Abstract
The evidence regarding the role of oral antibiotics alone (oA) or combined with mechanical bowel preparation (MoABP) for elective colorectal surgery remains controversial. A prospective database of 8359 colorectal resections gathered over a 32-month period from 78 Italian surgical units (the iCral 2 and 3 studies), reporting patient-, disease-, and procedure-related variables together with 60-day adverse events, was re-analyzed to identify a subgroup of 1013 cases (12.1%) that received either oA or MoABP. This dataset was analyzed using a 1:1 propensity score-matching model including 20 covariates. Two well-balanced groups of 243 patients each were obtained: group A (oA) and group B (MoABP). The primary endpoints were anastomotic leakage (AL) and surgical site infection (SSI) rates. Group A vs. group B showed a significantly higher AL risk [14 (5.8%) vs. 6 (2.5%) events; OR: 3.77; 95%CI: 1.22–11.67; p = 0.021], while no significant difference was recorded between the two groups regarding SSIs. These results strongly support the use of MoABP for elective colorectal resections.
1. Introduction
The earliest literature report on bowel decontamination and surgery dates back to 1899 [1]. During the last 80 years, the use of mechanical bowel preparation (MBP), oral antibiotics (oA), and perioperative intravenous antibiotic prophylaxis (PIVAP) to reduce the incidence of anastomotic leakage (AL) and surgical site infections (SSIs) in elective colorectal surgeries have shown time-related and geographic fluctuating trends, with clinical practice and guidelines remaining non-unique and inconclusive, despite the extraordinary number of published studies [2]. The use of MBP started at the beginning of the last century, becoming the usual practice in the 1930s until the beginning of the 1940s, when the use of multiple oral, non-absorbable sulfa derivatives, active only against aerobic species in the colon, was studied together with MBP [3]. After the Second World War, the discovery of several new oral, non-absorbable antibiotics active against aerobic and anaerobic species (aminoglycosides, tetracyclines, polimixines, macrolides, and, later on, nitroimidazoles) influenced bowel preparation before elective colorectal surgery, favoring oA combined with MBP (MoABP) and the diffusion of intraperitoneal resections with immediate anastomosis [4]. The landmark studies from surgeons in Chicago [5,6] using the oral administration of neomycin and erythromycin showed a dramatic reduction in AL and SSI rates, leading to the widespread diffusion of MoABP among North American surgeons, covering approximately 86% of cases at the end of the previous century [7]. At the same time, the introduction of parenteral cephalosporins and amoxicillin/clavulanate in the decades from the 1970s to 1980s shifted attention towards the major role of PIVAP in reducing SSI rates, and led to the current evidence [8] and the strong recommendation of the World Health Organization [9] for the administration of a single preoperative (30 to 120 min before the operation) intravenous dose of a cephalosporin and metronidazole, albeit with a conditional recommendation for the use of oA. At the beginning of the current century, several randomized controlled trials (RCTs) failed to demonstrate any clear benefit of MBP alone, supporting the concept of no bowel preparation (NBP), leading to the recommendation to avoid MBP in systematic reviews [10,11], in both the European [12] and Italian [13] Enhanced Recovery After Surgery (ERAS) society guidelines, and in the WHO guidelines [9]. Thereafter, the use of MoABP for colorectal surgery in North America dropped down to a 30–40% rate [14].
During the last ten years, however, the results of several large retrospective series stemming from the American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) has led to the resurgence of the belief that MoABP significantly decreases SSIs and overall morbidity (OM) rates compared to NBP [15,16,17,18,19,20,21,22]. Consequently, the guidelines of four large North American societies (The American Society of Colon and Rectal Surgeons, the Society of American Gastrointestinal and Endoscopic Surgeons, the American Society for Enhanced Recovery, and the Perioperative Quality Initiative) recommended MoABP [23,24,25]. Thereafter, the number of patients treated with MoABP in North America rose again up to 80% of cases [26]. The rate of adoption of MoABP among European surgeons seems to have been more variable. It is currently used by 50% of Austrian–German [27] surgeons, while its use is much more limited (about 10% of cases) in Italy [28]. These figures will probably change in the near future, as very recently the European Association of Endoscopic Surgery, the European Society of ColoProctology, together with the Society of American Gastrointestinal and Endoscopic Surgeons, published a joint guideline recommending MoABP [29], albeit supported by low-quality evidence, due to the variable adherence to PIVAP and the great heterogeneity regarding oral antibiotics schedules [30].
During the last five years, three RCTs on this topic have been published. The first one, comparing NBP with MoABP [31], failed to detect significant differences in SSIs and AL rates, but it was largely underpowered. Another RCT, comparing NBP with oA [32], showed that the oral administration of ciprofloxacin 750 mg b.i.d. and metronidazole 250 mg t.i.d. the day before colon surgery significantly reduced SSIs. This trial, however, received some criticism related to the very low AL and major morbidity rates in both study arms [33]; its authors launched another RCT comparing oA with MoABP, which is currently still recruiting participants [34]. Finally, the third study compared PIVAP alone with PIVAP combined with oA [35], using different MBP schedules. It showed significantly reduced SSI rates in the oA arm, particularly when oA was coupled with MBP, although the PIVAP schedule did not include metronidazole, as has been recommended since 2014 [8]. Moreover, although several other RCTs have been launched, only one study comparing MoABP with MBP for rectal cancer [36] has completed the planned enrollment, although its results are not yet available. Unfortunately, an interesting, long-awaited, four-arm RCT comparing NBP with oA, MBP, and MoABP for colon resections [37] was recently closed before completion owing to poor accrual.
The great heterogeneity of both oral and intravenous antibiotic prophylaxis schedules, coupled with the heterogeneity of mechanical bowel preparations (polyethylene glycol, sodium phosphate, picosulfate, etc.), result in an extraordinary number of possible combinations potentially evaluable by RCTs. The current evidence regarding the “optimal” bowel preparation for elective colorectal surgery, therefore, is inconclusive because of the following (1) MoABP probably reduces SSIs as well as anastomotic leakage compared to MBP alone; (2) oA alone might be as effective as MoABP, but this cannot be clearly determined yet; and (3) whether NBP compared to MoABP has an influence on morbidity cannot be determined yet [2]. When conclusive evidence from RCTs is lacking, or when researchers need to assess treatment effects based on real-life data, a propensity score-matching analysis (PSMA) performed on data from prospective observational studies offers an alternative approach for estimating treatment effects. Based on these considerations, the Italian ColoRectal Anastomotic Leakage (iCral) study group estimated the effects of oA plus PIVAP (the treatment variable) versus MoABP plus PIVAP before elective colorectal surgery, through a PSMA of the data derived from two prospective, open-label, observational multicenter studies [38,39].
2. Materials and Methods
2.1. Study Design
This was a secondary, unplanned, ad hoc propensity score-matched re-analysis of two prospective cohorts of patients who had undergone colorectal surgery for malignant and benign diseases.
2.2. Patient Population and Data Collection
The iCral2 [38] and iCral3 [39] studies prospectively enrolled 8359 patients who underwent colorectal resection with anastomosis, according to explicit inclusion/exclusion criteria, in 78 surgical centers in Italy from January 2019 to September 2021. Both studies followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [40].
The present PSMA study included 1013 (12.1%) patients selected from the parent studies according to explicit exclusion criteria (Figure 1) to control for data imbalance due to any treatment confounder. Most of the exclusions (81.8%) were based on a self-evident rationale (i.e., no bowel preparation, mechanical bowel preparation alone, no perioperative intravenous antibiotic prophylaxis, missing data regarding bowel preparation, perioperative steroids, mechanical bowel preparation different from PEG, and dialysis). The remaining criteria (neoadjuvant therapy, a proximal derivative stoma, urgency or delayed urgency, and an anastomosis within 6 cm from the external anal verge) accounted for 18.2% of the excluded cases, and were considered to limit the heterogeneity regarding one of the primary endpoints (anastomotic leakage). The descriptive variables considered for the 1013 patients are shown in Table 1. All 1013 patients were treated with PIVAP; however, the intravenous antibiotic schedules were not available.
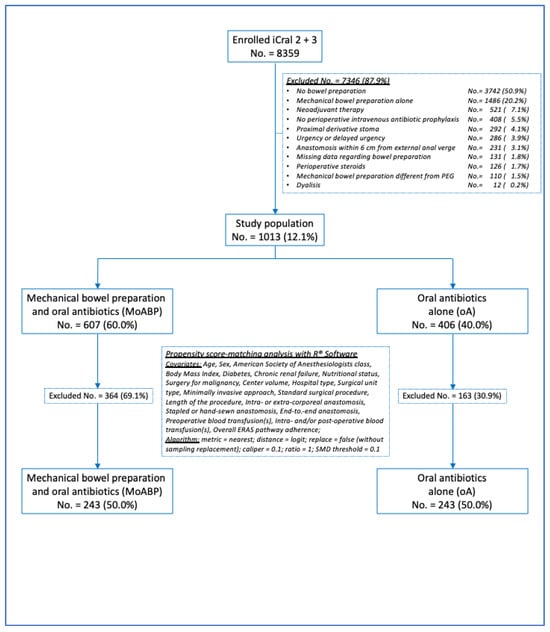
Figure 1.
Study flowchart.

Table 1.
The descriptive analysis of the variables considered in the entire population.
The continuous variables were categorized according to their median values to reduce the number of unmatched cases. The true population of interest—oA—included 406 patients (40.0%); the control population—MoABP—included 607 patients (60.0%). Significant differences in age, nutritional status, indications of malignancy, type of surgical procedure, end-to-end anastomosis, hospital type, unit type, and the percentage of adherence to ERAS items were detected between the oA and MoA groups (Table 1). The patients in the MoABP control population prepared their bowels by drinking products containing polyethylene glycol the day before surgery. The patients in both groups received several different oral antibiotic schedules, the majority of which contained metronidazole, all of which provided both aerobic and anaerobic coverage (Table 2).

Table 2.
Oral antibiotic schedules in oA and MoA groups before propensity score matching.
All the enrolled patients were followed up with for at least 8 weeks after surgery, recording and grading any adverse events [41,42], as well as any reoperations, readmissions, or death. Anastomotic leakage (AL) was defined according to the international consensus [43].
2.3. Outcomes
All the outcomes were calculated at 60 days after surgery. The primary outcomes were AL and SSIs, defined as superficial and/or deep surgical site infections (sdiSSIs), deep wound dehiscence, and/or abdominal collection/abscess [44]. The secondary outcomes were as follows: (1) overall morbidity (any adverse event), (2) major morbidity (any adverse event grade > II), and (3) reoperation (any unplanned operation) rates. In this retrospective study, mortality, sdiSSIs, deep wound dehiscence, and abdominal collection/abscess were not considered between the outcomes because the very small number of events in relation to the sample size (1013 patients) would make the statistical results of the comparison between the oA and MoABP groups burdened by inconsistency and unreliability [45,46].
2.4. Statistical Analysis
This was a retrospective PSMA of two prospective cohorts, with the sample sizes calculated and reported in the respective core papers [38,39]. The events per variable guideline were followed [45]. There were no missing data in the database for the 1013 patients. The target of the estimands was represented by the average treatment effect in the true population of interest (ATT). A propensity score-matching model [47,48] was used for the analysis (Figure 1). An adjusted logistic regression was used to estimate the propensity scores for the treatment and control groups. The exposure variable was a treatment that implied oA for the elective colorectal surgery. Twenty covariates potentially affecting the treatment [49] were selected: age, sex, American Society of Anesthesiologists (ASA) class, body mass index (BMI), diabetes, chronic renal failure, nutritional status measured through the Mini Nutritional Assessment—Short Form (MNA-SF) [50], surgery for malignancy, center volume, hospital type (academic/metropolitan versus local/regional), surgical unit type (general versus oncologic/colorectal), mini-invasive surgery, standard surgical procedure, operation length (minutes), intra- or extra-corporeal anastomosis, stapled versus handsewn anastomosis, end-to-end anastomosis, preoperative blood transfusion(s), intra- and/or postoperative blood transfusion(s), and overall ERAS pathway adherence rates. To ensure that the treatment groups were balanced [51], we performed the PSMA using the software “R©” (Version 4.2.2, The R Foundation© for Statistical Computing, Vienna, Austria, 2022). We used a nearest-neighbor approach with a logit distance metric and a caliper of 0.1 to minimize the differences between the groups. We also used an adjusted logistic regression to estimate the association between the treatment variable and outcomes. The balance of the matched groups was assessed by calculating the standardized mean difference (SMD) using a threshold of 0.1 (a standardized mean difference of less than 0.1 typically indicates a negligible difference between the means of the groups), and the general variance ratio (a variance ratio close to 1 indicates that variances are equal in the two groups). For outcome modeling, an adjusted logistic regression was performed based on a treatment variable represented by oA with elective colorectal surgery and on the same 20 covariates selected for the PSMA [52], which calculated the odds ratios (OR) and 95% confidence intervals (95%CI). The eventual effect of any unobserved confounder was tested through a sensitivity analysis [53] using the library “SensitivityR5” of the software R© (Version 4.2.2, The R Foundation© for Statistical Computing, Vienna, Austria, 2022), which calculated the values (each 0.1 increment in the value represents a 10% odds of a differential assignment to treatment due to any unobserved variable). Sidak–Bonferroni’s adjustment for multiple comparisons was applied, setting α = 0.025, because the two primary outcomes were not independent and were selected based on the literature evidence [2].
3. Results
In this series of 1013 patients undergoing elective colorectal surgery for malignant and benign diseases, mortality events occurred in 4 patients (0.4%), 2 in the oA group and 2 in the MoABP group. Before propensity score matching, a univariate analysis of the entire population of 1013 patients showed no statistically significant differences in the primary and secondary outcomes between the oA and MoABP groups (Table 3).

Table 3.
The univariate analysis of outcomes in the entire population.
After propensity score matching, 486 patients were included, and two groups of 243 patients each were generated (Figure 1): the oA group (the true population of interest) and the MoABP group (the control population). A good balance between the two groups was achieved (Figure 2 and Table 4), with a model variance ratio of 1.089.
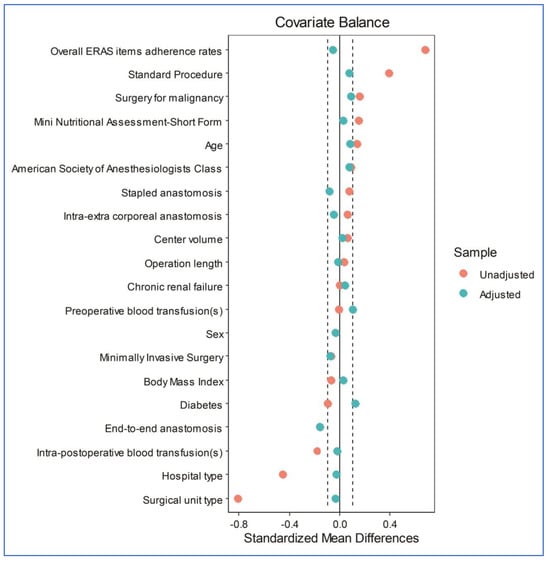
Figure 2.
A Love plot of the covariates’ standardized mean differences between the treatment and control groups before and after matching; the vertical lines represent an interval of ± 0.1 within which the balance is considered acceptable.

Table 4.
Variables’ distribution in control and treatment groups before and after propensity score matching.
After the multivariate logistic regression analysis for the endpoints for the 486 patients evaluated after score matching, oA versus MoABP was significantly associated with a higher risk of AL [14 (5.8%) vs. 6 (2.5%) events; OR: 3.77; 95%CI: 1.22–11.67; p = 0.021]. The sensitivity analysis calculated a Γ = 1 (p upper bound = 0.057). No difference was recorded between the two groups for SSIs [9 (3.7%) vs. 7 (2.9%) events; OR: 1.02; 95%CI: 0.31–3.29; p = 0.977]. The oA group was also significantly associated with a higher risk of major morbidity [25 (10.3%) vs. 9 (3.7%) events; OR: 4.55; 95%CI: 1.82–11.38; p = 0.001; Γ = 1.4; p upper bound = 0.038], and a higher risk of reoperation [16 (6.6%) vs. 5 (2.1%) events; OR: 5.05; 95%CI: 1.55–16.49; p = 0.007; Γ = 1.3; p upper bound = 0.037]. No significant differences were recorded between the two groups in terms of overall morbidity (Table 5).

Table 5.
The multivariate logistic regression analysis of the endpoints considered for the 486 patients evaluated using the PSMA.
According to the types of adverse events reported in the two groups (Table 6), the higher risk of major morbidity recorded in the oA group vs. MoABP group was significantly related to AL and superficial and/or deep surgical site infections (sdiSSIs).

Table 6.
Adverse events contributing to overall morbidity and major morbidity in 486 patients evaluated using PSMA.
4. Discussion
The effectiveness of oA and MoABP for reducing AL and SSI rates for elective colorectal resections remains largely controversial [2]. On the one hand, a well-designed RCT showed that oA alone is able to significantly reduce SSI rates compared to NBP, albeit with no influence on AL rates [32], while another large French RCT [33] showed the same finding, with the highest reduction achieved with MoABP, although the PIVAP schedule in this trial did not include metronidazole. On the other hand, two largely underpowered RCTs [54,55] showed inconclusive results. Analyses of the large retrospective databases of the ACS-NSQIP [17,18,20,21] and Veterans Affairs NSQIP [56] have suggested that both oA and MoABP may be equally effective in reducing AL and SSI rates compared to MBP alone or NBP. Therefore, while waiting for the results of the ongoing international RCT comparing oA to MoABP [34], it could be of particular interest to know how these different types of preoperative preparations work in real-life clinical practice.
To the best of our knowledge, the present study is the first PSMA to compare oA with PIVAP versus MoABP with PIVAP using the data derived from a prospective multicenter database, which represents a snapshot of the real-life clinical practice for 1013 Italian patients before elective colorectal surgery. There were no significant differences between the groups in terms of SSI rates, while there was a significantly higher risk of AL, MM, and reoperation in the group treated with oA (Table 5). The sensitivity analysis [53] showed Γ = 1 for AL, Γ = 1.4 for MM, and Γ = 1.3 for reoperation, meaning that 10%, 40%, and 30% of the patients in this study should have been treated with MoABP instead of oA, in order to alter the significant association between oA and the higher risk of AL, MM, and reoperation, respectively. The significantly higher MM risk in the oA group was significantly related to AL and sdiSSIs, among several other adverse events (Table 6), and the significantly lower risk of reoperation in the MoABP group may have been related to the causal link between AL and reoperation.
In summary, the results of this PSMA suggest that oA alone exposed the patients to a higher risk of AL and grade > II sdiSSIs. The reasons are mainly speculative, and rely on the conviction [57] that luminal feces may lead to the reduced efficiency of topically acting antibiotics. In 2016, Fry suggested that retained stool contains a large bulk of microbes, dietary fiber, and exfoliated cells that will not permit a reduction in the density of potential pathogens on the colonic mucosal surface with the use of oral antibiotics [58], supporting previous studies [3,5,59] performed in the 1940s and the 1970s. In patients treated with oA alone, it is possible that some members of the Bacteroidetes phylum [60] and other microbes, such as Enterococcus faecalis and Pseudomonas aeruginosa [57,61], can remain in the feces and colon mucosa and express enzymes that promote the degradation of synthesized tissue, leading to the vulnerability of the newly created anastomosis in response to the surgical trauma and resulting ischemia. The hypothesis that MBP could reduce the abundance of protective Bifidobacterium and Lactobacillus species, leading to higher rates of postoperative infections [62] appears less convincing, mainly because changes in the microbiota are only one of the factors that influence the rates of AL and postoperative complications after elective colorectal surgery [57,63].
Although the need for aerobic and anaerobic coverage is universally accepted, many different oral and intravenous antibiotic combinations have been previously reported [2], with prevalent geographic preferences. In the present study, many different antibiotics and administration schedules were used (Table 2), and because of the small number of AL events in each oral antibiotic and administration schedule subgroup, it was not possible to conclude which antibiotic and administration schedule is better for preventing AL. Over 100 trillion microorganisms (microbiota, including fungi, viruses, protozoans, and bacteria) are present in the gastrointestinal tracts of the hosts [60]. Approximately 80 bacterial species are present in the colorectal tract, differing between individuals according to many factors, including ethnicity, sex, age [60], cultural and social disparities [64], and antibiotic resistance due to extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales due to previous antibiotic therapies [65]. Considering the recent shift in European guidelines towards recommending MoABP instead of NBP [23], many European surgeons (just like the authors) are currently asking themselves which oA regimen (molecules and schedules) should be implemented in their clinical practice. Based on the results of the most recent RCTs [32,35], a short-term (on the preoperative day) oral administration of a nitroimidazolic (i.e., ornidazole or metronidazole) combined with a quinolone (i.e., ciprofloxacin) is appealing, due to their optimal aerobic and anaerobic coverage. However, even a short-term course of oral metronidazole and ciprofloxacin [66] produces profound changes in the gut microbiota shortly after administration, with a drop in microbial diversity, an overgrowth of the genera Streptococcus and Lactobacillus, and an early loss of anaerobic bacterial taxa with important roles in short-chain fatty acid metabolism (colonic butyrate-producing communities) that have been demonstrated to be of paramount importance for colorectal mucosal integrity and anastomotic healing in animal studies [64,67]. Moreover, these changes require several months to return back to the baseline [66], and quinolones may be involved in the worldwide increasing incidence of a plague of multidrug-resistant microorganisms [68,69,70]. A possible answer may come, in the near future, from the ongoing Human Microbiome Project [71], whose worldwide mapping will allow for perioperative microbiome manipulation through the targeted administration of antibiotics, probiotics, or symbiotics to restore the ideal bowel flora by selecting specific bowel strains rather than continuing to search for an impossible “one-size-fits-all” elimination of the intestinal microbiota.
Strengths and Limitations
The strengths of this study are the large number of enrolled patients in a well-defined time-lapse study, representing a real-life snapshot of the surgical units performing colorectal resections in Italy, and its PSMA methodology. Following recommendations for the use of propensity score methods [72,73], a rigorous patients selection from the parent population and the reasoned inclusion of 20 conditioning variables were performed to limit data imbalances. Moreover, both a clear and restrictive balance algorithm, together with the evaluation of the treatment effects through an adjusted multiple regression model including the same 20 covariates used for matching, were used (Figure 1). Finally, Rosenbaum’s sensitivity analysis for unmeasured confounders was applied [53].
On the other hand, this study has several limitations, and its results should be interpreted with caution: (a) a moderate heterogeneity of oral and intravenous antibiotic prophylaxis schedules, as reported by previous cohort studies [18,21,56]; (b) the exclusion criteria applied to the parent database (Figure 1) practically excluded any resection performed for low rectal cancers, making the results not applicable to this subgroup of patients; (c) several aspects of the health-acquired infections preventive bundle (preoperative whole-body bathing, hair removal, and skin decontamination) and each surgeon’s experience [74] were not measured in the parent studies; and (d) finally, further bias from residual unknown factors and potential measurement errors by the participating investigators may have had an impact on the results.
5. Conclusions
The present study contains an important warning, reporting that oA alone compared to MoABP before elective colorectal surgery was significantly associated with a higher risk of AL, MM, and reoperation.
Future clinical research should be aimed at tailoring the administration of oral antibiotics, probiotics, and symbiotics according to the individual’s microbiome, instead of trying to adapt a “one size fits all” strategy of bowel preparation for elective colorectal surgery.
Author Contributions
M.C., iCral Study Group coordinator, and S.G. share the first co-authorship. They had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: M.C., S.G., F.M., M.S. (Massimo Sartelli), L.A.M., G.L.B., G.D.T., F.B., P.M., and M.S. (Marco Scatizzi). Acquisition, analysis, and interpretation of the data: M.C., S.G., F.M., M.S. (Massimo Sartelli), L.A.M., G.L.B., G.D.T., F.B., P.M., and M.S. (Marco Scatizzi). Drafting of the manuscript: M.C., S.G., and F.M. Critical revision of the manuscript for important intellectual content: M.C., S.G., F.M., M.S. (Massimo Sartelli), L.A.M., G.L.B., G.D.T., F.B., P.M., and M.S. (Marco Scatizzi). Statistical analysis: F.M., S.G., and M.C. All the collaborators participated in the data acquisition and quality control for the iCral2 and iCral3 studies. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Both the iCral2 and iCral3 studies were conducted in accordance with the Declaration of Helsinki and the guidelines for good clinical practice E6 (R2). The study protocols were approved by the ethics committee of the coordinating center (Marche Regional Ethics Committee [CERM] 2018/334, released on 11/28/2018 for iCral2, and 2020/192, released on 07/30/2020 for iCral3) and were registered at ClinicalTrials.gov (NCT03771456 for iCral2 and NCT04397627 for iCral3). Subsequently, all the other centers were authorized to participate by their local ethics committees.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the parent studies.
Data Availability Statement
Individual participant-level anonymized datasets are available upon request by contacting the study coordinator.
Conflicts of Interest
Catarci reports personal fees from Baxter Spa separate from the submitted work. Guadagni, Masedu, Sartelli, Montemurro, Baiocchi, Tebala, Borghi, Marini, and Scatizzi have no competing interests to declare.
Appendix A
† Collaborators: iCral2 and iCral3 investigators are Paolo Ciano, MD, Michele Benedetti, MD, and Matteo Di Carlo, MD, General Surgery Unit, Sandro Pertini Hospital, ASL Roma 2; Marco Clementi, MD, General Surgery Unit, University of L’Aquila; Paolo Delrio, MD, Ugo Pace, MD, and Andrea Fares Bucci, MD, Colorectal Surgical Oncology, Istituto Nazionale per lo Studio e la Cura dei Tumori, “Fondazione Giovanni Pascale IRCCS-Italia”, Napoli; Gianluca Garulli, MD and Francesco Monari, MD, General Surgery Unit, Infermi Hospital, Rimini; Felice Pirozzi, MD and Antonio Sciuto, MD, General Surgery Unit, ASL Napoli 2 Nord, Pozzuoli (NA); Lorenzo Pandolfini, MD and Alessandro Falsetto, MD, General Surgery Unit, Santa Maria Annunziata & Serristori Hospital, Firenze; Giacomo Ruffo, MD, Elisa Bertocchi, MD, and Gaia Masini, MD, General Surgery Unit, IRCCS Sacro Cuore Don Calabria Hospital, Negrar di Valpolicella (VR); Massimo Giuseppe Viola, MD, Amedeo Altamura, MD, and Francesco Rubichi, MD, General Surgery Unit, Cardinale G. Panico Hospital, Tricase (LE); Ferdinando Ficari, MD, Francesco Giudici, MD, and Fabio Cianchi, MD, General Surgery and IBD Unit, Careggi University Hospital, Firenze; Desirée Cianflocca, MD and Marco Migliore, MD, General & Oncologic Surgery Unit, Department of Surgery, Santa Croce e Carle Hospital, Cuneo; Michele Simone, MD and Raffaele De Luca, MD, Department of Surgical Oncology, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari; Alessandro Rizzo, MD, Department of Medical Oncology, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari; Anna Albano, MS, Trial Office (AA), IRCCS Istituto Tumori “Giovanni Paolo II”, Bari; Alberto Patriti, MD and Marcella Lodovica Ricci, MD, Department of Surgery, Marche Nord Hospital, Pesaro e Fano (PU); Walter Siquini, MD and Alessandro Cardinali, MD, General Surgery Unit, S. Lucia Hospital, Macerata; Stefano D’Ugo, MD, PhD, FEBS, FACS and Marcello Spampinato, MD, PhD, FEBS (HPB), General Surgery Unit, “V. Fazzi” Hospital, Lecce; Stefano Scabini, MD, Alessandra Aprile, MD, and Domenico Soriero, MD, General & Oncologic Surgery Unit, IRCCS “San Martino” National Cancer Center, Genova; Marco Caricato, MD, FACS and Gabriella Teresa Capolupo, MD, FACS, Colorectal Surgery Unit, Policlinico Campus BioMedico, Roma; Giusto Pignata, MD, Jacopo Andreuccetti, MD, and Ilaria Canfora, MD, 2nd General Surgery Unit 2, Spedali Civili di Brescia; Andrea Liverani, MD and Andrea Scarinci, MD, General Surgery Unit, Regina Apostolorum Hospital, Albano Laziale (RM); Roberto Campagnacci, MD and Angela Maurizi, MD, General Surgery Unit, “C. Urbani” Hospital, Jesi (AN); Pierluigi Marini, MD and Grazia Maria Attinà, MD, General & Emergency Surgery Unit, San Camillo-Forlanini Hospital, Roma; Ugo Elmore, MD and Giulia Maggi, MD, Department of Gastrointestinal Surgery Unit, San Raffaele Research Hospital and “Vita-Salute” San Raffaele University, Milano; Francesco Corcione, MD, Umberto Bracale, MD, Roberto Peltrini, MD, and Maria Michela Di Nuzzo, MD, Minimally Invasive General and Oncologic and Surgery Unit, “Federico II” University, Napoli; Roberto Santoro, MD and Pietro Amodio, MD, General Oncologic Surgery Unit, Belcolle Hospital, Viterbo; Massimo Carlini, MD, FACS, Domenico Spoletini, MD, PhD, FACS, Rosa Marcellinaro, MD, and Giorgio Lisi, MD, General Surgery Unit, S. Eugenio Hospital, ASL Roma 2; Antonio Giuliani, MD and Giovanni Del Vecchio, MD, General Surgery Unit, S. Carlo Hospital, Potenza; Mario Sorrentino, MD and Massimo Stefanoni, MD, General Surgery Unit, Latisana-Palmanova Hospital, Friuli Centrale University (UD); Giovanni Ferrari, MD and Carmelo Magistro, MD, General Oncologic and Mininvasive Surgery Unit, Great Metropolitan Niguarda Hospital, Milano; Gianandrea Baldazzi, MD and Diletta Cassini, MD, General Surgery Unit, ASST Ovest Milanese, Nuovo Ospedale di Legnano, Legnano (MI); Alberto Di Leo, MD and Lorenzo Crepaz, MD, General and Minimally Invasive Surgery Unit, San Camillo Hospital, Trento; Augusto Verzelli, MD and Andrea Budassi, MD, General Surgery Unit, Profili Hospital, Fabriano (AN); Giuseppe Sica, MD and Bruno Sensi, MD, Minimally Invasive Surgery Unit, Policlinico Tor Vergata University Hospital, Roma; Stefano Rausei, MD and Silvia Tenconi, MD, General Surgery Unit, Gallarate Hospital (VA); Davide Cavaliere, MD, Leonardo Solaini, MD, and Giorgio Ercolani, MD, General & Oncologic Surgery Unit, AUSL Romagna, Forlì (FC); Gian Luca Baiocchi, MD, FACS and Sarah Molfino, MD, General Surgery Unit 3, Department of Clinical and Experimental Sciences, University of Brescia; Marco Milone, MD and Giovanni Domenico De Palma, MD, General & Endoscopic Surgery Unit, “Federico II” University, Napoli; Giovanni Ciaccio, MD and Paolo Locurto, MD, General Surgery Unit, S. Elia Hospital, Caltanissetta; Antonio Di Cintio, MD, General Surgery Unit, S. Maria Hospital, Terni; Luigi Boni, MD, FACS and Elisa Cassinotti, MD, General Surgery Unit, Fondazione IRCCS Ca’ Granda, Policlinico Maggiore Hospital, Milano; Stefano Mancini, MD, Andrea Sagnotta, MD, PhD, General & Oncologic Surgery Unit, San Filippo Neri Hospital, ASL Roma 1; Mario Guerrieri, MD and Monica Ortenzi, MD, Surgical Clinic, Torrette Hospital, University of Ancona; Roberto Persiani, MD and Alberto Biondi, MD, General Surgery Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma; Andrea Lucchi, MD, FACS and Alban Cacurri, MD, General Surgery Unit, “Ceccarini” Hospital, Riccione (RN); Dario Parini, MD and Maurizio De Luca, MD, General Surgery Unit, S. Maria della Misericordia Hospital, Rovigo; Antonino Spinelli, MD and Francesco Carrano, MD, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI) and IRCCS Humanitas Research Hospital, Rozzano (MI); Michele Genna, MD and Francesca Fior, MD, General Surgery Unit, University Hospital, Verona; Vincenzo Bottino, MD and Antonio Ferronetti, MD, General & Oncologic Surgery Unit, Evangelico Betania Hospital, Napoli; Andrea Coratti, MD, Giuseppe Giuliani, MD and Roberto Benigni, MD, General and Emergency Surgery Unit, Misericordia Hospital, Grosseto; Dario Scala, MD, Graziella Marino, MD, and Battistino Puppio, MD, Abdominal Oncologic Surgery Unit, IRCCS CROB Basilicata Referral Cancer Center, Rionero in Vulture (PZ); Andrea Muratore, MD, Patrizia Marsanic, MD, and Nicoletta Sveva Pipitone Federico, MD, General Surgery Unit, “E. Agnelli” Hospital, Pinerolo (TO); Maurizio Pavanello, MD and Carlo Di Marco, MD, General Surgery Unit, AULSS2 Marca Trevigiana, Conegliano Veneto (TV); Umberto Rivolta, MD and Camillo Leonardo Bertoglio, MD, PhD, General Surgery Unit, Fornaroli Hospital, ASST Ovest Milanese, Magenta (MI); Micaela Piccoli, MD, FACS and Francesca Pecchini, MD, General Surgery Unit, Civil Hospital, Baggiovara (MO); Carlo Talarico, MD and Vincenzo Greco, MD, General Surgery Unit, Villa dei Gerani Hospital, Vibo Valentia (VV); Alessandro Carrara, MD, Michele Motter, MD, and Giuseppe Tirone, MD, 1st General Surgery Unit, S. Chiara Hospital, Trento; Mauro Totis, MD and Nicolò Tamini, MD, Colorectal Surgery Unit, San Gerardo Hospital, ASST Monza; Franco Roviello, MD and Riccardo Piagnerelli, MD, General & Oncologic Surgery Unit, AOU Senese, Siena; Alessandro Anastasi, MD and Giuseppe Canonico, MD, General Surgery Unit, San Giovanni di Dio Hospital, Firenze; Gianluca Guercioni, MD and Simone Cicconi, MD, General Surgery Unit, “C. e G. Mazzoni” Hospital, Ascoli Piceno; Giuseppe Maria Ettorre, MD and Marco Colasanti, MD, General & Transplant Surgery Unit, San Camillo-Forlanini Hospital, Roma; Mauro Montuori, MD and Enrico Pinotti, MD, General & Mininvasive Surgery Unit, S. Pietro Hospital, Ponte San Pietro (BG); Pierpaolo Mariani, MD and Roberta Carminati, MD, General Surgery Unit, Pesenti Fenaroli Hospital, Alzano Lombardo (BG); Nicolò de Manzini, MD and Edoardo Osenda, MD, Surgical Clinic, University of Trieste; Annibale Donini, MD and Luigina Graziosi, MD, General & Emergency Surgery Unit, University of Perugia; Mariano Fortunato Armellino, MD, Ciro De Martino, MD, and Giovanna Ioia, MD, General & Emergency Surgery Unit, S. Giovanni di Dio e Ruggi d’Aragona Hospital, Salerno; Lucio Taglietti, MD and Arianna Birindelli, MD, General Surgery Unit, ASST Valcamonica, Esine (BS); Gabriele Anania, MD and Matteo Chiozza, MD, General & Laparoscopic Surgery Unit, University Hospital, Ferrara; Mariantonietta Di Cosmo, MD and Daniele Zigiotto, MD, General & Upper GI Surgery Unit, University Hospital, Verona; Carlo Vittorio Feo, MD and Fioralba Pindozzi, MD, General Surgery Unit, Delta Hospital, Lagosanto (FE); Paolo Millo, MD and Manuela Grivon, MD, General Surgery Unit, “U. Parini” Regional Hospital, Aosta; Corrado Pedrazzani, MD and Cristian Conti, MD, General & HPB Surgery Unit, University Hospital, Verona; Silvio Guerriero, MD and Lorenzo Organetti, MD, General Surgery Unit, “A. Murri” Hospital, Fermo; Andrea Costanzi, MD and Michela Monteleone, MD, General Surgery Unit, S. Leopoldo Hospital, Merate (LC); Nereo Vettoretto, MD and Emanuele Botteri, MD, General Surgery Unit, Spedali Civili of Brescia, Montichiari (BS); Federico Marchesi, MD and Giorgio Dalmonte, MD, Surgical Clinic, University of Parma; Massimo Basti, MD and Diletta Frazzini, MD, General Surgery Unit, Spirito Santo Hospital, Pescara; Graziano Longo, MD and Simone Santoni, MD, General Surgery Unit, Policlinico Casilino, Roma; Moreno Cicetti, MD and Gabriele La Gioia, MD, General Surgery Unit, S. Maria della Misericordia Hospital, Urbino (PU); Giuseppe Brisinda, MD, Maria Michela Chiarello, MD, and Maria Cariati, MD, General Surgery Unit, San Giovanni di Dio Hospital, Crotone; Stefano Berti, MD and Andrea Gennai, MD, General Surgery Unit, ASL 5 Liguria POLL, La Spezia; Italy.
References
- Yeo, B.; Harley, V.; Goodbody, F.; Pope, F.M.; Herschell, G.; Wild, R.B.; Haig, A. A discussion on intestinal antiseptics. BMJ 1899, 2, 1250–1257. [Google Scholar]
- Willis, M.A.; Toews, I.; Soltau, S.L.V.; Kal, J.C.; Meerpohl, J.J.; Vilz, T.O. Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database Syst. Rev. 2023, 2, CD014909. [Google Scholar] [PubMed]
- Poth, E.J.; Ross, C.A. The clinical use of phthalylsulfathiazole. J. Lab. Clin. Med. 1944, 29, 785–808. [Google Scholar]
- Lloyd-Davies, O.V.; Morgan, C.N.; Goligher, J.C. The treatment of carcinoma of the colon. In British Surgical Practice: Progress Volume; Carling, E.R., Ross, J.P., Eds.; Butterworth: London, UK, 1953; p. 71. [Google Scholar]
- Nichols, R.L.; Condon, R.E.; Gorbach, S.L.; Nyhus, L.M. Efficacy of preoperative antimicrobial preparation of the bowel. Ann. Surg. 1972, 176, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.L.; Broido, P.; Condon, R.E.; Gorbach, S.L.; Nyhus, L.M. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann. Surg. 1973, 178, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.L.; Smith, J.W.; Garcia, R.Y.; Waterman, R.S.; Holmes, J.W. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin. Infect. Dis. 1997, 24, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.L.; Gladman, E.; Barbateskovic, M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst. Rev. 2014, 2014, CD001181. [Google Scholar] [CrossRef]
- Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018.
- Guenaga, K.F.; Matos, D.; Castro, A.A.; Atallah, A.N.; Wille-Jørgensen, P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst. Rev. 2005, 1, CD001544. [Google Scholar]
- Guenaga, K.F.; Matos, D.; Wille-Jorgensen, P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst. Rev. 2011, 9, CD001544. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS©) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Ficari, F.; Borghi, F.; Catarci, M.; Scatizzi, M.; Alagna, V.; Bachini, I.; Baldazzi, G.; Bardi, U.; Benedetti, M.; Beretta, L.; et al. Enhanced recovery pathways in colorectal surgery: A consensus paper by the Associazione Chirurghi Ospedalieri Italiani (ACOI) and the PeriOperative Italian Society (POIS). G. Chir. 2019, 40 (Suppl. S4), 1–40. [Google Scholar]
- Markell, K.W.; Hunt, B.M.; Charron, P.D.; Kratz, R.J.; Nelson, J.; Isler, J.T.; Steele, S.R.; Billingham, R.P. Prophylaxis and management of wound infections after elective colorectal surgery: A survey of the American Society of Colon and Rectal Surgeons membership. J. Gastrointest. Surg. 2010, 14, 1090–1098. [Google Scholar] [CrossRef]
- Toneva, G.D.; Deierhoi, R.J.; Morris, M.; Richman, J.; Cannon, J.A.; Altom, L.K.; Hawn, M.T. Oral antibiotic bowel preparation reduces length of stay and readmissions after colorectal surgery. J. Am. Coll. Surg. 2013, 216, 756–763. [Google Scholar] [CrossRef]
- Kim, E.K.; Sheetz, K.H.; Bonn, J.; DeRoo, S.; Lee, C.; Stein, I.; Zarinsefat, A.; Cai, S.; Campbell, D.A., Jr.; Englesbe, M.J. A statewide colectomy experience: The role of full bowel preparation in preventing surgical site infection. Ann. Surg. 2014, 259, 310–314. [Google Scholar] [CrossRef]
- Morris, M.S.; Graham, L.A.; Chu, D.I.; Cannon, J.A.; Hawn, M.T. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann. Surg. 2015, 261, 1034–1040. [Google Scholar] [CrossRef]
- Scarborough, J.E.; Mantyh, C.R.; Sun, Z.; Migaly, J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: An analysis of colectomy-targeted ACS NSQIP. Ann. Surg. 2015, 262, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, R.; Abou-Khalil, J.; Morin, N.; Ghitulescu, G.; Vasilevsky, C.-A.; Gordon, P.; Demian, M.; Boutros, M. Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis. Colon Rectum. 2017, 60, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Koller, S.E.; Bauer, K.W.; Egleston, B.L.; Smith, R.; Philp, M.M.; Ross, H.M.; Esnaola, N.F. Comparative effectiveness and risks of bowel preparation before elective colorectal surgery. Ann. Surg. 2018, 267, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Midura, E.F.; Jung, A.D.; Hanseman, D.J.; Dhar, V.; Shah, S.A.; Rafferty, J.F.; Davis, B.R.; Paquette, I.M. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery 2018, 163, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Klinger, A.L.; Green, H.; Monlezun, D.J.; Beck, D.; Kann, B.; Vargas, H.D.; Whitlow, C.; Margolin, D. The role of bowel preparation in colorectal surgery. Ann. Surg. 2019, 269, 671–677. [Google Scholar] [CrossRef]
- Holubar, S.D.; Hedrick, T.; Gupta, R.; Kellum, J.; Hamilton, M.; Gan, T.J.; Mythen, M.G.; Shaw, A.D.; Miller, T.E.; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper. Med. 2017, 6, 4. [Google Scholar] [CrossRef]
- Carmichael, J.C.; Keller, D.S.; Baldini, G.; Bordeianou, L.; Weiss, E.; Lee, L.; Boutros, M.; McClane, J.; Feldman, L.S.; Steele, S.R. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis. Colon Rectum. 2017, 60, 761–784. [Google Scholar] [CrossRef]
- Migaly, J.; Bafford, A.C.; Francone, T.D.; Gaertner, W.B.; Eskicioglu, C.; Bordeianou, L.; Feingold, D.L.; Steele, S.R.; On behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis. Colon Rectum. 2019, 62, 3–8. [Google Scholar] [CrossRef]
- McChesney, S.L.; Zelhart, M.D.; Green, R.L.; Nichols, R.L. Current U.S. Pre-operative bowel preparation trends: A 2018 survey of the American Society of Colon and Rectal Surgeons Members. Surg. Infect. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Willis, M.A.; Keller, P.S.; Sommer, N.; Koch, F.; Ritz, J.-P.; Beyer, K.; Reißfelder, C.; Hardt, J.; Herold, A.; Buhr, H.J.; et al. Adherence to fast-track measures in colorectal surgery—A survey among German and Austrian surgeons. Int. J. Color. Dis. 2023, 38, 80. [Google Scholar] [CrossRef]
- Catarci, M.; Guadagni, S.; Masedu, F.; Ruffo, G.; Viola, M.G.; Borghi, F.; Baldazzi, G.; Pirozzi, F.; Delrio, P.; Garulli, G.; et al. Mechanical bowel preparation in elective colorectal surgery: A propensity score-matched analysis of the Italian colorectal anastomotic leakage (iCral) study group prospective cohorts. Updates Surg. 2024, 76, 107–117. [Google Scholar] [CrossRef]
- Antoniou, S.A.; Huo, B.; Tzanis, A.A.; Koutsiouroumpa, O.; Mavridis, D.; Balla, A.; Dore, S.; Kaiser, A.M.; Koraki, E.; Massey, L.; et al. EAES, SAGES, and ESCP rapid guideline: Bowel preparation for minimally invasive colorectal resection. Surg. Endosc. 2023, 37, 9001–9012. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Acheson, A.G.; Lobo, D.N. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery: A Meta-analysis. Ann. Surg. 2019, 270, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Koskenvuo, L.; Lehtonen, T.; Koskensalo, S.; Rasilainen, S.; Klintrup, K.; Ehrlich, A.; Pinta, T.; Scheinin, T.; Sallinen, V. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): A multicentre, randomised, parallel, single-blinded trial. Lancet 2019, 394, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Espin Basany, E.; Solís-Peña, A.; Pellino, G.; Kreisler, E.; Fraccalvieri, D.; Muinelo-Lorenzo, M.; Maseda-Díaz, O.; García-González, J.M.; Santamaría-Olabarrieta, M.; Codina-Cazador, A.; et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): A multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2020, 5, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Preoperative oral antibiotics in colon surgery (letters to the editor). Lancet Gastroenterol. Hepatol. 2020, 5, 800–803. [CrossRef]
- Pellino, G.; Solís-Peña, A.; KraP, M.; Huguet, B.M.; Espín-Basany, E. Preoperative oral antibiotics with versus without mechanical bowel preparation to reduce surgical site infections following colonic resection: Protocol for an international randomized controlled trial (ORALEV2). Color. Dis. 2021, 23, 2173–2181. [Google Scholar] [CrossRef]
- Futier, E.; Jaber, S.; Garot, M.; Vignaud, M.; Panis, Y.; Slim, K.; Lucet, J.-C.; Lebuffe, G.; Ouattara, A.; El Amine, Y.; et al. COMBINE study group. Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: Multicentre, randomised, double blind, placebo controlled trial. BMJ 2022, 379, e071476. [Google Scholar] [CrossRef] [PubMed]
- Assistance Publique—Hôpitaux de Paris. Mechanical Bowel Preparation and Oral Antibiotics Before Rectal Cancer Surgery (PREPACOL2). NCT03491540. ClinicalTrials.gov—NIH—US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03491540 (accessed on 11 January 2024).
- Assistance Publique—Hôpitaux de Paris. Mechanical Bowel Preparation and Oral Antibiotics Before Colon Cancer Surgery (COLONPREP). NCT03475680. ClinicalTrials.gov—NIH—US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03475680 (accessed on 11 January 2024).
- Catarci, M.; Ruffo, G.; Viola, M.G.; Pirozzi, F.; Delrio, P.; Borghi, F.; Garulli, G.; Baldazzi, G.; Marini, P.; Sica, G.; et al. ERAS program adherence-institutionalization, major morbidity and anastomotic leakage after elective colorectal surgery: The iCral2 multicenter prospective study. Surg. Endosc. 2022, 36, 3965–3984. [Google Scholar] [CrossRef] [PubMed]
- Italian ColoRectal Anastomotic Leakage (iCral) Study Group. Patient-reported outcomes, return to intended oncological therapy and enhanced recovery pathways after colorectal surgery: A prospective multicenter observational investigation by the Italian ColoRectal Anastomotic Leakage (iCral 3) study group. Ann. Surg. Open 2023, 4, e267. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Katayama, H.; Kurokawa, Y.; Nakamura, K.; Ito, H.; Kanemitsu, Y.; Masuda, N.; Tsubosa, Y.; Satoh, T.; Yokomizo, A.; Fukuda, H.; et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg. Today 2016, 46, 668–685. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Bujang, M.A.; Sa’at, N.; Sidik TMITAB; Joo, L.C. Sample Size Guidelines for Logistic Regression from Observational Studies with Large Population: Emphasis on the Accuracy Between Statistics and Parameters Based on Real Life Clinical Data. Malays. J. Med. Sci. 2018, 25, 122–130. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit. Anal. 2007, 15, 199–236. [Google Scholar] [CrossRef]
- Rosenbaum, P.R. The power of a sensitivity analysis and its limit. In Design of Observational Studies, 2nd ed.; Springer Series in Statistics; Springer Nature Switzerland, A.G.: Cham, Switzerland, 2020; pp. 317–336. [Google Scholar]
- Zmora, O.; Mahajna, A.; Bar-Zakai, B.; Rosin, D.; Hershko, D.; Shabtai, M.; Krausz, M.M.; Ayalon, A. Colon and rectal surgery without mechanical bowel preparation: A randomized prospective trial. Ann. Surg. 2003, 237, 363–367. [Google Scholar] [CrossRef]
- Suzuki, T.; Sadahiro, S.; Tanaka, A.; Okada, K.; Saito, G.; Miyakita, H.; Ogimi, T. Usefulness of preoperative mechanical bowel preparation in patients with colon cancer who undergo elective surgery: A prospective randomized trial using oral antibiotics. Dig. Surg. 2020, 37, 192–198. [Google Scholar] [CrossRef]
- Cannon, J.A.; Altom, L.K.; Deierhoi, R.J.; Moris, M.; Richman, J.S.; Vick, C.C.; Itani, K.M.F.; Hawn, M.T. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis. Colon Rectum. 2012, 55, 1160–1166. [Google Scholar] [CrossRef]
- Schardey, H.M.; Rogers, S.; Schopf, S.K.; Ahnen, T.; Wirth, U. Are gut bacteria associated with the development of anastomotic leaks ? A review of experimental and clinical studies. Coloproctology 2017, 39, 94–100. [Google Scholar] [CrossRef]
- Fry, D.E. Antimicrobial Bowel Preparation for Elective Colon Surgery. Surg. Infect. 2016, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Poth, E.J. Historical development of intestinal antisepsis. World J. Surg. 1982, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shang, F.; Jin, M.; Deng, S.; Gu, J.; Mao, F.; Qin, L.; Wang, J.; Xue, Y.; Jiang, Z.; et al. Changes in Bacteroides and the microbiota in patients with obstructed colorectal cancer: Retrospective cohort study. BJS Open 2023, 7, zrad105. [Google Scholar] [CrossRef] [PubMed]
- Shogun, B.D.; Smith, D.P.; Christley, S.; Gilbert, J.A.; Zaborina, O.; Alverdy, J.C. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, O.; Lobo, D.N. Bowel Preparation for Colorectal Surgery: Have All Questions Been Answered? JAMA Surg. 2022, 157, 41–42. [Google Scholar] [CrossRef]
- Sell, N.M.; Francone, T.D. Anastomotic Troubleshooting. Clin. Colon Rectal Surg. 2021, 34, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.; Alverdy, J.C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 43–54. [Google Scholar] [CrossRef]
- Kirby, A.; Santoni, N. Antibiotic resistance in Enterobacteriaceae: What impact on the efficacy of antibiotic prophylaxis in colorectal surgery? J. Hosp. Infect. 2015, 89, 259–263. [Google Scholar] [CrossRef]
- Haak, B.W.; Lankelma, J.M.; Hugenholtz, F.; Belzer, C.; de Vos, W.M.; Wiersinga, W.J. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 2019, 74, 782–786. [Google Scholar] [CrossRef]
- Hajjar, R.; Santos, M.M.; Dagbert, F.; Richard, C.S. Current evidence on the relation between gut microbiota and intestinal anastomotic leak in colorectal surgery. Am. J. Surg. 2019, 218, 1000–1007. [Google Scholar] [CrossRef]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Schwaber, M.J.; Navon-Venezia, S.; Schwartz, D.; Giladi, M.; Chmelnitsky, I.; Leavitt, A.; Carmeli, Y. Influx of extended-spectrum beta-lactamase-producing enterobacteriaceae into the hospital. Clin. Infect. Dis. 2006, 42, 925–934. [Google Scholar] [CrossRef]
- Sartelli, M.; Coccolini, F.; Labricciosa, F.M.; Al Omari, A.H.; Bains, L.; Baraket, O.; Catarci, M.; Cui, Y.; Ferreres, A.R.; Gkiokas, G.; et al. Surgical Antibiotic Prophylaxis: A Proposal for a Global Evidence-Based Bundle. Antibiotics 2024, 13, 100. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Yao, X.I.; Wang, X.; Speicher, P.J.; Hwang, E.S.; Cheng, P.; Harpole, D.H.; Berry, M.F.; Schrag, D.; Pang, H.H. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J. Natl. Cancer Inst. 2017, 109, djw323. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, G.; Pellegrini, F.; Debray, T.P.A.; Rouette, J.; Muñoz, J.; Platt, R.W.; Petkau, J.; Bohn, J.; Shen, C.; de Moor, C.; et al. Recommendations for the use of propensity score methods in multiple sclerosis research. Mult. Scler. J. 2022, 28, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- García-Granero, E.; Navarro, F.; Santacruz, C.C.; Frasson, M.; García-Granero, A.; Marinello, F.; Flor-Lorente, B.; Espí, A. Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: An institutional analysis of 800 patients. Surgery 2017, 162, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).