Chestnut Honey Is Effective against Mixed Biofilms at Different Stages of Maturity
Abstract
1. Introduction
- (1)
- From the combinations P. aeruginosa-MRSA, P. aeruginosa-S. epidermidis, and S. epidermidis-MRSA, which were the most sensitive to treatment with chestnut honey;
- (2)
- In which stage of biofilm formation could this honey type interfere the most.
2. Results
2.1. Melissopalynological Analysis
2.2. Minimum Inhibitory Concentration (MIC)
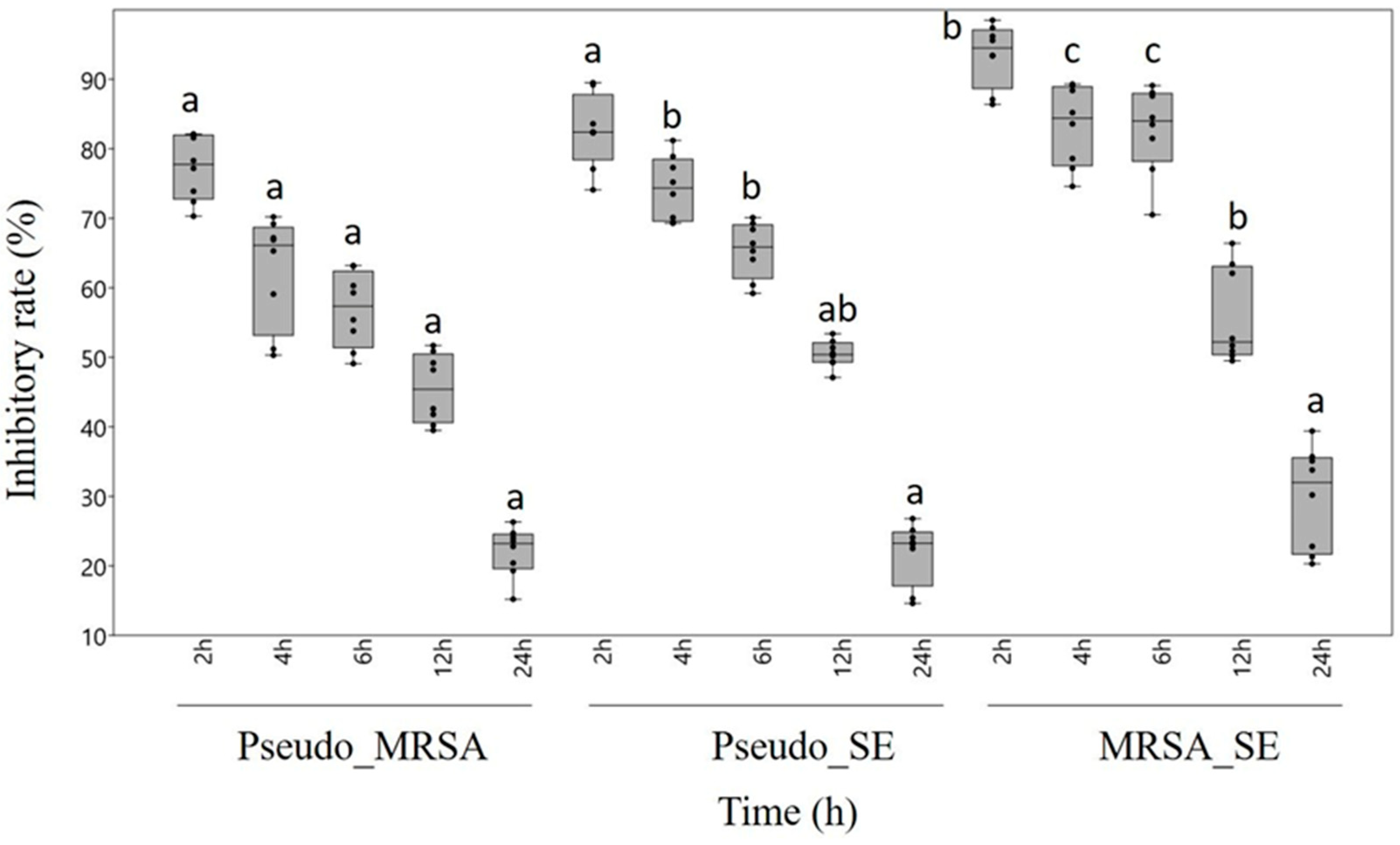
2.3. Antibiofilm Effect
3. Discussion
4. Materials and Methods
4.1. Honey Sample and Melissopalynological Analysis
4.2. Microbiological Assays
4.2.1. Bacterial Strains
4.2.2. Determination of Minimum Inhibitory Concentration
4.2.3. Antibiofilm Activity
Cell Viability in Biofilm Formation
Scanning Electron Microscopy (SEM)
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Grooters, K.E.; Ku, J.C.; Richter, D.M.; Krinock, M.J.; Minor, A.; Li, P.; Kim, A.; Sawyer, R.; Li, Y. Strategies for combating antibiotic resistance in bacterial biofilms. Front. Cell Infect Microbiol. 2024, 14, 1352273. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Nickel, J.C.; Ruseska, I.; Wright, J.B.; Costerton, J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985, 27, 619–624. [Google Scholar] [CrossRef]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef]
- Diggle, S.P.; Whiteley, M. Microbe Profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology 2020, 166, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Maranan, M.C.; Moreira, B.; Boyle-Vavra, S.; Daum, R.S. Antimicrobial resistance in staphylococci. Epidemiology, molecular mechanisms, and clinical relevance. Infect Dis. Clin. N. Am. 1997, 11, 813–849. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.; Otto, M. Understanding the significance of Staphylococcus epidermidis bacteremia in babies and children. Curr. Opin. Infect Dis. 2010, 23, 208–216. [Google Scholar] [CrossRef]
- Rodis, N.; Tsapadikou, V.K.; Potsios, C.; Xaplanteri, P. Resistance mechanisms in bacterial biofilm formations: A Review. J. Emerg. Intern. Med. 2020, 4, 30. [Google Scholar]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Oliveira, A.; Sousa, J.C.; Silva, A.C.; Melo, L.D.R.; Sillankorva, S. Chestnut Honey and Bacteriophage Application to Control Pseudomonas aeruginosa and Escherichia coli Biofilms: Evaluation in an ex vivo Wound Model. Front. Microbiol. 2018, 31, 1725. [Google Scholar] [CrossRef] [PubMed]
- León-Ruiz, V.; González-Porto, A.V.; Al-Habsi, N.; Vera, S.; San Andrés, M.P.; Jauregi, P. Antioxidant, antibacterial and ACE-inhibitory activity of four monofloral honeys in relation to their chemical composition. Food Funct. 2013, 4, 1617–1624. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.M.; Estevinho, L.; Teixeira-Santos, R.; Rodrigues, A.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Antibacterial Action Mechanisms of Honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 10, 1252. [Google Scholar] [CrossRef]
- Cumhur, A.; Hakan, A.; Tuğrul, Y.; Ahmet, T.; Nuray, T.D. Determination of the superior quality properties of randomly selected chestnut honey samples from the Sinop region. Spectrosc. Lett. 2023, 56, 353–363. [Google Scholar] [CrossRef]
- Balázs, V.L.; Nagy-Radványi, L.; Bencsik-Kerekes, E.; Koloh, R.; Szabó, D.; Kocsis, B.; Kocsis, M.; Farkas, Á. Antibacterial and Antibiofilm Effect of Unifloral Honeys against Bacteria Isolated from Chronic Wound Infections. Microorganisms 2023, 17, 509. [Google Scholar] [CrossRef]
- Persano, O.L.R.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect 2015, 21, S1–S25. [Google Scholar] [CrossRef]
- Dalton, T.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Watters, C.; Griswold, J.A.; Rumbaugh, K.P. An In Vivo Polymicrobial Biofilm Wound Infection Model to Study Interspecies Interactions. PLoS ONE 2011, 6, e27317. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rumbaugh, K.P.; James, G.; Schultz, G.; Phillips, P.; Yang, Q.; Watters, C.; Stewart, P.S.; Dowd, S.E. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J. Wound Care 2010, 19, 320–328. [Google Scholar] [CrossRef]
- Mandal, D.M.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.; Harizanis, P.C.; Polissiou, M. Investigation of Organic Extractives from Unifloral Chestnut (Castanea sativa L.) and Eucalyptus (Eucalyptus globulus Labill.) Honeys and Flowers to Identification of Botanical Marker Compounds. LWT-Food Sci. Technol. 2011, 44, 1042–1051. [Google Scholar] [CrossRef]
- Mayda, N.; Ozok, A.; Sorkun, K. Some Characteristic Properties of Chestnut and Rhododendron Honeys in Turkey. J. Biol. Chem. 2018, 1, 135–145. [Google Scholar] [CrossRef]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Tuska, J. A Review of the Health Benefits of Food Enriched with Kynurenic Acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef] [PubMed]
- Balázs, V.L.; Nagy-Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, Á. In Vitro Antibacterial and Antibiofilm Activity of Hungarian Honeys against Respiratory Tract Bacteria. Foods 2021, 10, 1632. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Á.; Balázs, V.L.; Kőszegi, T.; Csepregi, R.; Kerekes, E.; Horváth, G.; Szabó, P.; Gaál, K.; Kocsis, M. Antibacterial and Biofilm Degradation Effects of Hungarian Honeys Linked with Botanical Origin, Antioxidant Capacity and Mineral Content. Front. Nutr. 2022, 9, 953470. [Google Scholar] [CrossRef]
- Park, J.S.; An, S.J.; Jeong, S.I.; Gwon, H.J.; Lim, Y.M.; Nho, Y.C. Chestnut Honey Impregnated Carboxymethyl Cellulose Hydrogel for Diabetic Ulcer Healing. Polymers 2017, 27, 248. [Google Scholar] [CrossRef]
- Skadiņš, I.; Labsvārds, K.D.; Grava, A.; Amirian, J.; Tomsone, L.E.; Ruško, J.; Viksna, A.; Bandere, D.; Brangule, A. Antimicrobial and Antibiofilm Properties of Latvian Honey against Causative Agents of Wound Infections. Antibiotics 2023, 26, 816. [Google Scholar] [CrossRef]
- Peterson, L. Squeezing the antibiotic balloon: The impact of antimicrobial classes on emerging resistance. Clin. Microbiol. Infect. 2005, 11, 4–16. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; deLancey Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Hindler, J.A.; Jorgensen, J.H. Susceptibility Test Methods: Fastidious Bacteria. In Manual of Clinical Microbiology; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Kerekes, E.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm formating and anti-quorum sensing activity of selected essential oils and their main components on food related microorganisms. J. App. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, S.; Zhang, C.; Liu, Y.; Ma, L.; Zhang, X. Effects of sub-minimum inhibitory concentrations of lemon essential oil on the acid tolerance and biofilm formation of Streptococcus mutans. Arch. Oral. Biol. 2018, 87, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wachsmann, P.; Lamprecht, A. Polymeric nanoparticles for the selective therapy of inflammatory bowel disease. Methods Enzym. 2012, 508, 377–397. [Google Scholar] [CrossRef]
- Lü, L.; Zhang, L.; Wai, M.S.M.; Yew, D.T.W.; Xu, J. Exocytosis of MTT formazan could exacerbate cell injury. Toxicol. Vitr. 2012, 26, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]

| Pollen Type—Relative Frequency (%) | ||||||
|---|---|---|---|---|---|---|
| Honey type | Castanea | Tilia | Helianthus | Robinia | Brassica | Other |
| chestnut | 82.9 | 6.3 | 0.6 | 2.4 | 6.4 | 1.4 |
| Type of Biofilm | Incubation Time (h) | Viable Cells (%) |
|---|---|---|
| P. aeruginosa-MRSA | 2 | 89.1 ± 0.2 |
| 4 | 79.4 ± 0.5 | |
| 6 | 65.7 ± 0.6 | |
| 12 | 59.2 ± 0.2 | |
| 24 | 26.4 ± 0.1 | |
| P. aeruginosa-S.epidermidis | 2 | 86.6 ± 0.5 |
| 4 | 75.4 ± 0.4 | |
| 6 | 70.2 ± 0.9 | |
| 12 | 48.6 ± 0.7 | |
| 24 | 25.4 ± 0.6 | |
| MRSA-S. epidermidis | 2 | 87.4 ± 0.7 |
| 4 | 74.2 ± 0.8 | |
| 6 | 62.1 ± 0.3 | |
| 12 | 53.9 ± 0.5 | |
| 24 | 34.1 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koloh, R.; Balázs, V.L.; Nagy-Radványi, L.; Kocsis, B.; Kerekes, E.B.; Kocsis, M.; Farkas, Á. Chestnut Honey Is Effective against Mixed Biofilms at Different Stages of Maturity. Antibiotics 2024, 13, 255. https://doi.org/10.3390/antibiotics13030255
Koloh R, Balázs VL, Nagy-Radványi L, Kocsis B, Kerekes EB, Kocsis M, Farkas Á. Chestnut Honey Is Effective against Mixed Biofilms at Different Stages of Maturity. Antibiotics. 2024; 13(3):255. https://doi.org/10.3390/antibiotics13030255
Chicago/Turabian StyleKoloh, Regina, Viktória L. Balázs, Lilla Nagy-Radványi, Béla Kocsis, Erika Beáta Kerekes, Marianna Kocsis, and Ágnes Farkas. 2024. "Chestnut Honey Is Effective against Mixed Biofilms at Different Stages of Maturity" Antibiotics 13, no. 3: 255. https://doi.org/10.3390/antibiotics13030255
APA StyleKoloh, R., Balázs, V. L., Nagy-Radványi, L., Kocsis, B., Kerekes, E. B., Kocsis, M., & Farkas, Á. (2024). Chestnut Honey Is Effective against Mixed Biofilms at Different Stages of Maturity. Antibiotics, 13(3), 255. https://doi.org/10.3390/antibiotics13030255