Triple-Antibiotic Combination Exerts Effective Activity against Mycobacterium avium subsp. hominissuis Biofilm and Airway Infection in an In Vivo Murine Model
Abstract
1. Introduction
2. Results
2.1. The Combination of CLR, RFB, and CFZ Is More Effective Than a Single Antibiotic or a Double Combination against Bacterial Biofilms
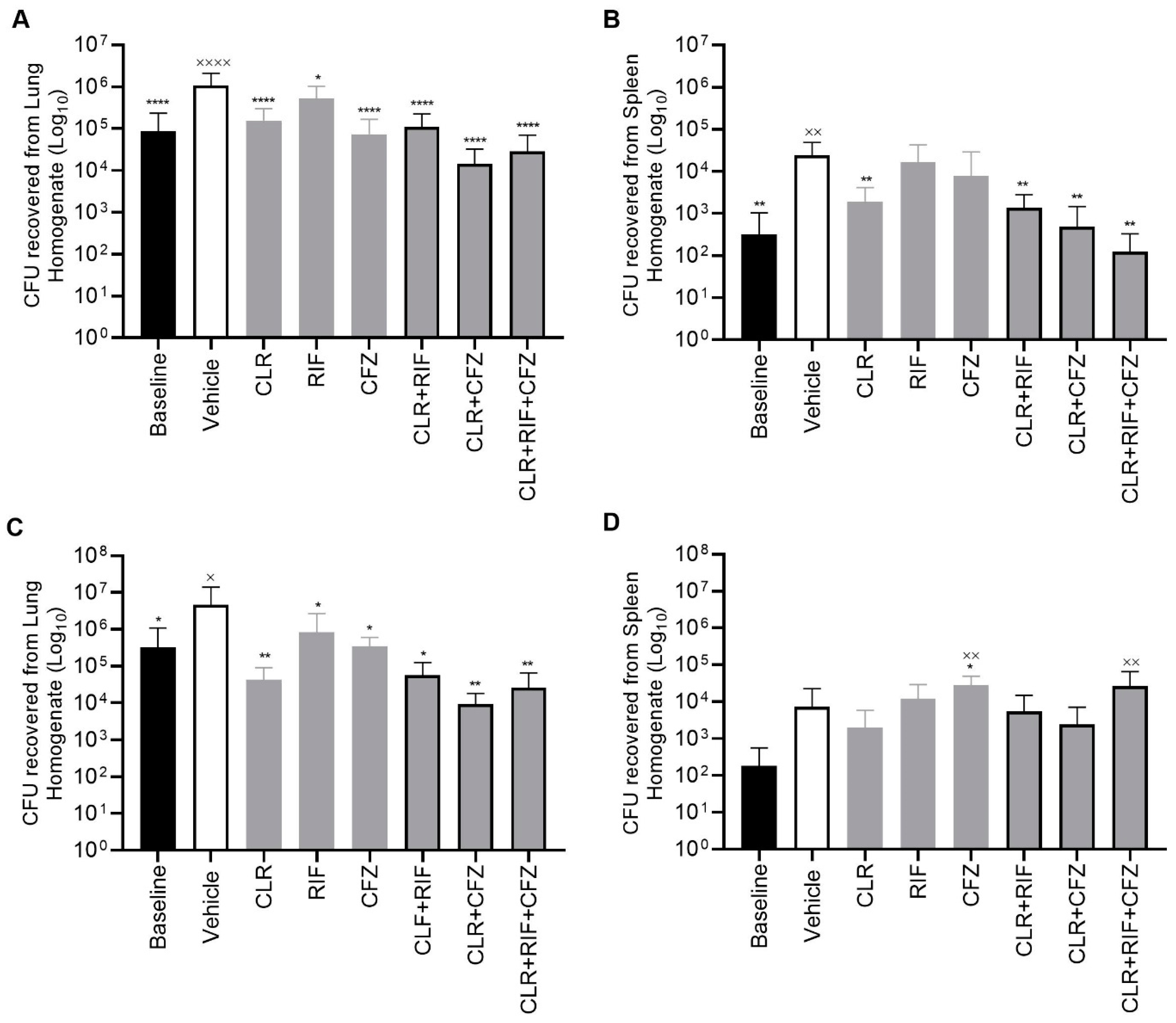
2.2. CLR-RFB-CFZ Is Effective in an In Vivo Murine Model of M. avium Infection with CLR-Susceptible and CLR-Resistant Strains of M. avium
2.3. Emergence of CLR-Resistant Strains in Mice
2.4. Lung Histopathology of Mice Infected with MAC ± after Four and Eight Weeks of Treatments (±CLR, ±RFB, ±CFZ Combinations) Reveals the Greatest Improvement for Treatment with the Triple Combination CLR-RFB-CFZ
2.5. Pharmacokinetic Parameters Confirm Drug Exposure Is Consistent with the RHB-204 Clinical Regimen Being Tested in Clinical Trials
3. Materials and Methods
3.1. Bacterial Strains
3.2. Antimicrobial Agent Preparation
3.3. Antimicrobial Susceptibility Testing
3.4. Biofilm Methodology
3.5. Animal Experiments
3.6. Emergence of Resistance Experiments
3.7. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horne, D.; Skerrett, S. Recent advances in nontuberculous mycobacterial lung infections. F1000Research 2019, 8, F1000 Faculty Rev-1710. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.L.; Limaye, A.; Pottinger, P.; Whimbey, E.; Goss, C.H.; Tonelli, M.R.; Cangelosi, G.A.; Dirac, M.A.; Olivier, K.N.; Brown-Elliott, B.A.; et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am. J. Respir. Crit. Care Med. 2012, 185, 231–232. [Google Scholar] [CrossRef] [PubMed]
- von Reyn, C.F.; Arbeit, R.D.; Horsburgh, C.R.; Ristola, M.A.; Waddell, R.D.; Tvaroha, S.M.; Samore, M.; Hirschhorn, L.R.; Lumio, J.; Lein, A.D.; et al. Sources of disseminated Mycobacterium avium infection in AIDS. J. Infect. 2002, 44, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Shaw, P.A.; Strickland, D.; Jackson, L.A.; Raebel, M.A.; Blosky, M.A.; Montes de Oca, R.; Shea, Y.R.; Seitz, A.E.; Holland, S.M.; et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 2010, 182, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; McNelley, E.; Kendall, B.; Marshall-Olson, A.; Morris, C.; Cassidy, M.; Saulson, A.; Hedberg, K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: An emerging public health disease. Am. J. Respir. Crit. Care Med. 2010, 182, 977–982. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; Bendien, S.A.; de Lange, W.C.M.; Hoefsloot, W.; Dekhuijzen, P.N.R.; Boeree, M.J.; van Soolingen, D. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax 2009, 64, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Babrak, L.; Bermudez, L.E. Mycobacterium avium Possesses Extracellular DNA that Contributes to Biofilm Formation, Structural Integrity, and Tolerance to Antibiotics. PLoS ONE 2015, 10, e0128772. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.; Drummond, D.; Bermudez, L.E. Characterization of biofilm formation by Mycobacterium avium strains. J. Med. Microbiol. 2003, 52, 747. [Google Scholar] [CrossRef]
- Carter, G.; Young, L.S.; Bermudez, L.E. Clarithromycin in a sub-inhibitory concentration inhibits Mycobacterium avium biofilm formation. Antimicrob. Agents Chemother. 2004, 48, 4907. [Google Scholar] [CrossRef]
- Diel, R.; Lipman, M.; Hoefsloot, W. High mortality in patients with Mycobacterium avium complex lung disease: A systematic review. BMC Infect. Dis. 2018, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, L.E.; Nash, K.; Petrofsky, M.; Young, L.S.; Inderlied, C.B. Effect of Ethambutol on the emergence of Clarithromycin-Resistant Mycobacterium avium in the beige mouse model. J. Infect. Dis. 1996, 174, 1218. [Google Scholar] [CrossRef]
- Miwa, S.; Shirai, M.; Toyoshima, M.; Shirai, T.; Yasuda, K.; Yokomura, K.; Yamada, T.; Masuda, M.; Inui, N.; Chida, K.; et al. Efficacy of Clarithromycin and Ethambotol for Mycobacterium avium complex Pulmonary Disease. Ann. Am. Thoracic. Soc. 2014, 11, 23–29. [Google Scholar] [CrossRef]
- Valinetz, E.; Stankiewicz Karita, H.; Pottinger, P.S.; Jain, R. Novel Administration of Clofazimine for the Treatment of Mycobacterium avium Infection. Open Forum Infect. Dis. 2020, 7, ofaa183. [Google Scholar] [CrossRef]
- Lanoix, J.-P.; Joseph, C.; Peltier, F.; Castelain, S.; Andrejak, C. Synergistic activity of clofazimine and clarithomycin in an aerosol mouse model of Mycobacterium avium infection. Antimicrob. Agents Chemother. 2020, 64, e02349-19. [Google Scholar] [CrossRef]
- Falkinham, J.O. Mycobacterium avium complex: Adherence as a way of life. AIMS Microbiol. 2018, 4, 428–438. [Google Scholar] [CrossRef]
- Rose, S.J.; Bermudez, L.E. Identification of Bicarbonate as a Trigger and Genes Involved with Extracellular DNA Export in Mycobacterial Biofilms. mBio 2016, 7, e01597-16. [Google Scholar] [CrossRef]
- Rose, S.J.; Bermudez, L.E. Mycobacterium avium Biofilm Attenuates Mononuclear Phagocyte Function by Triggering Hyperstimulation and Apoptosis during Early Infection. Infect. Immun. 2014, 82, 405–412. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Inderlied, C.B.; Kolonoski, P.; Wu, M.; Barbara-Burnham, L.; Young, L.S. Activities of bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob. Agents Chemother. 1996, 40, 546–551. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Kolonoski, P.; Wu, M.; Aralar, P.A.; Inderlied, C.B.; Young, L.S. Mefloquine is active in vitro and in vivo against Mycobacterium avium complex. Antimicrob. Agents Chemother. 1999, 43, 1870–1874. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Inderlied, C.B.; Kolonoski, P.; Petrofsky, M.; Young, L.S. Clarithromycin, dapsone, and a combination of both used to treat or prevent disseminated Mycobacterium avium infection in beige mice. Antimicrob. Agents Chemother. 1994, 38, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Bade, D.J.; Bébéar, C.; Brown, S.D.; Davidson, M.K.; Duffy, L.B.; Kenny, G.; Matlow, A.; Shortridge, D.; Talkington, D.; et al. Methods for Antimicrobial Susceptibility Testing for Human Mycoplasmas; Approved Guideline; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; Available online: http://www.ncbi.nlm.nih.gov/books/NBK544375/ (accessed on 17 March 2022).
- Kikuchi, E.; Yamazaki, K.; Kikuchi, J.; Hasegawa, N.; Hashimoto, S.; Ishizaka, A.; Nishimura, M. Pharmacokinetics of clarithromycin in bronchial epithelial lining fluid. Respirology 2008, 13, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gaohua, L.; Wedagedera, J.; Small, B.G.; Almond, L.; Romero, K.; Hermann, D.; Hanna, D.; Jamei, M.; Gardner, I. Development of a Multicompartment Permeability-Limited Lung PBPK Model and Its Application in Predicting Pulmonary Pharmacokinetics of Antituberculosis Drugs. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 605–613. [Google Scholar] [CrossRef]
- Kiem, S.; Schentag, J.J. Interpretation of Epithelial Lining Fluid Concentrations of Antibiotics against Methicillin Resistant Staphylococcus aureus. Infect. Chemother. 2014, 46, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Lenders, L.; Magombedze, G.; Srivastava, S.; Raj, P.; Arning, E.; Ashcraft, P.; Bottiglieri, T.; Wainwright, H.; Pennel, T.; et al. Drug-Penetration Gradients Associated with Acquired Drug Resistance in Patients with Tuberculosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, L.E.; Motamedi, N.; Kolonoski, P.; Chee, C.; Baimukanova, G.; Bildfell, R.; Wang, G.; Phan, L.T.; Young, L.S. The efficacy of clarithromycin and EDP-420 (a bicyclolide) against Mycobacterium avium in a mouse model of pulmonary infection. J. Infect. Dis. 2008, 197, 1506. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Griffith, D.E. Pulmonary non-tuberculous mycobacterial infections. Int. J. Tuberc. Lung Dis. 2010, 14, 665–671. [Google Scholar]
- Rojony, R.; Martin, M.; Campeau, A.; Wozniak, J.M.; Gonzalez, D.J.; Jaiswal, P.; Danelishvili, L.; Bermudez, L.E. Exposure of Mycobacterium avium subsp hominissuis to environmental conditions mimicking the host and antibiotics result in specific protein expression. Clin. Proteom. 2019, 16, 39. [Google Scholar] [CrossRef]
- Kunkel, M.; Doyle-Eisele, M.; Kuehl, P.; Rotermiund, K.; Ufer, S.; Reed, M.; Grant, M.; Hofmann, T. Clofazimine inhalation suspension demonstrates promising toxicology in canines for treating pulmonary Non-tuberculous mycobacteria infection. Antimicrob. Agents Chemother. 2023, 67, e01144-22. [Google Scholar] [CrossRef]

| CFU/mL (×107) | ||
|---|---|---|
| Treatment | 4 Days | 7 Days |
| HBSS (negative control) | 1.69 ± 0.4 a | 5.20 ± 0.5 |
| Clarithromycin 1 | 1.35 ± 0.3 | 1.07 ± 0.3 |
| Rifabutin 1 | 1.41 ± 0.5 | 1.64 ± 0.3 |
| Clofazimine 1 | 1.91 ± 0.6 | 1.42 ± 0.5 |
| Clarithromycin/Rifabutin 1,2,3 | 1.38 ± 0.3 | 0.75 ± 0.6 |
| Clarithromycin/Clofazimine 1 | 1.33 ± 0.4 | 1.08 ± 0.5 |
| Clarithromycin/Rifabutin/Clofazimine 1,2,3,4,5,6 | 0.30 ± 0.4 | 0.12 ± 0.5 |
| Total CFU | |||
|---|---|---|---|
| (% of Total CFU Resistant to Clarithromycin) 4 | |||
| Treatment | In Vivo Resistance | 4 Weeks Treatment | 8 Weeks Treatment |
| Clarithromycin | 1 × 10−9 (0.0001%) | 1.55 × 105 (0.129%) | 5.31 × 104 (1.129%) |
| Clarithromycin/Rifabutin | 1.11 × 105 (0.09%) | 5.7 × 104 (0.473%) 1 | |
| Clarithromycin/Clofazimine | 1.43 × 104 (0.139%) | 9.16 × 103 (0.469%) 1 | |
| Clarithromycin/Rifabutin/Clofazimine | 2.85 × 104 (0.035%) | 2.60 × 104 (0.0%) 1,2,3 | |
| Treatment Group | |
|---|---|
| Saline |
|
| |
| |
| Clarithromycin |
|
| |
| |
| Rifabutin |
|
| |
| |
| Clofazimine |
|
| |
| |
| |
| Clarithromycin |
|
| +Clofazimine |
|
| |
| Clarithromycin |
|
| +Rifabutin |
|
| Clarithromycin |
|
| +Rifabutin |
|
| +Clofazimine |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Offman, E.M.; Leestemaker-Palmer, A.; Fathi, R.; Keefe, B.; Bibliowicz, A.; Raday, G.; Bermudez, L.E. Triple-Antibiotic Combination Exerts Effective Activity against Mycobacterium avium subsp. hominissuis Biofilm and Airway Infection in an In Vivo Murine Model. Antibiotics 2024, 13, 475. https://doi.org/10.3390/antibiotics13060475
Offman EM, Leestemaker-Palmer A, Fathi R, Keefe B, Bibliowicz A, Raday G, Bermudez LE. Triple-Antibiotic Combination Exerts Effective Activity against Mycobacterium avium subsp. hominissuis Biofilm and Airway Infection in an In Vivo Murine Model. Antibiotics. 2024; 13(6):475. https://doi.org/10.3390/antibiotics13060475
Chicago/Turabian StyleOffman, Elliot M., Amy Leestemaker-Palmer, Reza Fathi, Bailey Keefe, Aida Bibliowicz, Gilead Raday, and Luiz E. Bermudez. 2024. "Triple-Antibiotic Combination Exerts Effective Activity against Mycobacterium avium subsp. hominissuis Biofilm and Airway Infection in an In Vivo Murine Model" Antibiotics 13, no. 6: 475. https://doi.org/10.3390/antibiotics13060475
APA StyleOffman, E. M., Leestemaker-Palmer, A., Fathi, R., Keefe, B., Bibliowicz, A., Raday, G., & Bermudez, L. E. (2024). Triple-Antibiotic Combination Exerts Effective Activity against Mycobacterium avium subsp. hominissuis Biofilm and Airway Infection in an In Vivo Murine Model. Antibiotics, 13(6), 475. https://doi.org/10.3390/antibiotics13060475








