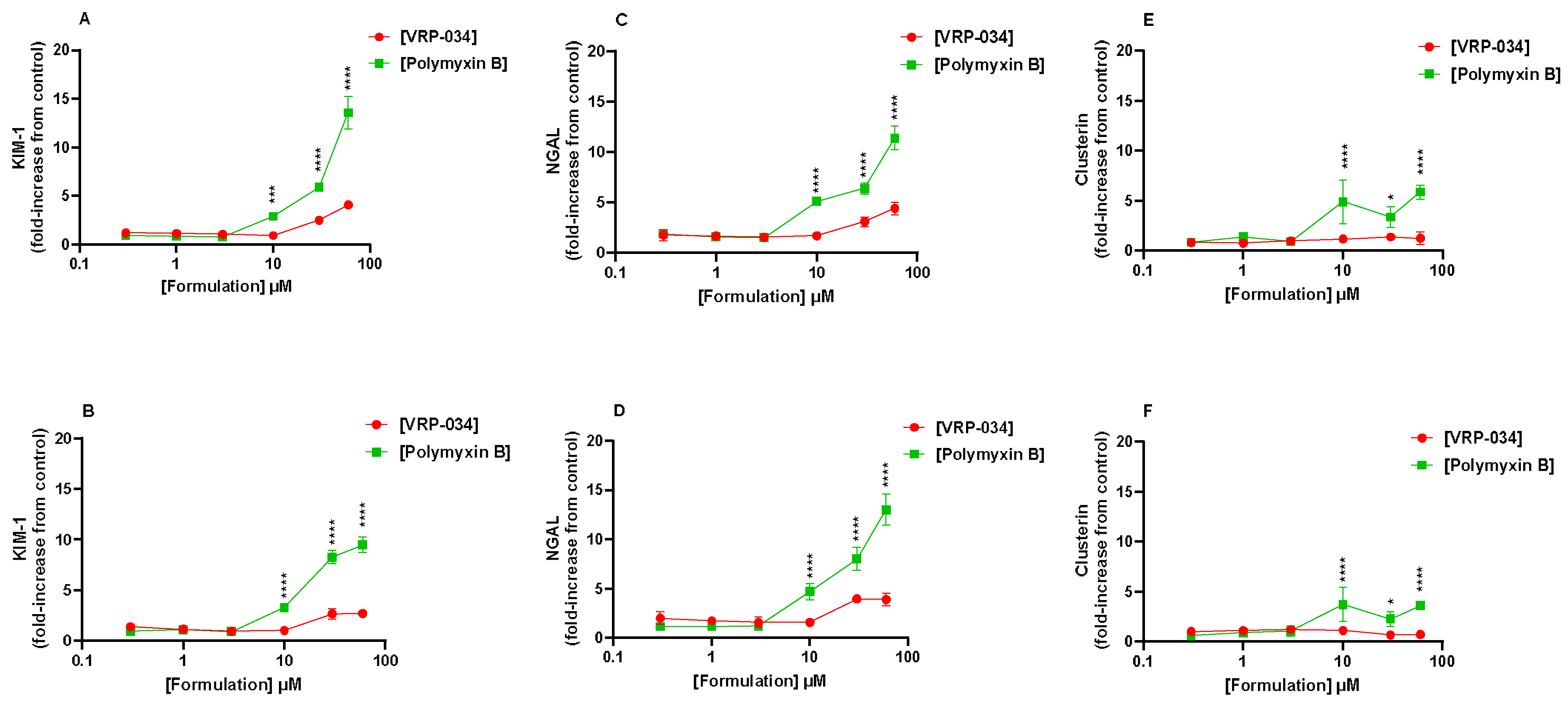
2.3. Comparison of VRP-034 and Polymyxin B Treatment on Biomarker Production from hPTC Monolayers
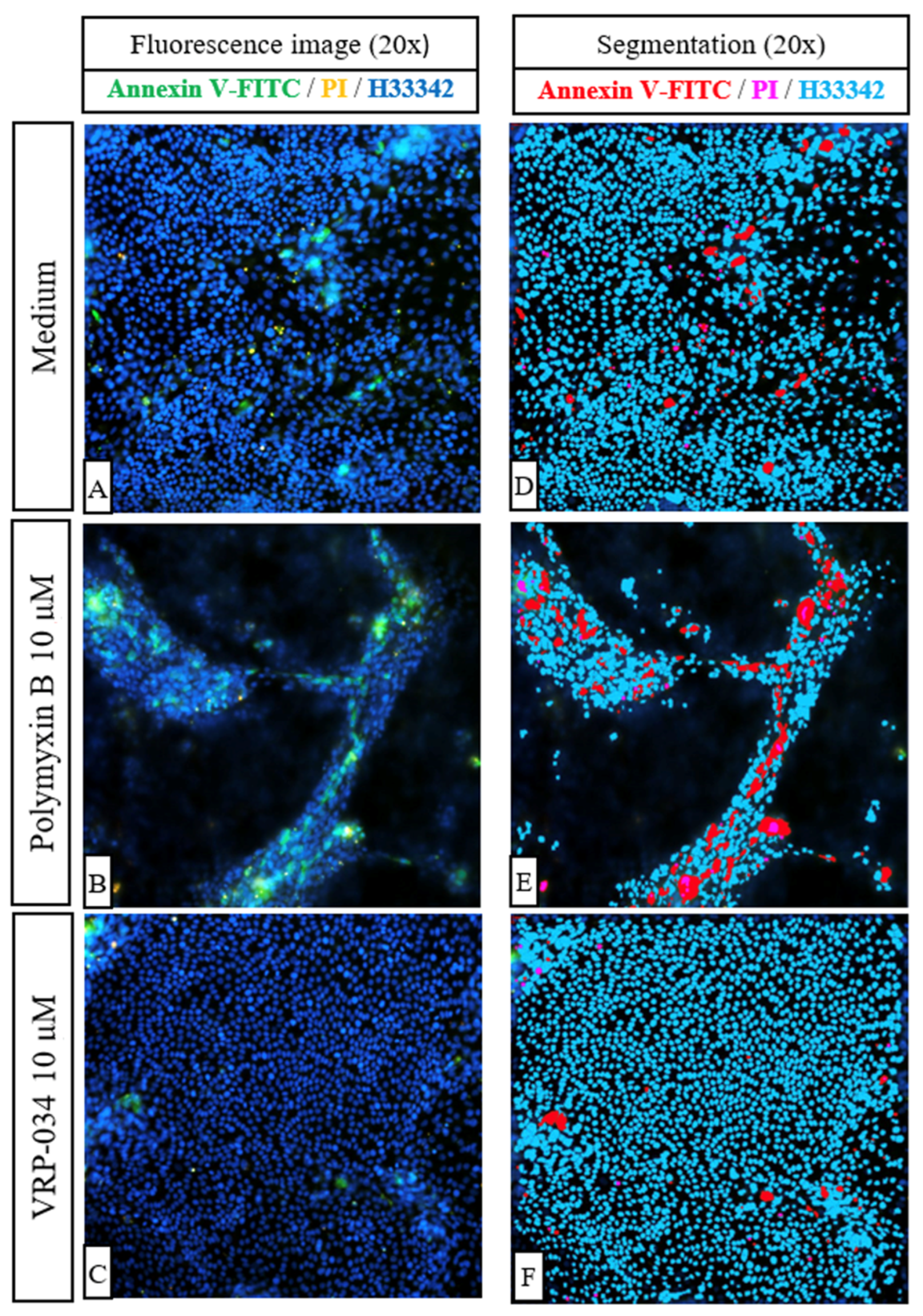
2.4. Comparison of Effects of VRP-034 and Polymyxin B Treatment on Apoptosis (Annexin V-FITC) and Necrosis (PI) in hPTC Monolayers
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Primary Human Proximal Tubule Monolayers
4.3. FITC-Albumin Uptake
4.4. TEER, ATP, and LDH Assays
4.5. Biomarker Quantification
4.6. Annexin V-FITC/PI Apoptosis/Necrosis Assay Imaging
4.7. Calculations, Data Processing, and Statistics
Biomarker Calculations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Miethke, M.; Pieroni, M.; Weber, T.; Bronstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Singh, O.; Juneja, D.; Tyagi, N.; Khurana, A.S.; Qamra, A.; Motlekar, S.; Barkate, H. Resurgence of Polymyxin B for MDR/XDR Gram-Negative Infections: An Overview of Current Evidence. Crit. Care Res. Pract. 2017, 2017, 3635609. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Hagos, B.; Edessa, D.; Tadiwos, Y.; Mekuria, A.N. Polymyxin-induced nephrotoxicity and its predictors: A systematic review and meta-analysis of studies conducted using RIFLE criteria of acute kidney injury. Pharmacol. Res. 2021, 163, 105328. [Google Scholar] [CrossRef] [PubMed]
- Kubin, C.J.; Ellman, T.M.; Phadke, V.; Haynes, L.J.; Calfee, D.P.; Yin, M.T. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J. Infect. 2012, 65, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [CrossRef] [PubMed]
- Vattimo Mde, F.; Watanabe, M.; da Fonseca, C.D.; Neiva, L.B.; Pessoa, E.A.; Borges, F.T. Polymyxin B Nephrotoxicity: From Organ to Cell Damage. PLoS ONE 2016, 11, e0161057. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Kulkarni, A.; Chaudhary, M.; Chaudhary, S.; Payasi, A.; Aggarwal, A. Polymyxin B-Induced Kidney Injury Assessment of a Novel Formulation of Polymyxin B (VRP-034) in Rats. Antibiotics 2021, 10, 359. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Payasi, A.; Kumar, S.; Sharma, A.; Chaudhary, S.; Aggarwal, A. P05 In vivo efficacy of VRP-034 in lung and thigh infection models. JAC-Antimicrob. Resist. 2022, 4 (Suppl. 1), dlac004-004. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Chilbule, R.; Chaudhary, M.; Sharma, A.; Chaudhary, S.; Aggarwal, A. Pharmacokinetics and Renal Accumulation of VRP-034; British Society for Antimicrobial Chemotherapy: London, UK, 2021. [Google Scholar]
- Murkunde, Y.; Chaudhary, S.; Vishwakarma, K.; Aggarwal, A.; Chilbule, R. Toxicokinetic Profile of VRP-034; British Society for Antimicrobial Chemotherapy: London, UK, 2021. [Google Scholar]
- Payasi, A.; Kumar, S.; Chaudhary, S.; Aggarwal, A. In Vitro Activity of VRP-034 Against Metallo-Beta-Lactamases Producing Gram-Negative Pathogens Responsible for Human Infections. In Proceedings of the European Congress of Clinical Microbiology and Infectious Disease, ECCMID, Lisbon, Portugal, 23–26 April 2022. [Google Scholar]
- Murkunde, Y.; Vishwakarma, K.; Chaudhary, S.; Kumar, P.; Kumar, S.; Aggarwal, A. Polymyxin B-Associated Kidney Injury Assessment of VRP-034 in Rats Using a Panel of Early Kidney Injury Biomarkers. In Proceedings of the American Society of Microbiology, ASM Microbe, Washington, DC, USA, 9–13 June 2022. [Google Scholar]
- Suzuki, T.; Yamaguchi, H.; Ogura, J.; Kobayashi, M.; Yamada, T.; Iseki, K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob. Agents Chemother. 2013, 57, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Chung, G.; Pye, K.; Yukawa, T.; Imanishi, A.; Takai, Y.; Brown, C.; Wagoner, M.P. Freshly isolated primary human proximal tubule cells as an in vitro model for the detection of renal tubular toxicity. Toxicology 2020, 442, 152535. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.R.; Faubel, S.; Edelstein, C.L. Biomarkers of Drug-Induced Kidney Toxicity. Ther. Drug Monit. 2019, 41, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Finnin, B.A.; Poudyal, A.; Davis, K.; Li, J.; Hill, P.A.; Nation, R.L.; Velkov, T.; Li, J. Polymyxin B Induces Apoptosis in Kidney Proximal Tubular Cells. Antimicrob. Agents Chemother. 2013, 57, 4329–4335. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Weyer, K.; Rbaibi, Y.; Long, K.R.; Tan, R.J.; Nielsen, R.; Christensen, E.I.; Baty, C.J.; Kashlan, O.B.; Weisz, O.A. Distinct functions of megalin and cubilin receptors in recovery of normal and nephrotic levels of filtered albumin. Am. J. Physiol. Renal Physiol. 2020, 318, F1284–F1294. [Google Scholar] [CrossRef] [PubMed]
- Vinken, P.; Starckx, S.; Barale-Thomas, E.; Looszova, A.; Sonee, M.; Goeminne, N.; Versmissen, L.; Buyens, K.; Lampo, A. Tissue Kim-1 and urinary clusterin as early indicators of cisplatin-induced acute kidney injury in rats. Toxicol. Pathol. 2012, 40, 1049–1062. [Google Scholar] [CrossRef]
- Bailly, V.; Zhang, Z.; Meier, W.; Cate, R.; Sanicola, M.; Bonventre, J.V. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 2002, 277, 39739–39748. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31. [Google Scholar] [CrossRef]
- Bonventre, J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003, 14 (Suppl. 1), S55–S61. [Google Scholar] [CrossRef]
- Nation, R.L.; Rigatto, M.H.P.; Falci, D.R.; Zavascki, A.P. Polymyxin Acute Kidney Injury: Dosing and Other Strategies to Reduce Toxicity. Antibiotics 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.D.; Sayer, R.; Windass, A.S.; Haslam, I.S.; De Broe, M.E.; D’Haese, P.C.; Verhulst, A. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol. Appl. Pharmacol. 2008, 233, 428–438. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pye, K.; Tasinato, E.; Shuttleworth, S.; Devlin, C.; Brown, C. Comparison of the Impact of VRP-034 and Polymyxin B upon Markers of Kidney Injury in Human Proximal Tubule Monolayers In Vitro. Antibiotics 2024, 13, 530. https://doi.org/10.3390/antibiotics13060530
Pye K, Tasinato E, Shuttleworth S, Devlin C, Brown C. Comparison of the Impact of VRP-034 and Polymyxin B upon Markers of Kidney Injury in Human Proximal Tubule Monolayers In Vitro. Antibiotics. 2024; 13(6):530. https://doi.org/10.3390/antibiotics13060530
Chicago/Turabian StylePye, Keith, Elena Tasinato, Siannah Shuttleworth, Claire Devlin, and Colin Brown. 2024. "Comparison of the Impact of VRP-034 and Polymyxin B upon Markers of Kidney Injury in Human Proximal Tubule Monolayers In Vitro" Antibiotics 13, no. 6: 530. https://doi.org/10.3390/antibiotics13060530
APA StylePye, K., Tasinato, E., Shuttleworth, S., Devlin, C., & Brown, C. (2024). Comparison of the Impact of VRP-034 and Polymyxin B upon Markers of Kidney Injury in Human Proximal Tubule Monolayers In Vitro. Antibiotics, 13(6), 530. https://doi.org/10.3390/antibiotics13060530




