Antimicrobial and Antibiofilm Effects of Bithionol against Mycobacterium abscessus
Abstract
1. Introduction
2. Results
2.1. Screening the FDA Drug Library for Activity against M. abscessus
2.2. Activity against M. abscessus Clinical Strains
2.3. MIC and MBC of Bithionol against M. abscessus in Different Media
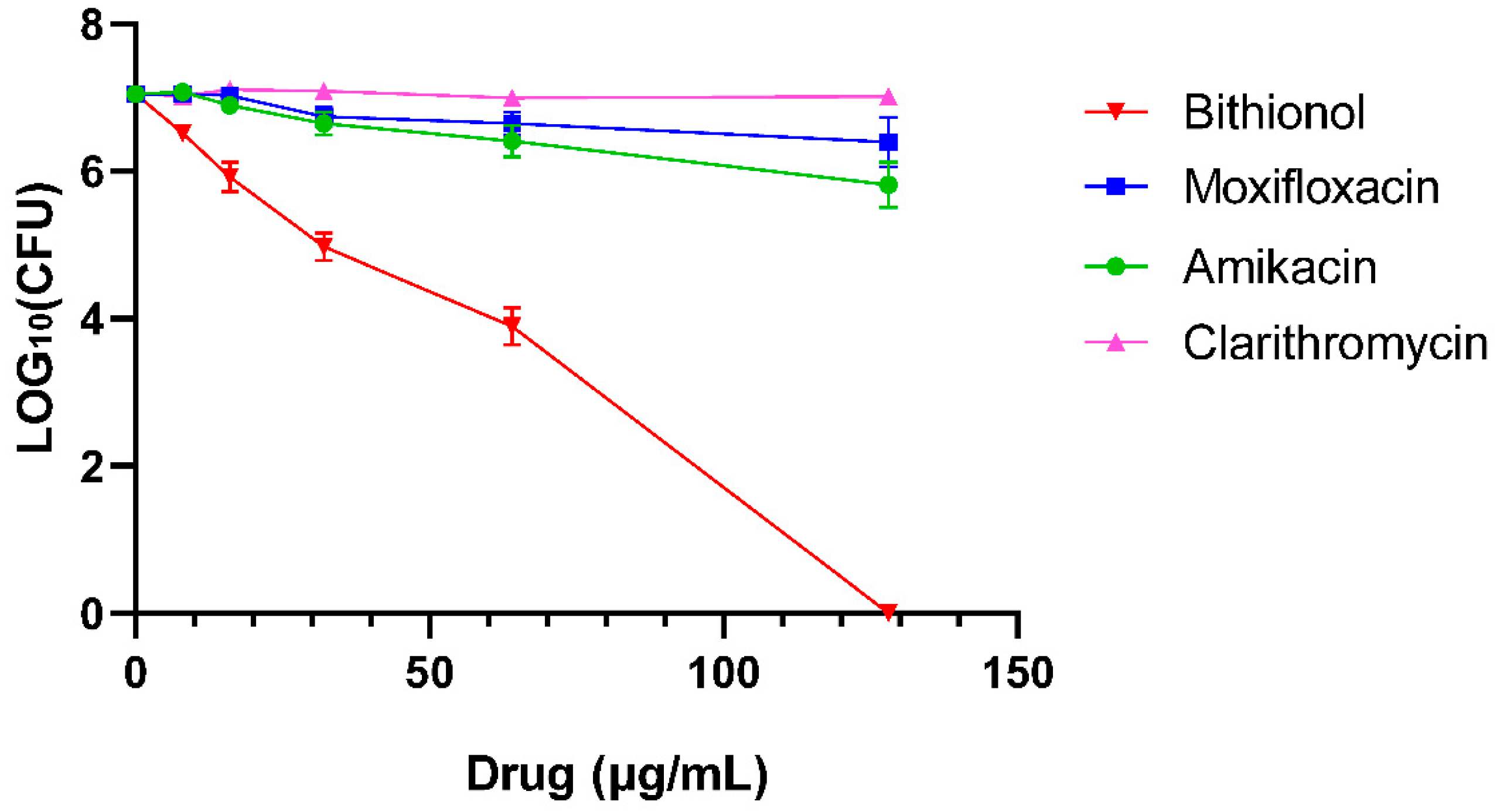
2.4. Time–Kill Assay
2.5. Antibiofilm Effect of Bithionol
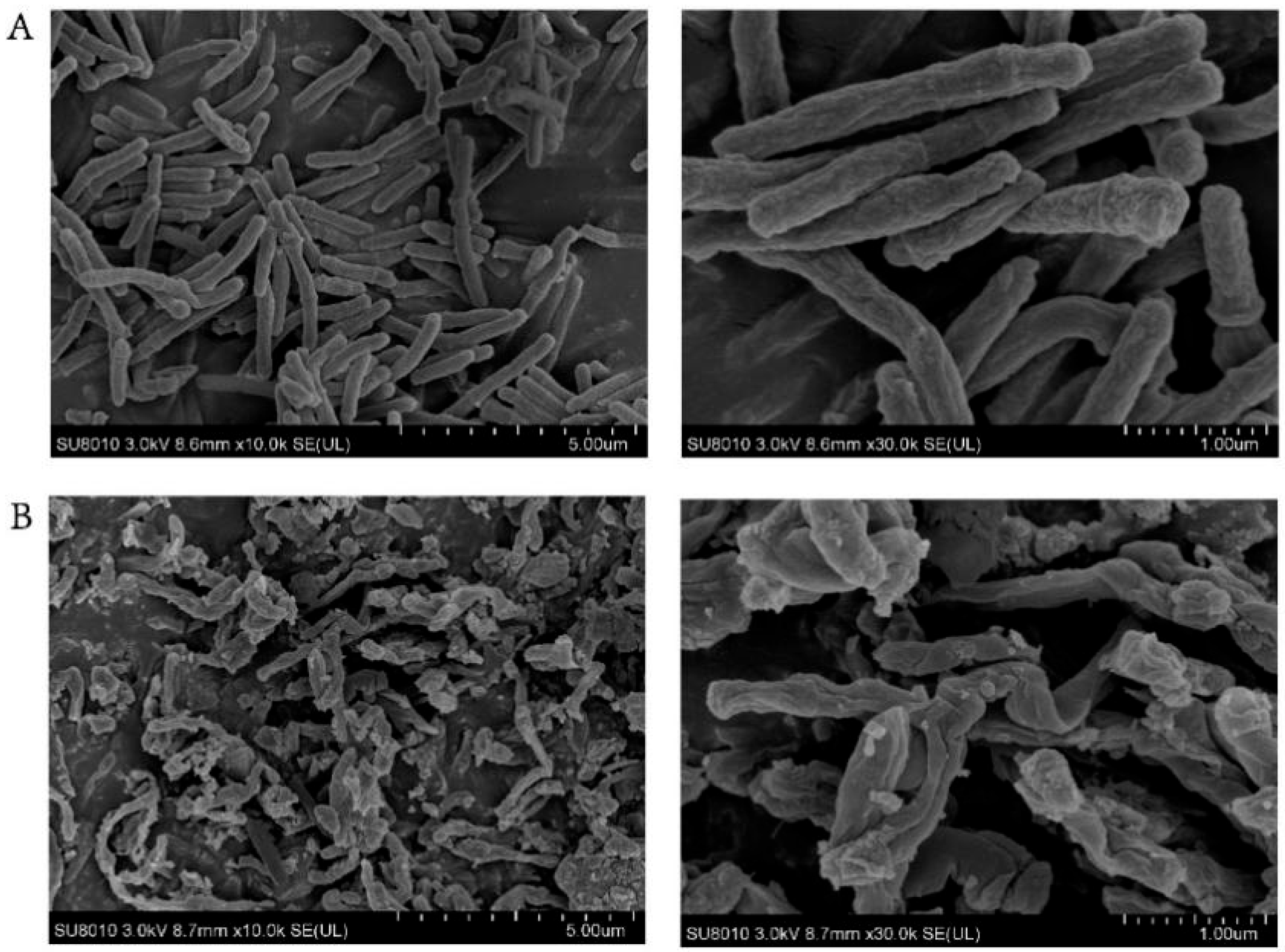
2.6. Scanning Electron Microscopy (SEM) Studies
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Compounds and Drug Library
4.3. Screening Assay
4.4. Minimal Inhibitory Concentration Assay against M. abscessus
4.5. Minimum Bactericidal Concentration against M. abscessus
4.6. Time–Kill Curve
4.7. Biofilm Assays
4.8. Scanning Electron Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.R.; Sheng, W.H.; Hung, C.C.; Yu, C.J.; Lee, L.N.; Hsueh, P.R. Mycobacterium abscessus Complex Infections in Humans. Emerg. Infect. Dis. 2015, 21, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.M.; Grogono, D.M.; Greaves, D.; Foweraker, J.; Roddick, I.; Inns, T.; Reacher, M.; Haworth, C.S.; Curran, M.D.; Harris, S.R.; et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet 2013, 381, 1551–1560. [Google Scholar] [CrossRef]
- Kam, J.Y.; Hortle, E.; Krogman, E.; Warner, S.E.; Wright, K.; Luo, K.; Cheng, T.; Manuneedhi Cholan, P.; Kikuchi, K.; Triccas, J.A.; et al. Rough and smooth variants of Mycobacterium abscessus are differentially controlled by host immunity during chronic infection of adult zebrafish. Nat. Commun. 2022, 13, 952. [Google Scholar] [CrossRef] [PubMed]
- Catherinot, E.; Roux, A.L.; Macheras, E.; Hubert, D.; Matmar, M.; Dannhoffer, L.; Chinet, T.; Morand, P.; Poyart, C.; Heym, B.; et al. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J. Clin. Microbiol. 2009, 47, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Ojano-Dirain, C.; Yang, Q.; Liu, L.; Lu, L.; Progulske-Fox, A.; Wang, G.P.; Antonelli, P.; Schultz, G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am. J. Respir. Crit. Care Med. 2016, 193, 692–693. [Google Scholar] [CrossRef]
- Qvist, T.; Eickhardt, S.; Kragh, K.N.; Andersen, C.B.; Iversen, M.; Høiby, N.; Bjarnsholt, T. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur. Respir. J. 2015, 46, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Hunt-Serracin, A.C.; Parks, B.J.; Boll, J.; Boutte, C.C. Mycobacterium abscessus Cells Have Altered Antibiotic Tolerance and Surface Glycolipids in Artificial Cystic Fibrosis Sputum Medium. Antimicrob. Agents Chemother. 2019, 63, e02488-18. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Guo, Q.; He, S.; Fan, J.; Xu, L.; Zhang, Z.; Wu, W.; Chu, H. Antibacterial peptide RP557 increases the antibiotic sensitivity of Mycobacterium abscessus by inhibiting biofilm formation. Sci. Total Environ. 2022, 807, 151855. [Google Scholar] [CrossRef]
- Cowman, S.; van Ingen, J.; Griffith, D.E.; Loebinger, M.R. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 2019, 54, 1900250. [Google Scholar] [CrossRef]
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Greendyke, R.; Byrd, T.F. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob. Agents Chemother. 2008, 52, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Matsuyama, K.; Hutson, C.; Madrid, P. Identification of antimicrobial activity among FDA-approved drugs for combating Mycobacterium abscessus and Mycobacterium chelonae. J. Antimicrob. Chemother. 2011, 66, 1533–1536. [Google Scholar] [CrossRef]
- Gupta, R.; Netherton, M.; Byrd, T.F.; Rohde, K.H. Reporter-Based Assays for High-Throughput Drug Screening against Mycobacterium abscessus. Front. Microbiol. 2017, 8, 2204. [Google Scholar] [CrossRef]
- Kim, T.; Hanh, B.T.; Heo, B.; Quang, N.; Park, Y.; Shin, J.; Jeon, S.; Park, J.W.; Samby, K.; Jang, J. A Screening of the MMV Pandemic Response Box Reveals Epetraborole as a New Potent Inhibitor against Mycobacterium abscessus. Int. J. Mol. Sci. 2021, 22, 5936. [Google Scholar] [CrossRef] [PubMed]
- Malin, J.J.; Winter, S.; van Gumpel, E.; Plum, G.; Rybniker, J. Extremely Low Hit Rate in a Diverse Chemical Drug Screen Targeting Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63, e01008-19. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, G.; Moon, C.; Kim, H.J.; Kim, T.H.; Jang, J. Pathogen Box screening for hit identification against Mycobacterium abscessus. PLoS ONE 2018, 13, e0195595. [Google Scholar] [CrossRef] [PubMed]
- Hanh, B.T.B.; Park, J.W.; Kim, T.H.; Kim, J.S.; Yang, C.S.; Jang, K.; Cui, J.; Oh, D.C.; Jang, J. Rifamycin O, An Alternative Anti-Mycobacterium abscessus Agent. Molecules 2020, 25, 1597. [Google Scholar] [CrossRef] [PubMed]
- Low, J.L.; Wu, M.L.; Aziz, D.B.; Laleu, B.; Dick, T. Screening of TB Actives for Activity against Nontuberculous Mycobacteria Delivers High Hit Rates. Front. Microbiol. 2017, 8, 1539. [Google Scholar] [CrossRef]
- Berube, B.J.; Castro, L.; Russell, D.; Ovechkina, Y.; Parish, T. Novel Screen to Assess Bactericidal Activity of Compounds Against Non-replicating Mycobacterium abscessus. Front. Microbiol. 2018, 9, 2417. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of Bedaquiline against Mycobacterium abscessus Complex. Antimicrob. Agents Chemother. 2019, 63, e01919-18. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, Q.; Zhao, L.; Xu, L.; Fan, J.; Wu, W.; Zhang, Z.; Li, B.; Chu, H. Sitafloxacin Expresses Potent Anti-Mycobacterium abscessus Activity. Front. Microbiol. 2021, 12, 779531. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Guo, Q.; He, S.; Xu, L.; Zhang, Z.; Ma, J.; Chu, H. In vitro activity of rifabutin against Mycobacterium abscessus, clinical isolates. Clin. Exp. Pharmacol. Physiol. 2022, 49, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Compain, F.; Soroka, D.; Heym, B.; Gaillard, J.L.; Herrmann, J.L.; Dorchène, D.; Arthur, M.; Dubée, V. In vitro activity of tedizolid against the Mycobacterium abscessus complex. Diagn. Microbiol. Infect. Dis. 2018, 90, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.M.; van Rossum, M.; Koeken, V.; Pennings, L.J.; Svensson, E.M.; Ruesen, C.; Bowles, E.C.; Wertheim, H.F.L.; Hoefsloot, W.; van Ingen, J. Auranofin Activity Exposes Thioredoxin Reductase as a Viable Drug Target in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2019, 63, e00449-19. [Google Scholar] [CrossRef] [PubMed]
- Bich Hanh, B.T.; Quang, N.T.; Park, Y.; Heo, B.E.; Jeon, S.; Park, J.W.; Jang, J. Omadacycline Potentiates Clarithromycin Activity Against Mycobacterium abscessus. Front. Pharmacol. 2021, 12, 790767. [Google Scholar] [CrossRef]
- Selchow, P.; Ordway, D.J.; Verma, D.; Whittel, N.; Petrig, A.; Hobbie, S.N.; Böttger, E.C.; Sander, P. Apramycin Overcomes the Inherent Lack of Antimicrobial Bactericidal Activity in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2022, 66, e0151021. [Google Scholar] [CrossRef] [PubMed]
- McParland, C.; Nunn, M.; Marras, T.K.; Chiasson, M. Eradication of Mycobacterium abscessus infection in cystic fibrosis with initiation of Elexacaftor/Tezacaftor/Ivacaftor. J. Cyst. Fibros. 2024, 23, 38–40. [Google Scholar] [CrossRef]
- Bacq, Y.; Besnier, J.M.; Duong, T.H.; Pavie, G.; Metman, E.H.; Choutet, P. Successful treatment of acute fascioliasis with bithionol. Hepatology 1991, 14, 1066–1069. [Google Scholar] [CrossRef]
- Guo, W.; Liu, Y.; Yao, Z.; Zhou, H.; Wang, X.; Huang, Z.; Zhang, X.; Wu, Q.; Zhou, T. Bithionol Restores Sensitivity of Multidrug-Resistant Gram-Negative Bacteria to Colistin with Antimicrobial and Anti-biofilm Effects. ACS Infect. Dis. 2023, 9, 1634–1646. [Google Scholar] [CrossRef]
- Kim, W.; Zou, G.; Hari, T.P.A.; Wilt, I.K.; Zhu, W.; Galle, N.; Faizi, H.A.; Hendricks, G.L.; Tori, K.; Pan, W.; et al. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2019, 116, 16529–16534. [Google Scholar] [CrossRef]
- She, P.; Wang, Y.; Li, Y.; Zhou, L.; Li, S.; Zeng, X.; Liu, Y.; Xu, L.; Wu, Y. Drug Repurposing: In vitro and in vivo Antimicrobial and Antibiofilm Effects of Bithionol Against Enterococcus faecalis and Enterococcus faecium. Front. Microbiol. 2021, 12, 579806. [Google Scholar] [CrossRef]
- Luo, Y.; Wen, Z.; Xiong, Y.; Chen, X.; Shen, Z.; Li, P.; Peng, Y.; Deng, Q.; Yu, Z.; Zheng, J.; et al. The potential target of bithionol against Staphylococcus aureus: Design, synthesis and application of biotinylated probes Bio-A2. J. Antibiot. 2023, 76, 406–415. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Dong, B.; Li, C.; Jian, L.; He, J.; Zeng, J.; Zhou, Q.; Jia, D.; Luo, Y.; et al. Discovery and Mechanistic Study of Mycobacterium tuberculosis PafA Inhibitors. J. Med. Chem. 2022, 65, 11058–11065. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; She, P.; Fu, J.; Peng, C.; Wu, Y. Identification of Eltrombopag as a Repurposing Drug Against Staphylococcus epidermidis and its Biofilms. Curr. Microbiol. 2021, 78, 1159–1167. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Hwang, J.; Park, S.; Kim, N.; Kim, K.; Lee, H.; Shum, D.; Jang, S. Repurposing Eltrombopag for Multidrug Resistant Staphylococcus aureus Infections. Antibiotics 2021, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H.; Del Rosso, J.Q.; Layton, A.M.; Schauber, J. Over 25 Years of Clinical Experience With Ivermectin: An Overview of Safety for an Increasing Number of Indications. J. Drugs Dermatol. 2016, 15, 325–332. [Google Scholar]
- Ashraf, S.; Chaudhry, U.; Raza, A.; Ghosh, D.; Zhao, X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob. Resist. Infect. Control 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; She, P.; Tan, F.; Li, S.; Zeng, X.; Chen, L.; Luo, Z.; Wu, Y. Repurposing Antispasmodic Agent Otilonium Bromide for Treatment of Staphylococcus aureus Infections. Front. Microbiol. 2020, 11, 1720. [Google Scholar] [CrossRef]
- Xu, C.; Liu, C.; Chen, K.; Zeng, P.; Chan, E.W.C.; Chen, S. Otilonium bromide boosts antimicrobial activities of colistin against Gram-negative pathogens and their persisters. Commun. Biol. 2022, 5, 613. [Google Scholar] [CrossRef]
- Heo, Y.A.; Deeks, E.D. Sofosbuvir/Velpatasvir/Voxilaprevir: A Review in Chronic Hepatitis C. Drugs 2018, 78, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Fusidic Acid: A Bacterial Elongation Factor Inhibitor for the Oral Treatment of Acute and Chronic Staphylococcal Infections. Cold Spring Harb. Perspect. Med. 2016, 6, a025437. [Google Scholar] [CrossRef] [PubMed]
- Cicek-Saydam, C.; Cavusoglu, C.; Burhanoglu, D.; Hilmioglu, S.; Ozkalay, N.; Bilgic, A. In vitro susceptibility of Mycobacterium tuberculosis to fusidic acid. Clin. Microbiol. Infect. 2001, 7, 700–702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Przybylski, P.; Pyta, K.; Stefańska, J.; Ratajczak-Sitarz, M.; Katrusiak, A.; Huczyński, A.; Brzezinski, B. Synthesis, crystal structures and antibacterial activity studies of aza-derivatives of phytoalexin from cotton plant—Gossypol. Eur. J. Med. Chem. 2009, 44, 4393–4403. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Zhang, S.; Shi, W.; Zhang, W.; Zhu, M.; Zhang, Y. Novel Mutations Associated with Clofazimine Resistance in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2018, 62, e00544-18. [Google Scholar] [CrossRef]

| Product Name | CAS No. | M.Wt | Research Area | Clinical Information | MIC (μM) | MIC (μg/mL) |
|---|---|---|---|---|---|---|
| Bleomycin (hydrochloride) | 67763-87-5 | 1451.00 | Cancer | Launched | 0.02 | 0.23 |
| Bleomycin (sulfate) | 9041-93-4 | 1512.62 | Cancer | Launched | 0.02 | 0.24 |
| Bedaquiline (fumarate) | 845533-86-0 | 671.58 | Infection | Launched | 0.625 | 0.42 |
| Closantel | 57808-65-8 | 663.07 | Infection | No Development Reported | 0.625 | 0.41 |
| Closantel (sodium) | 61438-64-0 | 685.06 | Infection | No Development Reported | 0.625 | 0.43 |
| Clarithromycin | 81103-11-9 | 747.95 | Infection; Cancer | Launched | 1.25 | 0.93 |
| Sitafloxacin (hydrate) | 163253-35-8 | 436.84 | Infection | Launched | 1.25 | 0.55 |
| Tigecycline (tetramesylate) | 970.07 | Infection; Cancer | Launched | 1.25 | 1.21 | |
| Bithionol | 97-18-7 | 356.05 | parasite | Launched | 2.5 | 0.89 |
| Auranofin | 34031-32-8 | 680.50 | Cancer; Infection; Inflammation/Immunology | Launched | 2.5 | 1.70 |
| Rifamycin (sodium) | 14897-39-3 | 719.75 | Infection | Launched | 2.5 | 1.80 |
| Regorafenib (Hydrochloride) | 835621-07-3 | 519.28 | Cancer | Launched | 5 | 2.60 |
| Gatifloxacin (hydrochloride) | 121577-32-0 | 411.86 | Infection | Launched | 5 | 2.06 |
| Rifamycin S | 13553-79-2 | 695.75 | Infection | No Development Reported | 5 | 3.48 |
| Omadacycline (hydrochloride) | 1196800-39-1 | 593.11 | Infection | Launched | 5 | 2.97 |
| Eltrombopag (Olamine) | 496775-62-3 | 564.63 | Cardiovascular Disease; Cancer | Launched | 5 | 2.82 |
| Eltrombopag | 496775-61-2 | 442.47 | Cardiovascular Disease; Cancer | Launched | 5 | 2.21 |
| Ivermectin | 70288-86-7 | 875.09 | Infection; Cancer | Launched | 5 | 4.38 |
| Rifabutin | 72559-06-9 | 847.00 | Infection | Launched | 5 | 4.24 |
| Doramectin | 117704-25-3 | 899.11 | Infection | No Development Reported | 5 | 4.50 |
| Oxyclozanide | 2277-92-1 | 401.46 | Infection | No Development Reported | 5 | 2.01 |
| Chlorhexidine | 55-56-1 | 505.45 | Infection | Launched | 5 | 2.53 |
| Hypericin | 548-04-9 | 504.44 | Cancer | Phase 1 | 5 | 2.52 |
| Lapatinib (ditosylate) | 388082-77-7 | 925.46 | Cancer | Launched | 10 | 9.25 |
| Entrectinib | 1108743-60-7 | 560.64 | Cancer | Launched | 10 | 5.61 |
| Ebselen | 60940-34-3 | 274.18 | Cancer; Infection; Inflammation/Immunology; Neurological Disease | Phase 3 | 10 | 2.74 |
| Mirodenafil (dihydrochloride) | 862189-96-6 | 604.59 | Others | Launched | 10 | 6.05 |
| Tedizolid | 856866-72-3 | 370.34 | Infection | Launched | 10 | 3.70 |
| Olverembatinib (dimesylate) | 1421783-64-3 | 724.77 | Cancer | Launched | 10 | 7.25 |
| Olverembatinib | 1257628-77-5 | 532.56 | Cancer | Launched | 10 | 5.33 |
| Sonidegib | 956697-53-3 | 485.50 | Cancer | Launched | 10 | 4.86 |
| Sonidegib (diphosphate) | 1218778-77-8 | 681.49 | Cancer | Launched | 10 | 6.81 |
| Idarubicin (hydrochloride) | 57852-57-0 | 533.95 | Cancer; Infection | Launched | 10 | 5.34 |
| Fidaxomicin | 873857-62-6 | 1058.04 | Infection | Launched | 10 | 10.58 |
| Marbofloxacin | 115550-35-1 | 362.36 | Infection | No Development Reported | 10 | 3.62 |
| Levofloxacin (hydrate) | 138199-71-0 | 370.38 | Infection | Launched | 10 | 3.70 |
| Ciprofloxacin (hydrochloride monohydrate) | 86393-32-0 | 385.82 | Infection | Launched | 10 | 3.86 |
| Roxithromycin | 80214-83-1 | 837.05 | Infection | Launched | 10 | 8.37 |
| Otilonium (bromide) | 26095-59-0 | 563.57 | Neurological Disease | Launched | 10 | 5.64 |
| Tilmicosin | 108050-54-0 | 869.13 | Infection | No Development Reported | 10 | 8.69 |
| Benzethonium chloride | 121-54-0 | 448.08 | Neurological Disease | Launched | 10 | 4.48 |
| Erythromycin Ethylsuccinate | 1264-62-6 | 862.05 | Infection | Launched | 10 | 8.62 |
| Cetrimonium (bromide) | 57-09-0 | 364.45 | Cancer | No Development Reported | 10 | 3.64 |
| Apramycin (sulfate) | 65710-07-8 | 637.66 | Infection; Cancer | Phase 1 | 10 | 6.38 |
| Aclacinomycin A hydrochloride | 75443-99-1 | 848.33 | Cancer | Launched | 10 | 8.48 |
| Gossypol | 303-45-7 | 518.55 | Infection, Cancer | Launched | 20 | 10.37 |
| Gossypol (acetic acid) | 12542-36-8 | 578.61 | Cancer | Launched | 20 | 11.57 |
| Abiraterone acetate | 154229-18-2 | 391.55 | Cancer | Launched | 20 | 7.83 |
| Fusidic acid | 6990-06-3 | 516.71 | Infection | Launched | 20 | 10.33 |
| Rifaximin | 80621-81-4 | 785.88 | Infection | Launched | 20 | 15.72 |
| Ivacaftor | 873054-44-5 | 392.49 | Endocrinology | Launched | 20 | 7.85 |
| Mitoxantrone | 65271-80-9 | 444.48 | Cancer | Launched | 20 | 8.89 |
| Tamoxifen (Citrate) | 54965-24-1 | 563.64 | Cancer | Launched | 20 | 11.27 |
| Voxilaprevir | 1535212-07-7 | 868.93 | Infection | Launched | 20 | 17.38 |
| Rafoxanide | 22662-39-1 | 626.01 | Infection | No Development Reported | 20 | 12.52 |
| Crystal Violet | 548-62-9 | 407.98 | Infection | Phase 3 | 20 | 8.16 |
| Novobiocin (Sodium) | 1476-53-5 | 634.61 | Infection; Cancer | Launched | 20 | 12.69 |
| Zinc Pyrithione | 13463-41-7 | 317.69 | Cardiovascular Disease | Launched | 20 | 6.35 |
| Nitroxoline | 4008-48-4 | 190.16 | Infection; Cancer | Launched | 20 | 3.80 |
| Fusidic acid (sodium salt) | 751-94-0 | 538.69 | Infection | Launched | 20 | 10.77 |
| Cetylpyridinium (chloride monohydrate) | 6004-24-6 | 358.00 | Infection | Launched | 20 | 7.16 |
| Amikacin (disulfate) | 39831-55-5 | 781.76 | Infection | Launched | 20 | 15.64 |
| Isolate | Subspecies | Morphotype | Clarithromycin Susceptibility | MIC (μM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bithionol | Eltrombopag | Ivermectin | Otilonium (Bromide) | Voxilaprevir | Fusidic Acid | Gossypol | ||||
| 49 | abscessuss | Smooth | Resistant (≥16 μg/mL) | 0.625 | 5 | 5 | 10 | 20 | 10 | 20 |
| 97 | abscessuss | Smooth | Resistant (≥16 μg/mL) | 1.25 | 5 | 5 | 10 | 20 | 20 | 20 |
| 2338 | abscessuss | Rough | Resistant (≥16 μg/mL) | 1.25 | 5 | 2.5 | 10 | 10 | 20 | 10 |
| 2136 | abscessuss | Smooth | Resistant (≥16 μg/mL) | 1.25 | 5 | 5 | 10 | 10 | 10 | 10 |
| 835 | abscessuss | Smooth | Sensitive (2 μg/mL) | 2.5 | 2.5 | 2.5 | 10 | 2.5 | 20 | 20 |
| 908 | abscessuss | Rough | Sensitive (1 μg/mL) | 1.25 | 2.5 | 10 | 10 | 20 | 2.5 | 20 |
| 905 | massilience | Smooth | Sensitive (0.0625 μg/mL) | 2.5 | 5 | 5 | 10 | 10 | 20 | 20 |
| 2075 | massilience | Rough | Sensitive (0.0625 μg/mL) | 1.25 | 1.25 | 5 | 10 | 20 | 2.5 | 20 |
| 1808 | bolletii | Smooth | Sensitive (2 μg/mL) | 2.5 | 5 | 2.5 | 10 | 10 | 20 | 10 |
| 1783 | bolletii | Smooth | Sensitive (0.5 μg/mL) | 2.5 | 10 | 2.5 | 10 | 10 | 20 | 20 |
| Isolate | MIC (7H9) μg/mL | MBC (7H9) μg/mL | MIC (CAMHB) μg/mL | MBC (CAMHB) μg/mL |
|---|---|---|---|---|
| 49 | 0.25 | 1 | 0.5 | 2 |
| 97 | 0.25 | 0.5 | 0.5 | 2 |
| 2136 | 0.25 | 0.5 | 0.5 | 2 |
| 2338 | 0.25 | 0.5 | 0.5 | 1 |
| 19977 | 0.5 | 1 | 2 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, D.; Yuan, X.; Jiang, X.; Wu, T.; Xiang, Y.; Ji, Z.; Liu, J.; Dong, X.; Bi, K.; Tønjum, T.; et al. Antimicrobial and Antibiofilm Effects of Bithionol against Mycobacterium abscessus. Antibiotics 2024, 13, 529. https://doi.org/10.3390/antibiotics13060529
Cao D, Yuan X, Jiang X, Wu T, Xiang Y, Ji Z, Liu J, Dong X, Bi K, Tønjum T, et al. Antimicrobial and Antibiofilm Effects of Bithionol against Mycobacterium abscessus. Antibiotics. 2024; 13(6):529. https://doi.org/10.3390/antibiotics13060529
Chicago/Turabian StyleCao, Dan, Xin Yuan, Xiuzhi Jiang, Tiantian Wu, Yanghui Xiang, Zhongkang Ji, Jiaying Liu, Xu Dong, Kefan Bi, Tone Tønjum, and et al. 2024. "Antimicrobial and Antibiofilm Effects of Bithionol against Mycobacterium abscessus" Antibiotics 13, no. 6: 529. https://doi.org/10.3390/antibiotics13060529
APA StyleCao, D., Yuan, X., Jiang, X., Wu, T., Xiang, Y., Ji, Z., Liu, J., Dong, X., Bi, K., Tønjum, T., Xu, K., & Zhang, Y. (2024). Antimicrobial and Antibiofilm Effects of Bithionol against Mycobacterium abscessus. Antibiotics, 13(6), 529. https://doi.org/10.3390/antibiotics13060529







