Listeria monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights
Abstract
1. Introduction
2. Results
2.1. Prevalence and L. monocytogenes Identification
2.2. Antibiogram of L. monocytogenes Isolates from Food Products and Food Associated Environments
2.3. L. monocytogenes Typing and Core-Genome Clustering Analysis
2.4. Whole-Genome Sequencing (Antimicrobial Resistance and Virulence Genes)
2.5. Evaluation of the Biofilm Formation of L. monocytogenes Isolates
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Processing
4.2. Isolation and Identification of L. monocytogenes
4.3. Taxonomic Identification of Bacterial Isolates
4.4. Antimicrobial Susceptibility Testing
4.5. L. monocytogenes Whole-Genome Sequencing
4.6. Core-Genome Clustering Analysis of L. monocytogenes Isolates
4.7. Biofilm Formation Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wartha, S.; Bretschneider, N.; Dangel, A.; Hobmaier, B.; Hörmansdorfer, S.; Huber, I.; Murr, L.; Pavlovic, M.; Sprenger, A.; Wenning, M.; et al. Genetic Characterization of Listeria from Food of Non-Animal Origin Products and from Producing and Processing Companies in Bavaria, Germany. Foods 2023, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Department of International Trade, TEI of West Macedonia, Kastoria, Greece Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial Resistance and Virulence Characterization of Listeria monocytogenes Strains Isolated from Food and Food Processing Environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef] [PubMed]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive Response of Listeria monocytogenes to the Stress Factors in the Food Processing Environment. Front. Microbiol. 2021, 12, 710085. [Google Scholar] [CrossRef]
- Grudlewska-Buda, K.; Bauza-Kaszewska, J.; Wiktorczyk-Kapischke, N.; Budzyńska, A.; Gospodarek-Komkowska, E.; Skowron, K. Antibiotic Resistance in Selected Emerging Bacterial Foodborne Pathogens—An Issue of Concern? Antibiotics 2023, 12, 880. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Mafuna, T.; Matle, I.; Magwedere, K.; Pierneef, R.E.; Reva, O.N. Whole Genome-Based Characterization of Listeria monocytogenes Isolates Recovered From the Food Chain in South Africa. Front. Microbiol. 2021, 12, 669287. [Google Scholar] [CrossRef] [PubMed]
- Matereke, L.T.; Okoh, A.I. Listeria monocytogenes Virulence, Antimicrobial Resistance and Environmental Persistence: A Review. Pathogens 2020, 9, 528. [Google Scholar] [CrossRef]
- Ulusoy, B.H.; Chirkena, K. Two Perspectives of Listeria monocytogenes Hazards in Dairy Products: The Prevalence and the Antibiotic Resistance. Food Qual. Saf. 2019, 3, 233–241. [Google Scholar] [CrossRef]
- Bahrami, A.; Moaddabdoost Baboli, Z.; Schimmel, K.; Jafari, S.M.; Williams, L. Efficiency of Novel Processing Technologies for the Control of Listeria monocytogenes in Food Products. Trends Food Sci. Technol. 2020, 96, 61–78. [Google Scholar] [CrossRef]
- Dos Santos, J.S.; Biduski, B.; Dos Santos, L.R. Listeria monocytogenes: Health Risk and a Challenge for Food Processing Establishments. Arch. Microbiol. 2021, 203, 5907–5919. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, E.E.; Mousa, W.S.; Harb, O.H.; Fath-Elbab, G.A.; Nooruzzaman, M.; Gaber, A.; Alsanie, W.F.; Abdeen, A. Prevalence, Antibiogram and Genetic Characterization of Listeria monocytogenes from Food Products in Egypt. Foods 2021, 10, 1381. [Google Scholar] [CrossRef]
- Nwaiwu, O.; Onyeaka, H.; Rees, C. Probing the Evolution of Genes Associated with DNA Methylation in Listeria monocytogenes. bioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Andriyanov, P.A.; Zhurilov, P.A.; Liskova, E.A.; Karpova, T.I.; Sokolova, E.V.; Yushina, Y.K.; Zaiko, E.V.; Bataeva, D.S.; Voronina, O.L.; Psareva, E.K.; et al. Antimicrobial Resistance of Listeria monocytogenes Strains Isolated from Humans, Animals, and Food Products in Russia in 1950–1980, 2000–2005, and 2018–2021. Antibiotics 2021, 10, 1206. [Google Scholar] [CrossRef]
- McLauchlin, J.; Grant, K.A.; Amar, C.F.L. Human Foodborne Listeriosis in England and Wales, 1981 to 2015. Epidemiol. Infect. 2020, 148, e54. [Google Scholar] [CrossRef] [PubMed]
- Kayode, A.J.; Okoh, A.I. Assessment of the Molecular Epidemiology and Genetic Multiplicity of Listeria monocytogenes Recovered from Ready-to-Eat Foods Following the South African Listeriosis Outbreak. Sci. Rep. 2022, 12, 20129. [Google Scholar] [CrossRef]
- Tao, Q.; Wu, Q.; Zhang, Z.; Liu, J.; Tian, C.; Huang, Z.; Malakar, P.K.; Pan, Y.; Zhao, Y. Meta-Analysis for the Global Prevalence of Foodborne Pathogens Exhibiting Antibiotic Resistance and Biofilm Formation. Front. Microbiol. 2022, 13, 906490. [Google Scholar] [CrossRef]
- Niamah, A.K. Detection of Listeria monocytogenes Bacteria in Four Types of Milk Using PCR. Pak. J. Nutr. 2012, 11, 1158–1160. [Google Scholar] [CrossRef][Green Version]
- Jobori, K.M.A.; Aboodi, A.H. Detection of Salmonella Spp. and Listeria monocytogenes in Soft White Cheese Using PCR Assays. Int. J. Orig. Res. 2015, 1, 1–7. [Google Scholar]
- Silva, A.; Silva, V.; Cintas, L.M.; Pereira, E.; Maltez, L.; Rahman, T.; Igrejas, G.; Valentão, P.; Falco, V.; Poeta, P. Listeria monocytogenes in Livestock and Derived Food-Products: Insights from Antibiotic-Resistant Prevalence and Genomic Analysis. J. Bacteriol. Mycol. 2024, 11, 1216. [Google Scholar]
- Kayode, A.J.; Okoh, A.I. Antibiotic Resistance Profile of Listeria monocytogenes Recovered from Ready-to-Eat Foods Surveyed in South Africa. J. Food Prot. 2022, 85, 1807–1814. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Skowron, K.; Kijewska, A.; Bernaciak, Z.; Bauza-Kaszewska, J.; Kraszewska, Z.; Gospodarek-Komkowska, E. Comparison of Selected Phenotypic Features of Persistent and Sporadic Strains of Listeria monocytogenes Sampled from Fish Processing Plants. Foods 2022, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Calero-Cáceres, W.; Ortuño-Gutiérrez, N.; Sunyoto, T.; Gomes-Dias, C.-A.; Bastidas-Caldes, C.; Ramírez, M.S.; Harries, A.D. Whole-Genome Sequencing for Surveillance of Antimicrobial Resistance in Ecuador: Present and Future Implications. Rev. Panam. Salud Pública 2023, 47, 1. [Google Scholar] [CrossRef]
- Parra-Flores, J.; Holý, O.; Bustamante, F.; Lepuschitz, S.; Pietzka, A.; Contreras-Fernández, A.; Castillo, C.; Ovalle, C.; Alarcón-Lavín, M.P.; Cruz-Córdova, A.; et al. Virulence and Antibiotic Resistance Genes in Listeria monocytogenes Strains Isolated From Ready-to-Eat Foods in Chile. Front. Microbiol. 2022, 12, 796040. [Google Scholar] [CrossRef]
- Ronholm, J.; Nasheri, N.; Petronella, N.; Pagotto, F. Navigating Microbiological Food Safety in the Era of Whole-Genome Sequencing. Clin. Microbiol. Rev. 2016, 29, 837–857. [Google Scholar] [CrossRef]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole Genome-Based Population Biology and Epidemiological Surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef] [PubMed]
- Mixão, V.; Pinto, M.; Sobral, D.; Pasquale, A.D.; Gomes, J.P.; Borges, V. ReporTree: A Surveillance-Oriented Tool to Strengthen the Linkage between Pathogen Genetic Clusters and Epidemiological Data. Genome Med. 2022, 15, 43. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.-F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carriço, J.A.; Achtman, M. GrapeTree: Visualization of Core Genomic Relationships among 100,000 Bacterial Pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef]
- Van Walle, I.; Björkman, J.T.; Cormican, M.; Dallman, T.; Mossong, J.; Moura, A.; Pietzka, A.; Ruppitsch, W.; Takkinen, J. European Listeria WGS typing group Retrospective Validation of Whole Genome Sequencing-Enhanced Surveillance of Listeriosis in Europe, 2010 to 2015. Eurosurveillance 2018, 23, 1700798. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, G.; Yang, J.; Zhao, L.; Jiang, Y.; Guo, D.; Wang, X.; Zhi, S.; Xu, X.; Dong, Q.; et al. Prevalence, Antibiotic Resistance, and Molecular Epidemiology of Listeria monocytogenes Isolated from Imported Foods in China during 2018 to 2020. Int. J. Food Microbiol. 2022, 382, 109916. [Google Scholar] [CrossRef]
- Szymczak, B.; Szymczak, M.; Trafiałek, J. Prevalence of Listeria Species and Listeria monocytogenes in Ready-to-Eat Foods in the West Pomeranian Region of Poland: Correlations between the Contamination Level, Serogroups, Ingredients, and Producers. Food Microbiol. 2020, 91, 103532. [Google Scholar] [CrossRef]
- Psareva, E.K.; Liskova, E.A.; Razheva, I.V.; Yushina, Y.K.; Grudistova, M.A.; Gladkova, N.A.; Potemkin, E.A.; Zhurilov, P.A.; Sokolova, E.V.; Andriyanov, P.A.; et al. Diversity of Listeria monocytogenes Strains Isolated from Food Products in the Central European Part of Russia in 2000–2005 and 2019–2020. Foods 2021, 10, 2790. [Google Scholar] [CrossRef]
- Arslan, S.; Özdemir, F. Prevalence and Antimicrobial Resistance of Listeria Species and Molecular Characterization of Listeria monocytogenes Isolated from Retail Ready-to-Eat Foods. FEMS Microbiol. Lett. 2020, 367, fnaa006. [Google Scholar] [CrossRef]
- Koskar, J.; Kramarenko, T.; Meremäe, K.; Kuningas, M.; Sõgel, J.; Mäesaar, M.; Anton, D.; Lillenberg, M.; Roasto, M. Prevalence and Numbers of Listeria monocytogenes in Various Ready-to-Eat Foods over a 5-Year Period in Estonia. J. Food Prot. 2019, 82, 597–604. [Google Scholar] [CrossRef]
- Papatzimos, G.; Kotzamanidis, C.; Kyritsi, M.; Malissiova, E.; Economou, V.; Giantzi, V.; Zdragas, A.; Hadjichristodoulou, C.; Sergelidis, D. Prevalence and Characteristics of Listeria monocytogenes in Meat, Meat Products, Food Handlers and the Environment of the Meat Processing and the Retail Facilities of a Company in Northern Greece. Lett. Appl. Microbiol. 2022, 74, 367–376. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.; Aspridou, Z.; Misiou, O.; Dong, P.; Zhang, Y. The Prevalence and Antibiotic-Resistant of Listeria monocytogenes in Livestock and Poultry Meat in China and the EU from 2001 to 2022: A Systematic Review and Meta-Analysis. Foods 2023, 12, 769. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P. Trends of Major Foodborne Outbreaks in the European Union during the Years 2015–2019. Hygiene 2021, 1, 106–119. [Google Scholar] [CrossRef]
- Vidovic, S.; Paturi, G.; Gupta, S.; Fletcher, G.C. Lifestyle of Listeria monocytogenes and Food Safety: Emerging Listericidal Technologies in the Food Industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 1817–1835. [Google Scholar] [CrossRef]
- Bouymajane, A.; Rhazi Filali, F.; Oulghazi, S.; Lafkih, N.; Ed-Dra, A.; Aboulkacem, A.; El Allaoui, A.; Ouhmidou, B.; Moumni, M. Occurrence, Antimicrobial Resistance, Serotyping and Virulence Genes of Listeria monocytogenes Isolated from Foods. Heliyon 2021, 7, e06169. [Google Scholar] [CrossRef]
- Anwar, T.M.; Pan, H.; Chai, W.; Ed-Dra, A.; Fang, W.; Li, Y.; Yue, M. Genetic Diversity, Virulence Factors, and Antimicrobial Resistance of Listeria monocytogenes from Food, Livestock, and Clinical Samples between 2002 and 2019 in China. Int. J. Food Microbiol. 2022, 366, 109572. [Google Scholar] [CrossRef]
- Lakicevic, B.Z.; Den Besten, H.M.W.; De Biase, D. Landscape of Stress Response and Virulence Genes Among Listeria monocytogenes Strains. Front. Microbiol. 2022, 12, 738470. [Google Scholar] [CrossRef]
- Panera-Martínez, S.; Rodríguez-Melcón, C.; Serrano-Galán, V.; Alonso-Calleja, C.; Capita, R. Prevalence, Quantification and Antibiotic Resistance of Listeria monocytogenes in Poultry Preparations. Food Control 2022, 135, 108608. [Google Scholar] [CrossRef]
- Kayode, A.J.; Okoh, A.I. Assessment of Multidrug-Resistant Listeria monocytogenes in Milk and Milk Product and One Health Perspective. PLoS ONE 2022, 17, e0270993. [Google Scholar] [CrossRef]
- Bland, R.; Brown, S.R.B.; Waite-Cusic, J.; Kovacevic, J. Probing Antimicrobial Resistance and Sanitizer Tolerance Themes and Their Implications for the Food Industry through the Listeria monocytogenes Lens. Comp. Rev. Food Sci. Food Safe 2022, 21, 1777–1802. [Google Scholar] [CrossRef]
- Gelbíčová, T.; Karpíšková, R. Population Structure of Listeria monocytogenes Isolated from Human Listeriosis Cases and from Ready-to-Eat Foods in the Czech Republic. J. Food Nutr. Res. 2019, 58, 2. [Google Scholar]
- Oxaran, V.; Lee, S.H.I.; Chaul, L.T.; Corassin, C.H.; Barancelli, G.V.; Alves, V.F.; De Oliveira, C.A.F.; Gram, L.; De Martinis, E.C.P. Listeria monocytogenes Incidence Changes and Diversity in Some Brazilian Dairy Industries and Retail Products. Food Microbiol. 2017, 68, 16–23. [Google Scholar] [CrossRef]
- Kurpas, M.; Osek, J.; Moura, A.; Leclercq, A.; Lecuit, M.; Wieczorek, K. Genomic Characterization of Listeria monocytogenes Isolated from Ready-to-Eat Meat and Meat Processing Environments in Poland. Front. Microbiol. 2020, 11, 1412. [Google Scholar] [CrossRef]
- Ebner, R.; Stephan, R.; Althaus, D.; Brisse, S.; Maury, M.; Tasara, T. Phenotypic and Genotypic Characteristics of Listeria monocytogenes Strains Isolated during 2011–2014 from Different Food Matrices in Switzerland. Food Control 2015, 57, 321–326. [Google Scholar] [CrossRef]
- Maury, M.M.; Bracq-Dieye, H.; Huang, L.; Vales, G.; Lavina, M.; Thouvenot, P.; Disson, O.; Leclercq, A.; Brisse, S.; Lecuit, M. Hypervirulent Listeria monocytogenes Clones’ Adaption to Mammalian Gut Accounts for Their Association with Dairy Products. Nat. Commun. 2019, 10, 2488. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, J.; Zhang, J.; Chen, Y.; Zeng, H.; Xue, L.; Lei, T.; Pang, R.; Wu, S.; Wu, H.; et al. Isolation, Potential Virulence, and Population Diversity of Listeria monocytogenes From Meat and Meat Products in China. Front. Microbiol. 2019, 10, 946. [Google Scholar] [CrossRef]
- Šteingolde, Ž.; Meistere, I.; Avsejenko, J.; Ķibilds, J.; Bergšpica, I.; Streikiša, M.; Gradovska, S.; Alksne, L.; Roussel, S.; Terentjeva, M.; et al. Characterization and Genetic Diversity of Listeria monocytogenes Isolated from Cattle Abortions in Latvia, 2013–2018. Vet. Sci. 2021, 8, 195. [Google Scholar] [CrossRef]
- Wieczorek, K.; Bomba, A.; Osek, J. Whole-Genome Sequencing-Based Characterization of Listeria monocytogenes from Fish and Fish Production Environments in Poland. IJMS 2020, 21, 9419. [Google Scholar] [CrossRef]
- Lu, B.; Yang, J.; Gao, C.; Li, D.; Cui, Y.; Huang, L.; Chen, X.; Wang, D.; Wang, A.; Liu, Y.; et al. Listeriosis Cases and Genetic Diversity of Their Listeria monocytogenes Isolates in China, 2008–2019. Front. Cell. Infect. Microbiol. 2021, 11, 608352. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. Multi-country Outbreak of Listeria monocytogenes Sequence Type 8 Infections Linked to Consumption of Salmon Products. EFS3 2018, 15, 1496E. [Google Scholar] [CrossRef]
- Praça, J.; Furtado, R.; Coelho, A.; Correia, C.B.; Borges, V.; Gomes, J.P.; Pista, A.; Batista, R. Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal. Microorganisms 2023, 11, 322. [Google Scholar] [CrossRef]
- Matle, I.; Mafuna, T.; Madoroba, E.; Mbatha, K.R.; Magwedere, K.; Pierneef, R. Population Structure of Non-ST6 Listeria monocytogenes Isolated in the Red Meat and Poultry Value Chain in South Africa. Microorganisms 2020, 8, 1152. [Google Scholar] [CrossRef]
- Vilchis-Rangel, R.E.; Espinoza-Mellado, M.D.R.; Salinas-Jaramillo, I.J.; Martinez-Peña, M.D.; Rodas-Suárez, O.R. Association of Listeria monocytogenes LIPI-1 and LIPI-3 Marker llsX with Invasiveness. Curr. Microbiol. 2019, 76, 637–643. [Google Scholar] [CrossRef]
- Shi, D.; Anwar, T.M.; Pan, H.; Chai, W.; Xu, S.; Yue, M. Genomic Determinants of Pathogenicity and Antimicrobial Resistance for 60 Global Listeria monocytogenes Isolates Responsible for Invasive Infections. Front. Cell. Infect. Microbiol. 2021, 11, 718840. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Kayode, A.J.; Semerjian, L.; Osaili, T.; Olapade, O.; Okoh, A.I. Occurrence of Multidrug-Resistant Listeria monocytogenes in Environmental Waters: A Menace of Environmental and Public Health Concern. Front. Environ. Sci. 2021, 9, 737435. [Google Scholar] [CrossRef]
- Saraiva, C.; Silva, A.C.; García-Díez, J.; Cenci-Goga, B.; Grispoldi, L.; Silva, A.F.; Almeida, J.M. Antimicrobial Activity of Myrtus Communis L. and Rosmarinus Officinalis L. Essential Oils against Listeria monocytogenes in Cheese. Foods 2021, 10, 1106. [Google Scholar] [CrossRef]
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. ISO: Geneva, Switzerland, 2017.
- Moura-Alves, M.; Machado, C.; Silva, J.A.; Saraiva, C. Shelf-life Determination of an Egg-based Cake, Relating Sensory Attributes Microbiological Characteristics and Physico-chemical Properties. Int. J. Food Sci. Tech. 2022, 57, 6580–6590. [Google Scholar] [CrossRef]
- de Vasconcelos Byrne, V.; Hofer, E.; Vallim, D.C.; de Castro Almeida, R.C. Occurrence and Antimicrobial Resistance Patterns of Listeria monocytogenes Isolated from Vegetables. Braz. J. Microbiol. 2016, 47, 438–443. [Google Scholar] [CrossRef][Green Version]
- Morobe, I.C.; Obi, C.L.; Nyila, M.A.; Gashe, B.A.; Matsheka, M.I. Prevalence, Antimicrobial Resistance Profiles of Listeria monocytogenes from Various Foods in Gaborone, Botswana. Afr. J. Biotechnol. 2009, 8, 6383–6387. [Google Scholar] [CrossRef]
- Llarena, A.; Ribeiro-Gonçalves, B.F.; Nuno Silva, D.; Halkilahti, J.; Machado, M.P.; Da Silva, M.S.; Jaakkonen, A.; Isidro, J.; Hämäläinen, C.; Joenperä, J.; et al. INNUENDO: A Cross-sectoral Platform for the Integration of Genomics in the Surveillance of Food-borne Pathogens. EFS3 2018, 15, 1498. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. CP Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Langmead, B. Aligning Short Sequencing Reads with Bowtie. CP Bioinform. 2010, 32, 11.7.1–11.7.14. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast Metagenomic Sequence Classification Using Exact Alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. chewBBACA: A Complete Suite for Gene-by-Gene Schema Creation and Strain Identification. Microb. Genom. 2018, 4, e000166. [Google Scholar] [CrossRef]
- Mamede, R.; Vila-Cerqueira, P.; Silva, M.; Carriço, J.A.; Ramirez, M. Chewie Nomenclature Server (Chewie-NS): A Deployable Nomenclature Server for Easy Sharing of Core and Whole Genome MLST Schemas. Nucleic Acids Res. 2021, 49, D660–D666. [Google Scholar] [CrossRef]
- Silva, V.; Correia, E.; Pereira, J.E.; González-Machado, C.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Exploring the Biofilm Formation Capacity in S. Pseudintermedius and Coagulase-Negative Staphylococci Species. Pathogens 2022, 11, 689. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef]
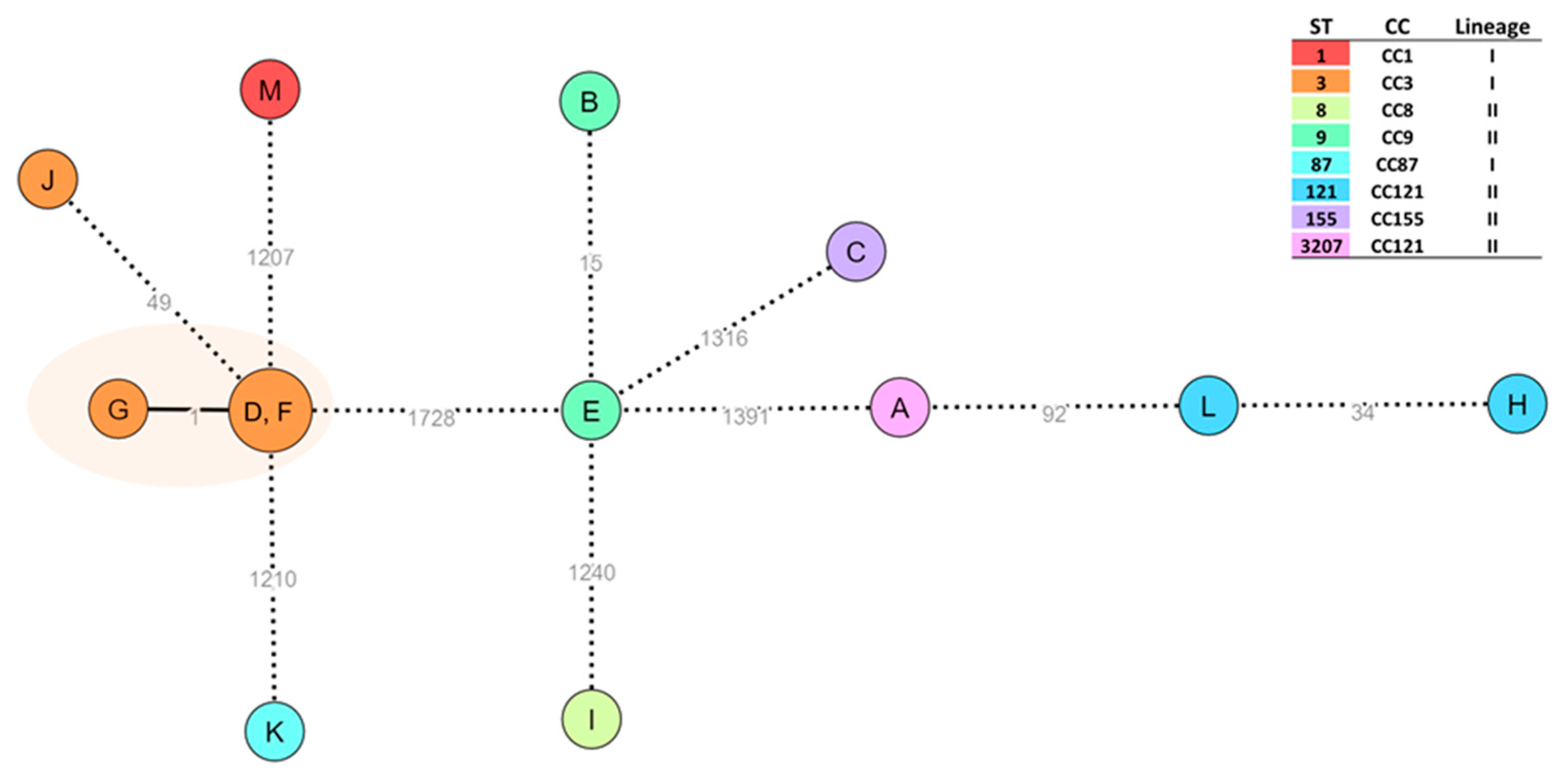

| Strain | Isolation Year | Source | Antibiotics Tested | Multi-Resistance Profile | Sequence Type | cgMLST | Clonal Complex | Lineage | |
|---|---|---|---|---|---|---|---|---|---|
| Resistant | Susceptible | ||||||||
| A | 2021 | Meat preparation | STX | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ | No | ST3207 | CC121 | II | |
| B | 2022 | Alheira | DA-SXT | MRP-AMP-CIP-E-K-CN-P-RD-VA-LNZ | No | ST9 | CC9 | II | |
| C | 2022 | Turkey kebab with chorizo and peppers | P | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ-SXT | No | ST155 | CC155 | II | |
| D | 2014 | Alheira pasta packaged in aerobiosis | P | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ-SXT | No | ST3 | cluster_1 (≤7 ADs) | CC3 | I |
| E | 2014 | Alheira dough in vacuum | - | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ-SXT | No | ST9 | CC9 | II | |
| F | 2014 | Alheira pasta packaged in aerobiosis | P | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ-SXT | No | ST3 | cluster_1 (≤7 ADs) | CC3 | I |
| G | 2014 | Fresh alheira pasta | - | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ-SXT | No | ST3 | cluster_1 (≤7 ADs) | CC3 | I |
| H | 2019 | Slaughterhouse floor | E-SXT | MRP-AMP-CIP-DA- K-CN-P-RD-VA-LNZ | No | ST121 | CC121 | II | |
| I | 2018 | Catering establishments | - | MRP-AMP-CIP-DA-E-K-CN-P- | No | ST8 | CC8 | II | |
| J | 2018 | Catering establishments | - | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ-SXT | No | ST3 | CC3 | I | |
| K | 2018 | Catering establishments | MRP-DA-E-CN-RD-VA-SXT-LNZ | AMP-CIP-K-P | Yes | ST87 | CC87 | I | |
| L | 2018 | Catering establishments | SXT | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ | No | ST121 | CC121 | II | |
| M | 2018 | Catering establishments | - | MRP-AMP-CIP-DA-E-K-CN-P-RD-VA-LNZ-SXT | No | ST1 | CC1 | I | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Silva, V.; Gomes, J.P.; Coelho, A.; Batista, R.; Saraiva, C.; Esteves, A.; Martins, Â.; Contente, D.; Diaz-Formoso, L.; et al. Listeria monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights. Antibiotics 2024, 13, 447. https://doi.org/10.3390/antibiotics13050447
Silva A, Silva V, Gomes JP, Coelho A, Batista R, Saraiva C, Esteves A, Martins Â, Contente D, Diaz-Formoso L, et al. Listeria monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights. Antibiotics. 2024; 13(5):447. https://doi.org/10.3390/antibiotics13050447
Chicago/Turabian StyleSilva, Adriana, Vanessa Silva, João Paulo Gomes, Anabela Coelho, Rita Batista, Cristina Saraiva, Alexandra Esteves, Ângela Martins, Diogo Contente, Lara Diaz-Formoso, and et al. 2024. "Listeria monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights" Antibiotics 13, no. 5: 447. https://doi.org/10.3390/antibiotics13050447
APA StyleSilva, A., Silva, V., Gomes, J. P., Coelho, A., Batista, R., Saraiva, C., Esteves, A., Martins, Â., Contente, D., Diaz-Formoso, L., Cintas, L. M., Igrejas, G., Borges, V., & Poeta, P. (2024). Listeria monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights. Antibiotics, 13(5), 447. https://doi.org/10.3390/antibiotics13050447











