Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties
Abstract
1. Introduction
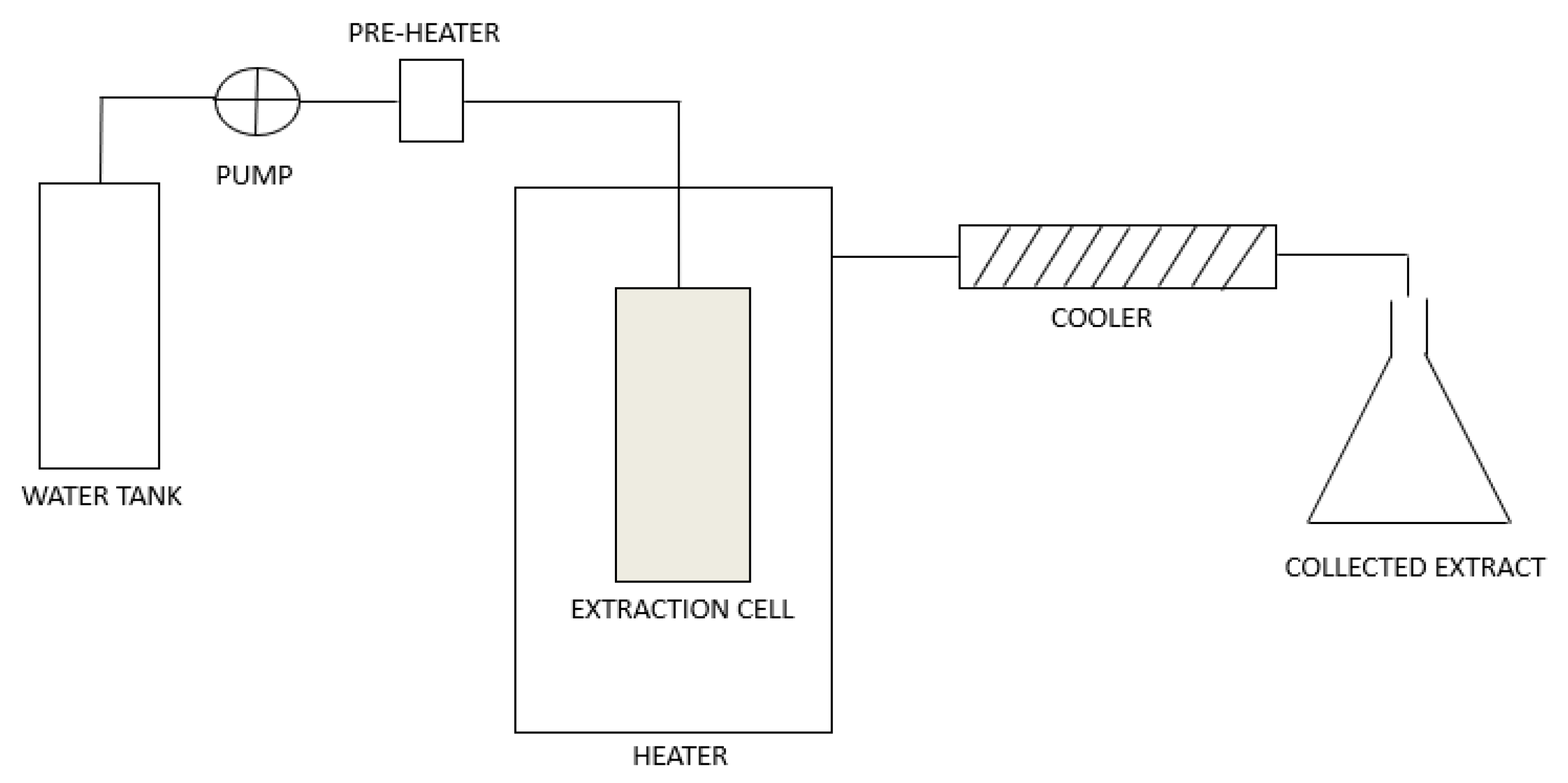
1.1. Subcritical Water Extraction
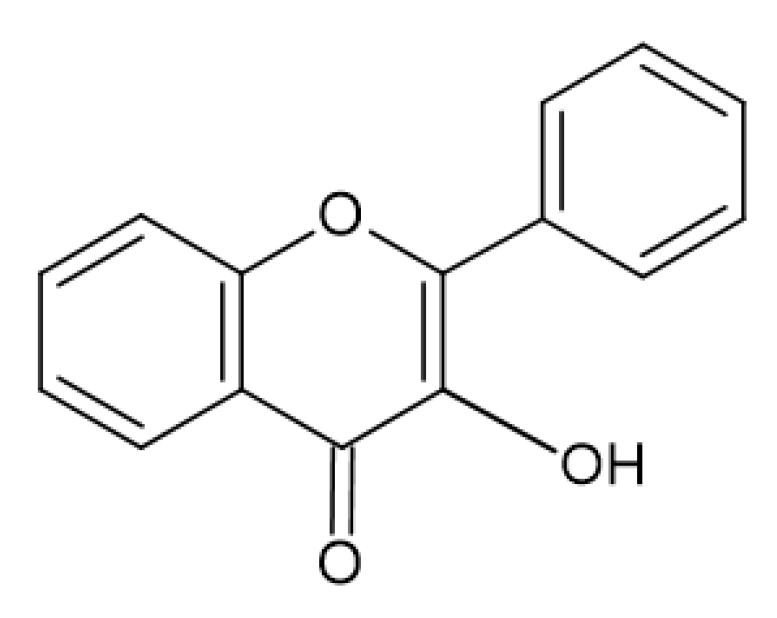
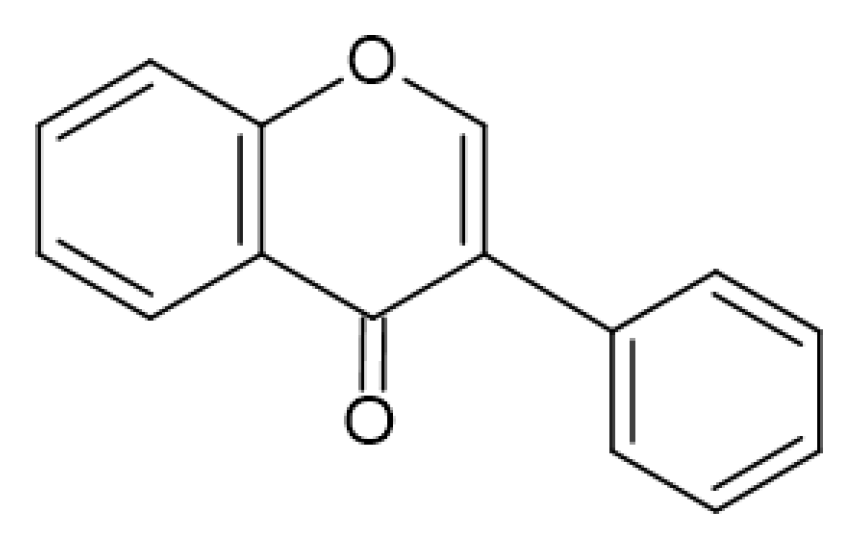
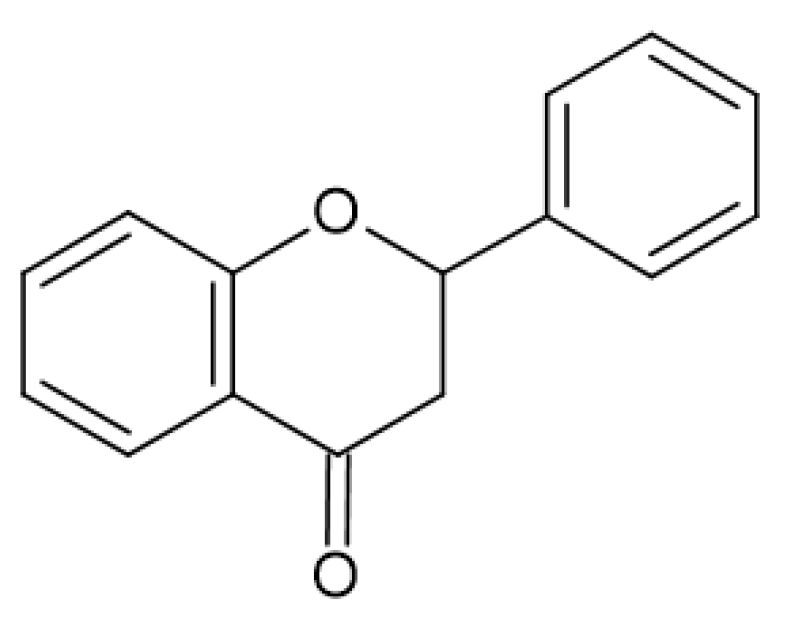
1.2. Phenolic Compounds
1.2.1. Biosynthesis of Phenolic Compounds
1.2.2. Biological Activity of Phenolic Compounds
1.2.3. Antimicrobial Activity of Phenolic Compounds
1.3. Subcritical Water for the Extraction of Phenol-Rich Extracts with Antimicrobial Activity
2. Discussion
| Plant and Plant Part | Extraction Method | Extraction Conditions | Extraction Efficiency/Yield (%) | TPC | TFC | Method of Identification and Quantification and Quantified Number of Phenolic Compounds | MIC Value or Width of Inhibition Zone on Tested Bacterial Strains |
|---|---|---|---|---|---|---|---|
| Urtica dioica L.—leaf [37] | UAE | water, sample to solvent ratio = 1:30, t = 30 min (156 W) | 18.30 | mg CAE/g DW: 147.46 (±18.31) | mg of CE/g DW: 5.34 (±0.09) | qualitative analysis: UHPLC-DAD-HESI-MS/MS 24 phenolic compounds quantitative analysis: HPLC-DAD µg/g of extract: 920.97 sinapic acid (50.49), p-hydroxybenzoic acid (37.38), p-coumaric acid (18.52), ferulic acid (18.36), syringic acid (16.72); rutin (578.36), quercetin (80.65), apigenin (37.05), kaempferol (26.56), apigenin glycoside (20.00), naringenin (15.08), rosmarinic acid (11.15), luteolin (10.65) | µg/mL: positive control: amracin (A) S. aureus ATCC 25923: 78.12 (A: 0.97) E. coli ATCC 25922: 156.25 (A: 0.49) P. vulgaris ATCC 13315: 156.25 (A: 0.49) K. pneumoniae ATCC 13883: 156.40 (A: 0.97) B. subtilis ATCC 6633: 156.40 (A: 0.24) P. mirabilis ATCC 14153: 312.50 (A: 0.49) |
| MAE | water, sample to solvent ratio = 1:30, t = 30 min (450 W) | 30.75 | 380.08 (±14.91) | 10.99 (±0.12) | qualitative analysis: UHPLC-DAD-HESI-MS/MS 27 phenolic compounds quantitative analysis: HPLC-DAD µg/g of extract: 1146.13 sinapic acid (63.12), p-hydroxybenzoic acid (48.68), p-coumaric acid (23.12), ferulic acid (21.07), syringic acid (20.29); rutin (722.83), quercetin (95.71), apigenin (46.34), kaempferol (32.58), apigenin glycoside (26.44), naringenin (21.46), rosmarinic acid (13.27), luteolin (11.22) | S. aureus: 39.10, P. mirabilis: 78.12, E. coli: 156.25, P. vulgaris: 312.50, K. pneumoniae: 312.50, B. subtilis: 625.00 | |
| SWE | T = 125 °C, p = 35 bar, t = 30 min, sample to solvent ratio = 1:30 | 42.81 | 463.59 (± 15.60) | 11.00 (±0.03) | qualitative analysis: UHPLC-DAD-HESI-MS/MS 22 phenolic compounds quantitative analysis: HPLC-DAD µg/g of extract: 336.23 sinapic acid (18.08), p-hydroxybenzoic acid (10.72), ferulic acid (10.30), syringic acid (7.71), p-coumaric acid (4.84); rutin (215.49), quercetin (25.51), apigenin (10.02), kaempferol (9.95), apigenin glycoside (9.95), rosmarinic acid (6.03), naringenin (4.48), luteolin (3.15) | S. aureus: 9.76, E. coli: 78.12, K. pneumoniae: 78.25, P. vulgaris: 156.25, B. subtilis: 312.50, P. mirabilis: 312.50 | |
| Allium ursinum L.—leaf [38] | SWE | T = 180 °C, p = 1500 psi, t = 10 min | mg GAE/g DW: 4.23 | mg QE/g DW: 0.73 | HPLC-DAD 11 phenolic compounds µg/mL of extract: gallic acid (32.97), gallic acid derivate (9.10), gallic acid derivate (7.24), kaempferol derivate (8.96), kaempferol derivate (16.76), kaempferol derivate (9.48), kaempferol derivate (20.45), kaempferol derivate (29.95), catechin derivate (7.24), catechin derivate (6.89), catechin derivate (3.44) | mg/mL: L. monocytogenes: 28 S. enteritidis, E. coli 10536, E. coli 8739, P. hauseri, E. faecalis: 29 | |
| Matricaria chamomilla L.—flower [39] | Soxhlet | 70% EtOH, t = 40 min | 25.75 | mg CAE/g DW: 141.41 | mg RE/g DW: 64.32 | µg/mL: positive control: amracin (A) E. coli ATCC 25922: 39.10 (A: 0.97) P. vulgaris ATCC 13315: 78.125 (A: 0.49) P. mirabilis ATCC 14153: 78.125 (A: 0.49) B. subtilis ATCC 6633: 78.125 (A: 0.24) S. aureus ATCC 25923: 156.25 (A:0.97) K. pneumoniae ATCC 13883: 156.25 (A: 0.49) | |
| MAE | 70% EtOH, t = 40 min | 31.64 | 117.31 | 58.23 | E. coli: 78.125, P. mirabilis: 78.125, B. subtilis: 156.25, S. aureus: 156.25, K. pneumoniae: 312.5, P. vulgaris: 312.5 | ||
| UAE | 70% EtOH, t = 40 min | 11.09 | 123.40 | 51.60 | E. coli: 39.10, P. mirabilis: 39.10, B. subtilis: 78.125, S. aureus: 78.125, P. vulgaris: 78.125, K. pneumoniae: 156.25 | ||
| SWE | T = 200 °C, p = 1,6 bar, t = 40 min, sample to solvent ratio = 1:50 | 42.10 | 151.45 | 49.70 | UHPLC-DAD-HESI-MS/MS 28 detected compounds apigenin-7-O-glucoside, caffeic acid phenylethyl ester, catechin, gallic acid, proanthocyanidin dimer, ferulic acid glucoside, 5-O-feruloylquinic acid, chlorogenic acid, luteolin, epicatechin, luteolin-7-O-glucoside, caffeoyl-hexoside-methylglutarate, quercetin-3-glucoside, 3-p-coumaroylquinic acid, pelargonidin-succinylarabinoside or pelargonidin-malonylrhamnoside, p-coumaroyl-hexosidemethylglutarate, apigenin, dicaffeolyquinic acid, pinobanksin-3-O-butyrate, hyperfirin, apigenin O-glucuronide, adhyperfirin | E. coli: 39.10, P. mirabilis: 78.125, P. vulgaris: 78.125, B. subtilis: 156.25, K. pneumoniae: 156.25, S. aureus: 312.50 | |
| Satureja hortensis L.—herb [40] | Soxhlet | 96% EtOH, t = 8 h | mg GAE/g DW: 119.28 (±0.50) | mg RE/g DW: 5.23 (±0.76) | HPLC-DAD 15 phenolic compounds µg/g of extract: 752.54 rosmarinic acid (301.66), quercetin (155.15), apigenin (52.78), kaempferol (46.63), luteolin (40.54), chlorogenic acid (36.06), rutin (33.54), apigenin-glycoside (24.39), p-coumaric acid (14.81), p-hydroxybenzoic acid (12.87), vanillic acid (11.03), naringenin (7.51), sinapic acid (7.09), caffeic acid (6.27), ferulic acid (2.15) | µg/mL: positive control: amracin (A) E. coli ATCC 25922: 7.81 (A: 0.49) S. aureus ATCC 25923: 7.81 (A: 0.97) L. ivanovii ATCC 19119: 31.25 (A: 0.49) S. typhimurium ATCC 14028: 31.25 (A: 0.24) E. faccalis ATCC 2912: 62.50 (A: 0.49) E. aerogenus ATCC 13048: 62.50 (A: 0.49) S. saprophiticus ATCC 15035: 125.00 (A: 0.24) L. inocun ATCC 33090: 125.00 (A: 0.97) L. monocytogenes ATCC 19112: 125.00 (A: 0.49) B. spieizeneii ATCC 6633: 125.00 (A: 0.24) S. enteritidas ATCC 13076: 125.00 (A: 0.97) C. freundi ATCC 43864: 125.00 (A: 0.49) P. aeroginosa ATCC 27853: 125.00 (A: 0.97) E. faccium ATCC 6057: 250.00 (A: 0.97) P. mirabilis ATCC 35659: 250.00 (A: 0.49) | |
| maceration | 96% EtOH, t = 7 days, T = 22 °C | 125.34 (±0.13) | 16.27 (±0.34) | HPLC-DAD 13 phenolic compounds µg/g of extract: 351.92 rosmarinic acid (287.59), chlorogenic acid (17.30), kaempferol (11.75), rutin (10.75), vanillic acid (9.02), apigenin (3.15), p-coumaric acid (2.84), p-hydroxybenzoic acid (2.35), apigenin-glycoside (2.16), quercetin (1.77), sinapic acid (1.47), luteolin (1.26), naringenin (0.46) | E. aerogenus: 7.81, S. saprophiticus: 15.82, E. faccium: 15.82, S. typhimurium: 15.82, P. mirabilis: 31.25, E. coli: 62.50, L. inocun: 62.50, L. monocytogenes: 62.50, C. freundi: 62.50, S. aureus: 125.00, B. spieizeneii: 125.00, P. aeroginosa: 125.00, L. ivanovii: 250.00, E. faccalis: 250.00, S. enteritidas: 250.00 | ||
| UAE | 96% EtOH, t = 30 min (216 W), frequency = 40 kHz | 132.40 (±0.65) | 19.68 (±0.50) | HPLC-DAD 12 phenolic compounds µg/g of extract: 43.22 rutin (24.04), quercetin (6.42), sinapic acid (4.24), apigenin (1.44), rosmarinic acid (1.34), kaempferol (1.24), p-coumaric acid (1.08), ferulic acid (0.90), luteolin (0.82), apigenin-glycoside (0.82), syringic acid (0.56), naringenin (0.32) | S. saprophiticus: 15.82, S. typhimurium: 15.82, C. freundi: 15.82, P. aeroginosa: 15.82, E. faccalis: 15.82, E. faccium: 31.25, L. inocun: 31.25, L. monocytogenes: 31.25, S. aureus: 31.25, B. spieizeneii: 31.25, L. ivanovii: 31.25, E. aerogenus: 62.50, P. mirabilis: 62.50, E. coli: 62.50, S. enteritidas: 500.00 | ||
| MAE | t = 30 min (600 W) | 147.21 (±0.15) | 23.10 (±0.18) | HPLC-DAD 12 phenolic compounds µg/g of extract: 95.22 quercetin (41.26), rutin (28.48), rosmarinic acid (9.62), sinapic acid (4.88), apigenin-glycoside (2.62), apigenin (2.36), kaempferol (1.96), luteolin (1.10), ferulic acid (1.08), naringenin (0.94), syringic acid (0.48), p-coumaric acid (0.44) | P. aeroginosa: 31.25, L. monocytogenes: 31.25, S. aureus: 31.25, S. saprophiticus: 62.50, E. faccalis: 62.50, S. typhimurium: 125.00, E. faccium: 125.00, L. inocun: 125.00, E. coli: 125.00, C. freundi: 250.00, B. spieizeneii: 250.00, L. ivanovii: 250.00, P. mirabilis: 250.00, S. enteritidas: 250.00, E. aerogenus: 500.00 | ||
| SWE | T = 140 °C, p = 40 bar, t = 30 min | 151.54 (±0.85) | 28.42 (±0.29) | HPLC-DAD 12 phenolic compounds µg/g of extract: 43.32 rutin (16.56), quercetin (11.12), p-hydrohybenzoic acid (7.58), rosmarinic acid (2.66), sinapic acid (1.42), kaempferol (1.12), apigenin-glycoside (0.88), apigenin (0.78), luteolin (0.46), ferulic acid (0.26), naringenin (0.26), luteolin-glycoside (0.22) | S. saprophiticus: 7.81, B. spieizeneii: 31.25, P. aeroginosa: 62.50, L. monocytogenes: 62.50, S. aureus: 62.50, S. enteritidas: 62.50, E. faccium: 125.00, L. inocun: 125.00, C. freundi: 125.00, L. ivanovii: 125.00, E. faccalis: 250.00, S. typhimurium: 250.00, E. coli: 250.00, E. aerogenus: 250.00, P. mirabilis: 500.00 | ||
| Satureja kitaibelii Wierzb. Ex Heuff—flower [41] | SWE | T = 130 °C, t = 30 min, sample to solvent ratio = 1:30 | 25.5 | HPLC 10 phenolic compounds mg/g of extract: 89.3 syringic acid (37.88), caffeic acid (18.06), epicatechin (10.04), protocatechuic acid (7.87), vanillic acid (6.20), ferulic acid (5.93), rutin (1.68), apigenin (1.05), chlorogenic acid (0.56), luteolin (0.06) | mg/mL: positive control: cefotaxime + clavulanic acid E. faecalis ATCC 19433: 1.04, S. aureus ATCC 25923: 2.08, L. monocytogenes ATCC 35152: 2.08, B. cereus ATCC 11778: 8.325, E. coli ATCC 25922: > 33.3, P. aeruginosa ATCC 27853: > 33.3, S. typhimurium ATCC 13311: > 33.3 | ||
| Crocus sativus L.—corm [42] | SWE | T: 100, 140, 180 °C, t: 10, 20, 30 min Optimal conditions: T = 180 °C, t = 22 min | 43.55 | mg GAE/g DW: 8.08 | mg QE/g DW: 0.12 | GC-MS | mg/mL: positive control: gentamicin S. aureus PTCC 1764: 150, E. coli PTCC 1330: 300 |
| Castanea sativa Mill.—shell [43] | SWE | T: 110, 120, 140, 160, 180 °C, p = 20 bar, t = 30 min, sample to solvent ratio = 1:30 | 110 °C: 20.88 (±0.78) 120 °C: 21.00 (±1.49) 140 °C: 20.83 (±0.62) 160 °C: 20.97 (±1.74) 180 °C: 20.29 (±0.93) | mg GAE/g DW: 110 °C: 239.53 (±23.17) 120 °C: 201.75 (±13.02) 140 °C: 162.52(±7.14) 160 °C: 122.31 (±5.89) 180 °C: 126.19 (±6.33) | HPLC-PDA 24 detected phenolic compounds mg/g of extract: 110 °C: 19.01 120 °C: 15.58 140 °C: 11.84 160 °C: 6.75 180 °C: 6.92 3,5-dicaffeolquinic acid, 4-O-caffeyolquinic acid, caffeoylquinic acid, caftaric acid, caffeic acid, chlorogenic acid, p-coumaric acid, ellagic acid, gallic acid, ferulic acid, neochlorogenic acid, protocatechuic acid, sinapic acid, syringic acid, vanillic acid, quercetin-3-O-galactoside, quercetin-3-O-glucopyranoside, naringin, rutin, catechin, epicatechin, phloridzin, resveratrol, trans-polydatin | mg/mL: S. aureus MRSA: 110 °C: 4, 120 °C: 8, 140 °C: 32, 160 °C: 4, 180 °C: 4 S. aureus ATCC25913: 110 °C: /, 120 °C: 64, 140 °C: 4, 160 °C: 4, 180 °C: 4 E. coli ATCC25922: 110 °C: 8, 120 °C: 8, 140 °C: 4, 160 °C: 4, 180 °C: 8 E. coli CTXM2: 110 °C: 4, 120 °C: 8, 140 °C: 4, 160 °C: 4, 180 °C: / E. coli ATCC8739: 110 °C: 4, 120 °C: 4, 140 °C: 16, 160 °C: 4, 180 °C: 8 E. faecalis: 110 °C: 64, 120 °C: 2, 140 °C: 64, 160 °C: 8, 180 C: 16 | |
| Morus nigra L.—leaf [44] | SWE | T: 60, 100, 130, 160, 200 °C, p = 10 bar, t = 30 min, sample to water ratio = 1:40 | mg GAE/g DW (160 °C): 61.89 TPC (160 °C) > TPC (200 °C) > TPC (130 °C) > TPC (100 °C) > TPC (60 °C) | HPLC-DAD 9 phenolic compounds mg/g of extract: chlorogenic acid (183), caffeic acid (91.1), protocatechuic acid (79.5), β-resorcylic acid (62.0), naringin (56.8), rutin (43.7), catechin (38.0), gallic acid (26.3), p-coumaric acid (9.57) | µg/mL: positive control: amracin (A) S. aureus ATCC 25923: 39.1 (A: 0.97) K. pneumoniae ATCC 13883: 78.125 (A: 0.49) P. vulgaris ATCC 13315: 78.125 (A: 0.49) E. coli ATCC 25922: 312.5 (A: 0.97) P. mirabilis ATCC 14153: 312.5 (A: 0.49) B. subtilis ATCC 6633: 312.5 (A: 0.24) | ||
| Teucrium chamaedrys L.—flower [44] | SWE | T: 60, 100, 130, 160, 200 °C, p = 10 bar, t = 30 min, sample to water ratio = 1:40 | mg GAE/g DW (160 °C): 176.74 TPC (160 °C) > TPC (200 °C) > TPC (130 °C) > TPC (100 °C) ≈ TPC (60 °C) | HPLC-DAD 9 phenolic compounds mg/g of extract: gallic acid (217), catechin (101), chlorogenic acid (76.3), protocatechuic acid (73.7), caffeic acid (54.2), vanillic acid (42.2), epicatechin (38.1), ferulic acid (29.1), sinapic acid (19.2) | µg/mL: positive control: amracin (A) S. aureus ATCC 25923: 78.125 (A: 0.97) K. pneumoniae ATCC 13883: 78.125 (A: 0.49) P. mirabilis ATCC 14153: 78.125 (A: 0.49) B. subtilis ATCC 6633: 156.25 (A: 0.24) P. vulgaris ATCC 13315: 156.4 (A: 0.49) E. coli ATCC 25922: 312.5 (A: 0.97) | ||
| Geranium macrorrhizum L.—leaf [44] | SWE | T: 60, 100, 130, 160, 200 °C, p = 10 bar, t = 30 min, sample to water ratio = 1:40 | TPC (130 °C) > TPC (100 °C) > TPC (160 °C) > TPC (60 °C) > TPC (200 °C) | HPLC-DAD 7 phenolic compounds mg/g of extract: gallic acid (1512), protocatechuic acid (234), ferulic acid (128), chlorogenic acid (106.9), catechin (97.7), vanillic acid (14.3), p-coumaric acid (8.64) | µg/mL: positive control: amracin (A) S. aureus ATCC 25923: 19.53 (A: 0.97) B. subtilis ATCC 6633: 78.125 (A: 0.24) K. pneumoniae ATCC 13883: 156.4 (A: 0.49) P. vulgaris ATCC 13315: 312.5 (A: 0.49) E. coli ATCC 25922: 312.5 (A: 0.97) P. mirabilis ATCC 14153: 312.5 (A: 0.49) | ||
| Symphytum officinale L.—leaf [44] | SWE | T: 60, 100, 130, 160, 200 °C, p = 10 bar, t = 30 min, sample to water ratio = 1:40 | TPC (130 °C) > TPC (160 °C) > TPC (100 °C) > TPC (60 °C) > TPC (200 °C) | HPLC-DAD 11 phenolic compounds mg/g of extract: p-coumaric acid (157), protocatechuic acid (135), gallic acid (122), caffeic acid (89.6), rutin (53.5), β-resorcylic acid (30.6), naringin (28.4), ferulic acid (14.1), sinapic acid (10.6), naringenin (3.36), cinnamic acid (1.27) | µg/mL: positive control: amracin (A) S. aureus ATCC 25923: 39.1 (A: 0.97) B. subtilis ATCC 6633: 78.125 (A: 0.24) E. coli ATCC 25922: 156.25 (A: 0.97) K. pneumoniae ATCC 13883: 156.4 (A: 0.49) P. vulgaris ATCC 13315: 312.5 (A: 0.49) P. mirabilis ATCC 14153: 625 (A: 0.49) | ||
| Aronia melanocarpa (Michx.) Elliott [45] | SWE—leaves | T = 130 °C, t = 20 min, p = 35 bar, sample to solvent ratio = 1:20 | mg CAE/g DW: 131.53 (±0.96) | mg RE/g DW: 88.64 (±0.31) | HPLC-DAD 14 phenolic compounds mg/g of extract: rutin (0.693), sinapic acid (0.547), luteolin (0.334), apigenin (0.210), rosmarinic acid (0.155), p-hydroxybenzoic acid (0.128), quercetin (0.105), p-coumaric acid (0.093), kaempferol (0.069), syringic acid (0.055), ferulic acid (0.046), vanillic acid (0.041), chlorogenic acid (0.029), naringenin (0.026) | µg/mL: positive control: amracin (A) S. aureus ATCC 25923: 39.10 (A: 19.53) B. subtilis ATCC 6633: 78.12 (A: 78.12) E. coli ATCC 25922: 312.50 (A: 19.53) K. pneumoniae ATCC 13883: 312.50 (A: 39.10) P. vulgaris ATCC 13315: 39.10 (A: 156.25) P. mirabilis ATCC 14153: 19.53 (A: 312.50) | |
| SWE—berries | 13.88 (±0.02) | 10.00 (±0.25) | HPLC-DAD 14 phenolic compounds mg/g of extract: rutin (5.544), quercetin (1.396), sinapic acid (1.072), p-hydroxybenzoic acid (0.469), syringic acid (0.419), kaempferol (0.271), apigenin (0.242), vanillic acid (0.238), rosmarinic acid (0.236), p-coumaric acid (0.175), ferulic acid (0.173), narigenin (0.172), chlorogenic acid (0.171), luteolin (0.154) | S. aureus: 78.20, B. subtilis: 312.50, E. coli: 312.50, K. pneumoniae: 312.50, P. vulgaris: 78.20, P. mirabilis: 78.125 | |||
| SWE—stems | 49.96 (±0.15) | 25.10 (±0.38) | HPLC-DAD 15 phenolic compounds mg/g of extract: rutin (1.264), luteolin (0.364), sinapic acid (0.290), quercetin (0.247), p-hydroxybenzoic acid (0.161), apigenin (0.151), protocatechuic acid (0.150), rosmarinic acid (0.115), naringenin (0.065), syringic acid (0.056), ferulic acid (0.051), chlorogenic acid (0.050), kaempferol (0.049), p-coumaric acid (0.033), caffeic acid (0.019) | S. aureus: 156.25, B. subtilis: 625.00, E. cColi: 19.53, K. pneumoniae: 625.00, P. vulgaris: 78.12, P. mirabilis: 312.50 | |||
| Pseuderanthemum palatiferum (Nees) Radlk.—leaf [46] | MeOH extraction | T = 25 °C, t = 19 h | g/g: 0.06 (±0.01) | mg CE/g DW: 6.94 (±0.54) | mg RE/g DW: 6.22 (±0.16) | HPLC mg/g of extract: apigenin: 1.90 kaempferol: 0.73 | Inhibition zone (mm): positive control: ampicillin >6 mm: S. aureus ATCC 6538P, E. coli ATCC 25922, P. putida 10464, P. aeruginosa KCCM 9027, L. monocytogenes ATCC 7644 |
| hot water extraction | T = 80 °C, t = 30 min | 0.34 (±0.02) | 15.27 (±0.11) | 14.71 (±0.14) | apigenin: 2.05 kaempferol: 0.89 | >6 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes | |
| Soxhlet | 70% EtOH, t = 7 h | 0.13 (±0.04) | 10.77 (±0.72) | 8.28 (±0.26) | apigenin: 2.11 kaempferol: 0.63 | >6 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes | |
| SWE | T: 130, 150, 170, 190, 210, 230, 250, 270 °C, p = 80 bar, t = 15 min, solid liquid ratio = 1:70 | 110 °C: 0.31 (±0.02) 130 °C: 0.32 (±0.01) 150 °C: 0.39 (±0.01) 170 °C: 0.43 (±0.02) 190 °C: 0.44 (±0.01) 210 °C: 0.46 (±0.01) 230 °C: 0.48 (±0.01) 250 °C: 0.49 (±0.01) 270 °C: 0.50 (±0.01) | 110 °C: 23.03 (±0.79) 130 °C: 24.35 (±0.45) 150 °C: 28.98 (±1.01) 170 °C: 29.47 (±0.04) 190 °C: 33.68 (±0.29) 210 °C: 25.29 (±0.02) 230 °C: 23.09 (±0.43) 250 °C: 18.53 (±1.76) 270 °C: 9.48 (±2.38) | 110 °C: 18.94 (±0.80) 130 °C: 20.71 (±0.42) 150 °C: 19.38 (±0.63) 170 °C: 18.38 (±0.15) 190 °C: 18.33 (±0.43) 210 °C: 16.58 (±0.12) 230 °C: 15.27 (±0.36) 250 °C: 11.47 (±0.41) 270 °C: 8.16 (±0.13) | 110 °C: apigenin: 2.63; kaempferol: 1.44 130 °C: apigenin: 2.93; kaempferol: 1.61 150 °C: apigenin: 3.23; kaempferol: 1.90 170 °C: apigenin: 3.46; kaempferol: 2.31 190 °C: apigenin: 3.34; kaempferol: 2.43 210 °C: apigenin: 2.80; kaempferol: 1.92 230 °C: apigenin: 2.47; kaempferol: 1.53 250 °C: apigenin: 1.14; kaempferol: 1.25 270 °C: apigenin: 0.97; kaempferol: 1.01 | 110 °C: >6 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes 130 °C: >6 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes 150 °C: >6 mm: P. putida, L. monocytogenes; >8 mm: S. aureus, E. coli, P. aeruginosa 170 °C: >8 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes 190 °C: >8 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes 210 °C: >8 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes 230 °C: >8 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes 250 °C: >6 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes 270 °C: >6 mm: S. aureus, E. coli, P. putida, P. aeruginosa, L. monocytogenes |
2.1. Influence of Extraction Methods on Phenol and Flavonoid Content
2.2. Influence of Extraction Methods on Yield
2.3. Influence of Extraction Methods on Antimicrobial Activity
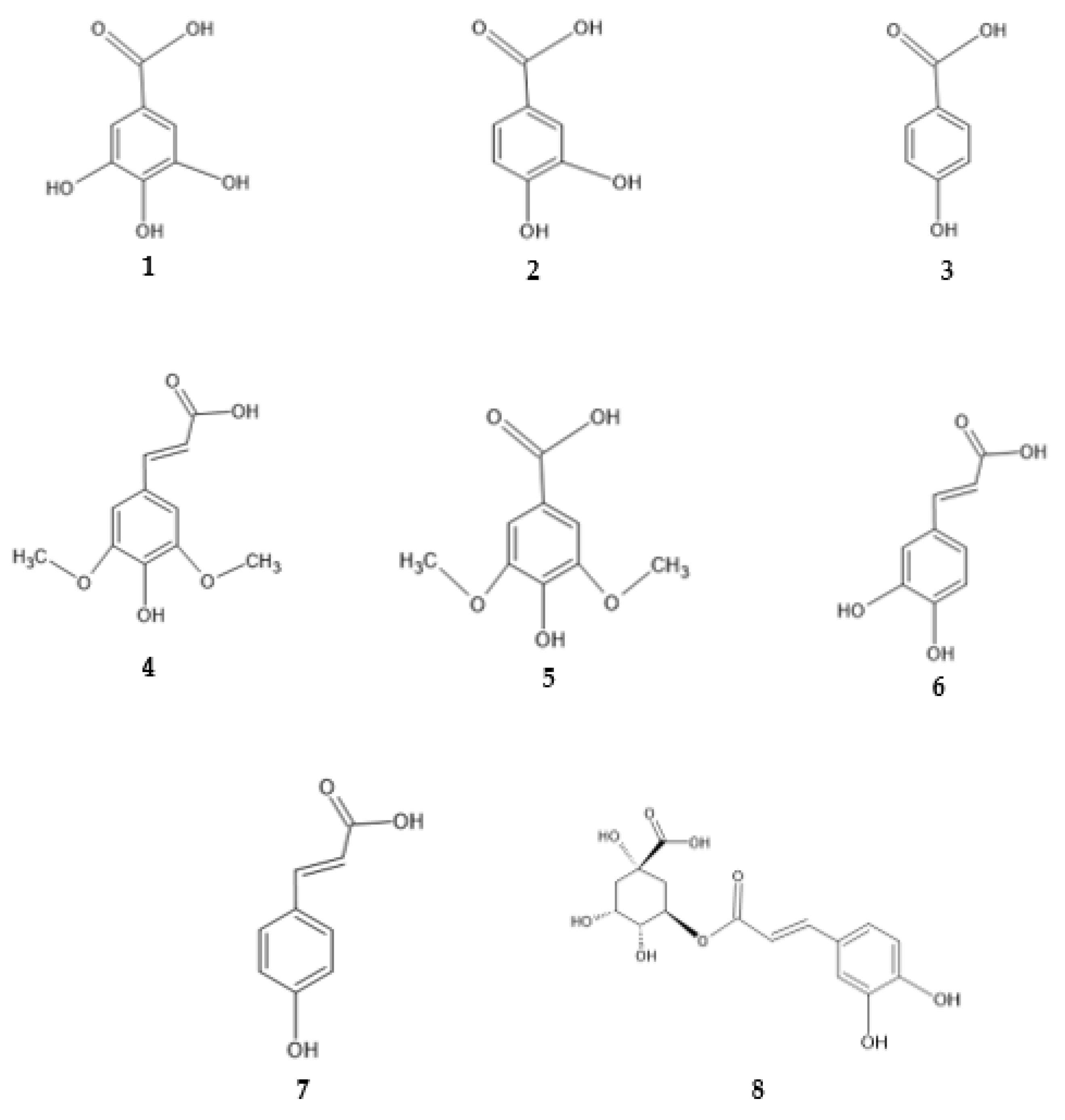
2.4. Antimicrobial Activity of Phenolic Compounds—SAR
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/concept-paper-development-reflection-paper-modern-manufacturing-techniques-used-herbal-preparations_.pdf (accessed on 13 November 2023).
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fan, Q. Application of sub-critical water extraction in pharmaceutical industry. J. Mater. Sci. Chem. Eng. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidovic, S.; Redovnikovic, I.R.; Jokic, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Schoss, K.; Glavač, N.K. Ekstrakcija s subkritično vodo za pridobivanje rastlinskih ekstraktov. Farm. Vestn. 2021, 72, 167–172. [Google Scholar]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2016, 46, 21–34. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Jokić, S.; Aladić, K.; Šubarić, D. Subcritical water extraction laboratory plant design and application. Environ. Sci. 2018, 21, 247–258. [Google Scholar]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Mantu, D.A.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practise. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in plants: Structure, biosynthesis, abiotic stress regulation and practical applications (review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Abram, V.; Simčič, M. Fenolne spojine kot antioksidanti (Phenolics as antioxidants). Farm. Vestn. 1997, 48, 573–589. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Durgo, K.; Hudek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar]
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Encinar, J.A.; Rodriguez-Diaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against Gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valero, J.R. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-Garcia, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2019; pp. 253–271. [Google Scholar]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals, 2nd ed.; Yahia, E.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; Volume 2, pp. 115–124. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, T.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Anilkumar, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Gorniak, I.; Bartoszewski, R.; Kroliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Cushine, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Chibane, L.B.; Forquet, V.; Lantéri, P.; Clément, Y.; Akkari, L.L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Zeković, Z.; Cvetanović, A.; Gajić, J.Š.; Gorjanović, S.Ž.; Sužnjević, D.; Masković, P.; Savić, S.R.; Radojković, M.; Đurović, S. Chemical and biological screening of stinging nettle leaves extracts obtained by modern extraction techniques. Ind. Crop. Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- Stupar, A.; Šarić, L.; Vidović, S.; Bajić, A.; Kolarov, V.; Šarić, B. Antibacterial potential of Allium ursinum extract prepared by the green extraction method. Microorganisms 2022, 10, 1358. [Google Scholar] [CrossRef]
- Cvetanović, A.; Gajić, J.Š.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crop. Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Mašković, P.; Veličković, V.; Mitić, M.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Gajić, J.Š.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted in enhanced biological. Ind. Crop. Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
- Aćimović, M.; Šeregelj, V.; Sovljanski, O.; Šaponjac, V.T.; Gajić, J.Š.; Borjan, T.B.; Pezo, L. In vitro antioxidant, antihyperglycemic, anti-inflammatory, and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff. subcritical water extract. Ind. Crop. Prod. 2021, 169, 113672. [Google Scholar] [CrossRef]
- Esmaeelian, M.; Jahani, M.; Einafshar, S.; Feizy, J. Optimization of experimental parameters in subcritical water extraction of bioactive constituents from the safron (Crocus sativus L.) corm based on response surface methodology. J. Food Meas. Charact. 2020, 14, 1822–1832. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Silva, A.M.; Pinto, P.; Moreira, M.M.; Ferraz, R.; Gajić, J.Š.; Costa, P.C.; Matos, C.D.; Rodrigues, F. New perspectives on the sustainable employment of chestnut shells as active ingredient against oral mucositis: A first screening. Int. J. Mol. Sci. 2022, 23, 14956. [Google Scholar] [CrossRef]
- Nastić, N.; Gajić, J.Š.; Matos, C.D.; Barroso, M.F.; Soares, C.; Moreira, M.M.; Morais, S.; Mašković, P.; Srček, V.G.; Slivac, I.; et al. Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants. Ind. Crop. Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chun, B.S. Extraction of bioactive compounds from Pseuderanthemum palatiferum (Nees) Radlk. using subcritical water and conventional solvents: A comparison study. J. Food Sci. 2019, 84, 1201–1207. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Mammen, D. Chemical perspective and drawbacks in flavonoid estimation assays. Front. Nat. Prod. Chem. 2022, 10, 189–228. [Google Scholar]
- Drinić, Z.; Vladic, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Tomić, M.; Vidović, S. Application of conventional and high-pressure extraction techniques for the isolation of bioactive compounds from the aerial part of hemp (Cannabis sativa L.) assortment Helena. Ind. Crop. Prod. 2021, 171, 113908. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Majdanik, M.M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wasik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus Aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Halake, K.; Birajdar, M.; Lee, J. Structural implications of polyphenolic antioxidants. J. Ind. Eng. Chem. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Thebti, A.; Meddeb, A.; Ben Salem, I.; Bakary, C.; Ayari, S.; Rezgui, F.; Essafi-Benkhadir, K.; Boudabous, A.; Ouzari, H.I. Antimicrobial activities and mode of flavonoid actions. Antibiotics 2023, 12, 225. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthehic flavonoids with antimicrobial activity: A review. J. Aplied Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žagar, T.; Frlan, R.; Kočevar Glavač, N. Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties. Antibiotics 2024, 13, 334. https://doi.org/10.3390/antibiotics13040334
Žagar T, Frlan R, Kočevar Glavač N. Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties. Antibiotics. 2024; 13(4):334. https://doi.org/10.3390/antibiotics13040334
Chicago/Turabian StyleŽagar, Tjaša, Rok Frlan, and Nina Kočevar Glavač. 2024. "Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties" Antibiotics 13, no. 4: 334. https://doi.org/10.3390/antibiotics13040334
APA StyleŽagar, T., Frlan, R., & Kočevar Glavač, N. (2024). Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties. Antibiotics, 13(4), 334. https://doi.org/10.3390/antibiotics13040334








