Abstract
Antimicrobial resistance (AMR) jeopardizes the effectiveness of essential antimicrobial agents in treating infectious diseases. Accelerated by human activities, AMR is prevalent in Sub-Saharan Africa, including Kenya, due to indiscriminate antibiotic use and limited diagnostics. This study aimed to assess Kenya’s AMR efforts through a situational analysis of policy efficacy, interventions, and implementation, culminating in recommendations for strengthening mitigation. Employing two methodologies, this study evaluated Kenya’s AMR endeavors. A systematic scoping review summarized AMR dynamic, and an expert validated the findings, providing an on-the-ground perspective. Antibiotic resistance is driven by factors including widespread misuse in human medicine due to irrational practices, consumer demand, and substandard antibiotics. Heavy antibiotic use in the agricultural sector leads to contamination of the food chain. The National Action Plan (NAP) reflects a One Health approach, yet decentralized healthcare and funding gaps hinder its execution. Although AMR surveillance includes multiple facets, diagnostic deficiencies persist. Expert insights recognize proactive NAP but underscore implementation obstacles. Kenya grapples with escalating resistance, but commendable policy efforts exist. However, fragmented implementations and complexities persist. Addressing this global threat demands investment in healthcare infrastructure, diagnostics, international partnerships, and sustainable strategies.
1. Introduction
Antimicrobial agents, such as antibiotics, antivirals, antifungals, and antiparasitics, play a crucial role in preventing and treating infectious diseases. However, the emergence of antimicrobial resistance among pathogens poses a significant threat to public health worldwide []. In a 1945 interview, Sir Alexander Fleming, renowned for his discovery of the first highly effective antibiotic, penicillin, forewarned about the potential emergence of resistant bacteria resulting from excessive antibiotic use, with this prediction now materializing []. AMR is increasingly recognized as a major global health and economic burden, with far-reaching effects on mortality, morbidity, healthcare costs, and productivity []. Economically, AMR has been projected to decrease the global gross domestic product by approximately 1% by 2050, in addition to causing the loss of millions of lives if the current AMR trends continue [].
AMR occurs naturally over time as microbes adapt and develop genetic changes; however, human activities, such as overuse and misuse of antimicrobial agents and inadequate infection prevention measures, have expedited this process []. Resistant bacteria can spread globally through cross-reservoir transmission, making the One Health approach essential for addressing AMR []. The One Health approach acknowledges collaborative efforts among various disciplines to tackle issues concerning human, animal, and environmental health. Recognizing these connections, One Health mobilizes various disciplines and communities across various sectors to collaborate and promote health by tackling AMR-related challenges. In response to the gravity of the situation, the World Health Organization (WHO) developed a Global Action Plan (GAP) for AMR in 2015. The plan emphasizes a collaborative One Health approach, urging member states to develop a NAP aligned with the WHO’s strategies.
These strategies include improving awareness, enhancing surveillance, implementing effective infection prevention measures, reducing the use of antibiotics for human and animal health, and increasing investment in research and development []. To enhance surveillance efforts, the WHO established the Global Antimicrobial Resistance and Use Surveillance System (GLASS) in 2014. GLASS advocates for a holistic approach to surveillance, incorporating epidemiological, clinical, and population-level data, to provide a standardized framework that enables countries to collect analyze, interpret, and share data [].
AMR is a global challenge affecting all countries regardless of their socioeconomic status [,]. However, in Africa, the problem of AMR is exacerbated by several factors, including indiscriminate antibiotic use, poor sanitary conditions, inadequate healthcare systems with limited diagnostic capabilities, and a lack of access to quality antibiotics [,,]. Kenya, situated in East Africa, faces significant challenges, with over 43% of its population living in poverty. The country grapples with pressing health issues, including elevated rates of maternal and child mortality, along with a substantial burden of infectious diseases such as HIV, tuberculosis and malaria []. Consequently, there is concern regarding the rise in AMR to commonly used first-line drugs and increasing infections from life-threatening pathogens [,].
Alarmingly, carbapenem-resistant Enterobacterales (CRE) and extended-spectrum-lactamase-producing organisms, which are associated with poor clinical outcomes in invasive infections, are increasingly prevalent in Kenya. A recent study found that 7–17% of hospitalized patients had infections caused by these bacteria, with 39% of isolates meeting the “difficult-to-treat resistance” definition []. This situation poses a significant public health challenge requiring urgent attention. Compounding this is the lack of routine surveillance data and methodological limitations that severely constrain the applicability of local data on antibiotic usage and resistance []. Kenya has actively engaged in AMR surveillance since the 1980s; however, the lack of microbiology laboratories in most healthcare facilities hinders the accurate measurement of AMR [].
Despite the abundance of policies addressing AMR in Kenya, there is a noticeable lack of studies assessing policy implementation progress and the necessary actions for swift execution. This paints a complex and fragmented picture of the AMR landscape in Kenya. To address this gap, this study aimed to conduct a systematic situational analysis encompassing policy effectiveness and the implementation of AMR interventions. Furthermore, insights from an expert in the field will offer an on-the-ground evaluation of ongoing initiatives. We envision that researchers can use our paper to navigate the diverse responsibilities associated with AMR, particularly in LMICs, ensuring that their data have a substantial impact. While we acknowledge the existing uncertainties in the landscape, particularly in the global south, our manuscript is crafted to initiate a meaningful discussion and set a trajectory for future research. The objective is to contribute valuable insights to the ongoing dialogue on resolving drug resistance issues in the global south, where vulnerability to such challenges is pronounced. Our emphasis lies in establishing the groundwork for continued exploration and understanding, aiming to foster a holistic approach to addressing AMR.
2. Results
The search process identified potential articles that were screened for language and duplicates based on titles and abstracts. After the initial screening, potentially relevant papers were selected for further review. Subsequently, full-text screening was conducted by applying the inclusion criteria, leading to the exclusion of articles. During the data extraction stage, articles were excluded if they did not mention an AMR variable of interest or if their geographic location did not include Kenya. This thorough process resulted in the final set of articles (n = 19) being included in the review, as depicted in Figure 1.
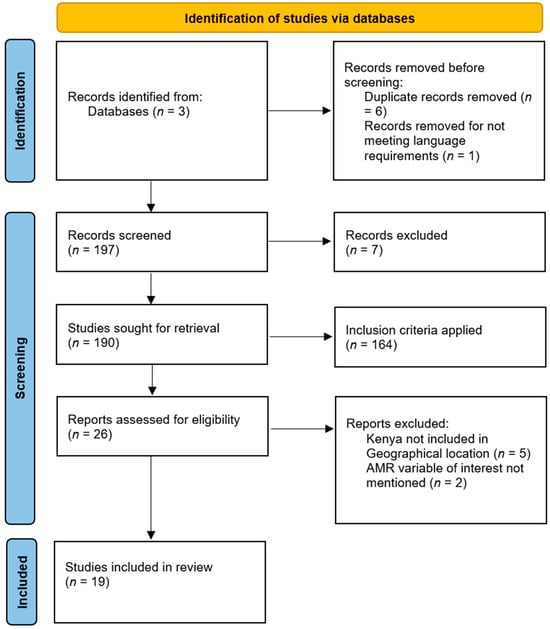
Figure 1.
PRISMA-ScR flow diagram of the literature selection process [].
Among the included studies, regarding AMR variables of interest, most studies focused on an AMR situational analysis (n = 9). Three studies analyzed AMR surveillance and diagnostics, as well as Antimicrobial Stewardship (AMS). Two studies concentrated on AMR from a One Health perspective and an AMR communications strategy, respectively. Detailed information regarding the characteristics of all included studies is shown in Table 1.

Table 1.
Summarized below are the chosen publications, categorized by color, with bold highlighting non-peer-reviewed articles.
3. Drivers of Antibiotic Resistance
3.1. Socio-Economic Factors and Related Behaviors
Rapid socioeconomic development in Kenya has not only enhanced the overall quality of life but also exacerbated wealth inequity among differing regions and demographic groups. Beyond its direct influence on healthcare quality and availability, SES profoundly impacts an individual’s lifestyle and behavior, including the use of antibiotics. A positive association has been shown between factors such as warmer temperatures, poorer administrative governance, higher urbanization levels, and a higher ratio of private to public health expenditure with the development and spread of AMR []. In the context of Kenya, these characteristics are evident as the nation faces the challenges of limited resources, sanitation and food safety issues, lenient regulations, high population density, and socioeconomic disparities. The convergence of these factors creates an environment conducive to detrimental behaviors and practices related to antibiotic use [].
3.2. Antibiotic Consumption
3.2.1. Antibiotic Use in Human Medicine
A combination of behavioral factors and economic incentives drives inappropriate prescription, dispensing, and purchasing of antibiotics. In Kenya, key drivers of AMR were highlighted in the overuse of antibiotics in the medical, veterinary, and agricultural fields []. Further evidence from a study in KNH CCUs showed that only 18.5% of antibiotic usage demonstrated a rational approach, with common irrational practices including the inappropriate selection of antibiotics (51%) and incorrect duration of treatment (32.3%) []. Consumer demand for antibiotics is fueled by a desire for rapid symptom eradication. Informal pharmacies, consisting of approximately 66% of the estimated 12,000 private pharmacies in Kenya, vastly outnumber certified branches [].
Although laws and regulations specify that antimicrobial agents should only be dispensed, sold, and used with prescriptions from licensed clinicians or animal health professionals [], antimicrobials are readily available over the counter or via unregulated supply chains []. Overall patient encounters with antimicrobial agents in East Africa was 57%, surpassing the WHO recommended value of ≤20% []. Another institutional factor contributing to the rise of AMR in Kenya is the influx of counterfeit antibiotics into the global pharmaceutical market []. Counterfeit products, often originating from Southeast Asia and Africa, are widely circulated. In Kenya, the quality of antibiotics on the market is largely unknown, and a significant proportion (up to 30%) could fail tests for labeled potency []. For instance, amoxicillin samples from different brands sourced from pharmacies in Nairobi were examined, and it was found that a significant 37.7% of these samples did not comply with the standards set by the United States Pharmacopoeia [].
Adding to the fragmented AMR landscape in Kenya is the distinct disparity in healthcare service delivery. The private for-profit healthcare sector, represented by entities like Aga Khan Healthcare, MP Shah, and African Air Rescue, has expanded significantly, especially in urban areas, offering flexibility and efficiency. In addition to the wide array of operating CSOs and private non-profit organizations, despite their diverse services attracting lower-income individuals, they constitute a disjointed system []. Challenges include higher healthcare costs, limited health literacy among patients, inadequate self-management support, and ineffective referral systems [].
Furthermore, the lack of vertical integration between healthcare levels impedes coordination, worsening AMR management in public and private facilities. Disjointed communication and skewed interactions among actors, coupled with limited government oversight, exacerbate the issue.
3.2.2. Antibiotic Use in Animal Health and Agriculture
The increasing demand for animal protein, primarily in Asia and Africa, has led to the routine use of antimicrobials in veterinary practice and livestock production []. In Kenya, antibiotics are commonly used for therapeutic and prophylactic purposes, with therapeutic applications accounting for approximately 90% of all antibiotic purchases. Unfortunately, a multitude of farmers rely on antibiotics to treat animal illnesses rather than implementing proper hygiene and feeding practices []. Moreover, the Kenya Veterinary Association estimates that 33% of antibiotics available for animal use are substandard and/or counterfeit, with over 78% of veterinary medicine outlets operating illegally throughout the country []. This misuse of antibiotics has the potential to lead to the accumulation of antibiotics in animal products sold for human consumption, thereby contributing to the spread of antibiotic-resistant bacteria through the food chain.
For instance, backyard poultry and cattle farming are essential to household economies in rural areas. Antibiotic use in poultry is particularly high, accounting for nearly 20% of the mean consumption per year [,]. This is primarily through the use of antimicrobial growth promoters (AGPs), which are believed to prevent disease, optimize feed conversion, promote growth and improve gut health []. However, excessive use of antibiotics in animal husbandry and close proximity to animals can lead to direct zoonotic transmission, with additional environmental contamination occurring through farm manure-contaminated water [].
3.3. Antibiotic Resistance
3.3.1. AMR Surveillance
To enhance the availability of AMR data through surveillance and research, Kenya has developed a national strategy complementing the NAP on AMR. The strategy aimed to strengthen data collection, promote routine AST, establish national databases and biobanks, monitor AMR trends, and inform clinical treatment guidelines []. The Kenya Medical Research Institute primarily leads AMR data generation, supported by central reference laboratories, national hospitals, and sentinel sites focusing on eight priority pathogens aligned with the WHO []. As of 2021, Kenya has ten facilities, including five surveillance sites and five healthcare facilities, participating in the national surveillance system reporting to GLASS. These facilities rely on clinical isolates collected from tertiary-level surveillance facilities. In the animal health sector, six laboratories have contributed data to the national database, with plans to integrate human and animal health databases in the future [].
3.3.2. AMR Laboratory and Diagnostic Capacity
In 2021, the MOH issued guidelines stressing the importance of performing antimicrobial susceptibility testing (AST) before initiating antibiotic treatment to curb unnecessary prescriptions []. Unfortunately, limited access to microbiological services, particularly in severe cases, leads to widespread administration of broad-spectrum antibiotics, as obtaining culture results within a timely intervention window proves challenging []. The inadequacy of laboratory infrastructure, especially in rural areas where basic requirements are rarely met, poses a significant barrier to reliable pathogen detection and AST []. Moreover, there exists a persistent healthcare delivery imbalance across counties, with over twice the laboratory technology accessibility in urban areas compared to their rural counterparts in Kenya [].
Furthermore, challenges persist in AMR data management due to understaffing, the absence of professional standards, and insufficient training for clinical and laboratory staff. This exacerbates issues like shortages in essential items, the use of low-quality diagnostics, and the lack of local manufacturing capabilities, all of which compromise the accuracy of laboratory results. Additionally, the absence of clear guidance for specimen selection, transportation, and quality assurance, coupled with difficulties in updating procedures due to language and cultural barriers, further hinders progress []. The integration of internationally standardized criteria for bacteria, as advocated by organizations like the Clinical and Laboratory Standards Institute, remains lacking in low- and middle-income countries such as Kenya [].
3.4. Antimicrobial Stewardship
3.4.1. AMS and the One Health Approach
Kenya’s One Health approach gained prominence during the Rift Valley Fever Outbreak of 2006–2007. This crisis fostered collaboration among international organizations, researchers, and government entities. As a result, the adoption of the One Health approach in Kenya has enabled swift detection and effective control of zoonotic disease outbreaks at their source []. Integral to Kenya’s AMR strategy is its NAP on AMR (Figure 2). Developed in 2017, the NAP aimed to enhance healthcare quality, mitigate the economic ramifications of AMR, and align with the World Health Organization’s Global Action Plan on AMR [].
Rooted in evidence-based recommendations from situational analyses conducted in 2011 and 2016, the NAP embraces a holistic approach by integrating One Health principles []. Acknowledging Kenya’s commitment to the One Health concept, the Global One Health (GOHI) index was utilized; this comprehensive tool assesses and ranks nations based on their efforts to address global AMR trends. Kenya received a high-ranking value of 41.30–52.63, surpassing the average ranking of 32.99 for LMICs. This positioning showcases Kenya as a prominent example of One Health advocacy within the region, underscoring the nation’s dedication to addressing AMR through a collaborative and integrated approach [].
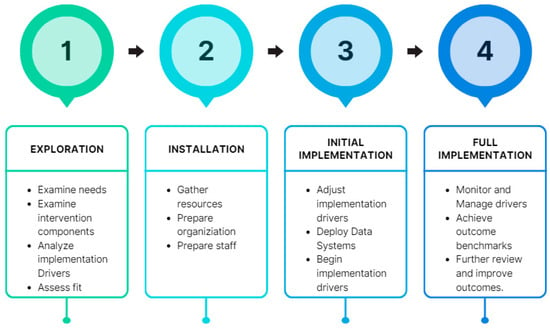
Figure 2.
Implementation stages of the NAP and follow-up in Kenya [,].
The NAP on AMR assigns crucial responsibilities to various stakeholders, including national and county governments. To facilitate effective implementation, a well-defined hierarchy is in place (Figure 3), which includes a two-tiered coordination system established at both the national and county levels:
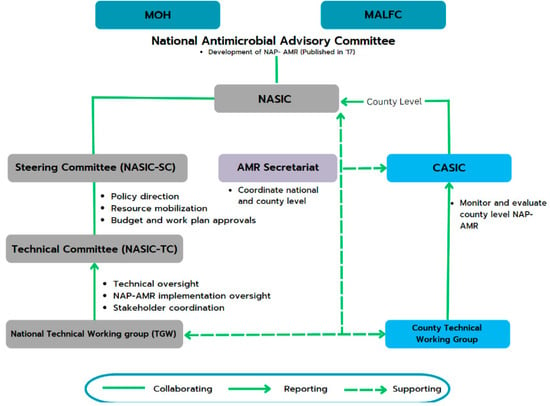
Figure 3.
Kenya’s national- and county-level governance structure for implementing and monitoring the NAP on AMR []. Abbreviations: AMR, Antimicrobial Resistance; CASIC, County Antimicrobial Stewardship Interagency Committees; MALFC, Ministry of Agriculture, Livestock, Fisheries, and Cooperatives; MOH, Ministry Of Health; NAP, National Action Plan; NASIC, National Antimicrobial Stewardship Interagency Committee.
- At the national level, the National Antimicrobial Stewardship Interagency Committee (NASIC) and Technical Committees oversee and guide AMR-related activities. These committees report to the MOH and the Ministry of Agriculture, Livestock, Fisheries, and Cooperatives for guidance and funding.
- At the county level, 8 of Kenya’s 47 counties have County Antimicrobial Stewardship Interagency Committees (CASICs). These committees play a pivotal role in monitoring NAP implementation and allocating resources for AMR-related efforts [].
Despite considerable progress, several challenges hinder the full realization of the NAP’s objectives []. The NAP’s update, originally scheduled for 2022, has been delayed owing to the unexpected emergence of the COVID-19 pandemic. This crisis disrupted AMR-related activities across the nation, diverting resources and attention from AMR mitigation efforts []. Notably, Kenya’s decentralized health system has posed challenges, with numerous counties not actively engaging in AMR mitigation efforts [].
3.4.2. AMR Awareness and Communication
The question “does AMR have a face?” arose in a sensitization session for journalists in Kenya, revealing the challenge of visualizing AMR as a distinct condition compared to diseases like TB and HIV/AIDS []. Despite its complexity, the Kenyan government aims to raise awareness through campaigns to promote understanding among stakeholders. In Nairobi’s Kibera settlement, a study revealed widespread misconceptions about antimicrobials, with 66% mistakenly believing they could treat influenza and the common cold. Less than half received proper information on antibiotic use, recognizing clinicians and pharmacists as trusted sources []. Efforts to address limited medical training include integrating AMR education into healthcare professionals’ curriculums. Despite initiatives, a lack of awareness persists, as outlined in Kenya’s NAP on AMR. A national communication strategy aimed to support AMR awareness campaigns but faced funding challenges like a myriad of other state health initiatives [].
Another factor that contributes to the ambiguity surrounding AMR in Kenya is the notable absence of equity and local representation in AMR research. Although AMR research is on the rise in Kenya and other LMICs, the trend in authorship disparity persists. Studies focusing on African infectious disease research have revealed persistent imbalances, with the majority of lead authors coming from high-income countries []. Collaborations between high- and low-income institutions in international health research often lead to imbalances because high-income partners tend to drive primary research objectives and provide funding. Consequently, low-income-country-affiliated authors face barriers such as limited promotion and leadership opportunities, less emphasis on local health priorities, and restricted access to academic and medical literature [].
3.4.3. IPC, Water, Sanitation and Hygiene (WASH) and Immunization
The convergence of IPC, WASH, and immunization efforts represents a multi-faceted strategy to combat the escalating challenge of AMR []. The establishment of a national IPC program, complete with guidelines, reflects the country’s commitment to curtailing infections and subsequent antibiotic demand. However, the intricate link between WASH and IPC cannot be overlooked, as suboptimal WASH infrastructure, particularly limited access to clean water and sanitation facilities, poses a significant obstacle to effective control measures []. This deficiency potentially exacerbates AMR concerns by creating environments that are conducive to the proliferation of resistant pathogens.
Kenya’s impressive national vaccination rate is pivotal for the reduction in infections that require antibiotic treatment. The imminent transition toward self-funded immunization underscores the need for ongoing efforts to ensure equitable access to vaccines, thereby perpetuating lowered infection rates and consequently diminishing the overall burden of antibiotic consumption [].
3.4.4. Funding for Programs
Concurrently, a recurring theme in AMR interventions is the substantial dependence on external funding from institutions such as the Fleming Fund, WHO, and USAID, which are pivotal in bolstering AMS initiatives via training, material support, and laboratory equipment procurement [,]. These initiatives are well aligned with WHO guidelines and include capacity-building, procurement of necessary equipment, and mentorship. However, a persistent challenge faced by AMR-focused programs in Kenya is their heavy reliance on foreign aid []. Scrutinized over the past decade for its perceived failure to achieve desired outcomes, the effectiveness of foreign aid in promoting growth is heavily contingent on a favorable policy environment.
This situation can be likened to a double-edged sword: while aid can be highly beneficial in supporting progress when policy and economic conditions are conducive, it can be ineffective or even counterproductive when such conditions are lacking []. At its worst, aid can inadvertently sustain corrupt or incompetent governments, as the reliance on foreign intervention nurtures a mentality of “let the West handle it” [].
4. Expert Opinion
Exploring Kenya’s AMR initiatives requires understanding expert perspectives on obstacles and pathways for progress. Insights from this interview highlight current challenges and strategies shaping Kenya’s regulatory landscape and on-the-ground situation. Kenya’s pursuit to combat AMR is underpinned by a multifaceted strategy. The nation’s proactive measures, as seen through the development of an extensive NAP on AMR, set it apart from the other countries in the region. Notably, the NAP integrates NASIC and CASIC to ensure a coordinated country-wide approach. However, challenges persist in spurring the NAP into action, particularly in humble healthcare facilities with limited resources and awareness.
This gap stems from an imbalance in the focus on public health facilities, creating hurdles for consistent NAP implementation across all healthcare settings. While urban facilities benefit from better-trained healthcare professionals, rural areas often grapple with a lack of expertise, even extending to nurses dispensing medications due to the lack of urban–rural migration. To address gaps, Kenya embraced collaboration with civil society organizations (CSOs) and faith-based groups. These partnerships aim for inclusivity, fostering discussions among CSOs, the government’s AMR focal person, and diverse healthcare professionals. The strategy focuses on aligning initiatives with the NAP framework.
Central to this approach is establishing AMS programs at health facilities, recognizing them as key service delivery sites. AMR stewardship committees play a crucial role in overseeing antibiotic use, improving governance, and ensuring IPC practices. Proactively, CSOs and faith-based organizations designate AMR and IPC champions, providing specialized training to develop action plans for enhancing these aspects within their facilities. Nevertheless, challenges in laboratory capacity hinder data collection on AMR trends.
Limited financial resources for reagents and equipment, coupled with a reluctance towards testing practices among healthcare professionals, impede diagnostics. Additionally, addressing substandard medications has emerged as a crucial yet overlooked issue. Faith-based organizations are employing innovative solutions, like county mini-labs functioning as compact testing facilities for culture and sensitivity tests. These mini-labs exclusively focus on detecting the presence or absence of active pharmaceutical ingredients and other impurities in medicines.
Community-level misuse of drugs, especially antibiotics obtained without prescriptions, is concerning. Financial constraints often lead individuals to compromise prescribed treatment durations when visiting pharmacies. Corruption further undermines regulatory effectiveness through bribery and evasion. Extensive educational programs target healthcare professionals, including clinicians, lab technicians, and pharmacy staff, focusing on AMR and stewardship. Training courses prioritize capacity building using an approach focuses on the training of trainers. Specialized tools assist AMR champions in prescription assessments, promoting appropriate medication use. General awareness efforts also target the wider population.
5. Potential Ways Forward
Despite prevailing challenges, this study draws inspiration from successful global examples, notably the Bangladesh Rural Advancement Committee (BRAC)’s initiative in sustainable rural poultry development. This model, designed to empower women in village settings, holds promise for adaptation in Kenya. It encompasses thorough training in poultry rearing, vaccinations, financial support, and the provision of high-yielding variety cocks for crossbreeding, resulting in increased income, crop yield, safer hygiene practices, food security, and farmers being empowered towards self-reliance [,,]. Importantly, this approach has the potential to reduce antibiotic dependence in poultry farming.
Additionally, the study emphasizes the need for sustainable investments to boost research and development in novel medicines, diagnostics, vaccines, and interventions. Aligned with Kenya’s NAP, prioritizing affordable and user-friendly point-of-care diagnostics is crucial. To tailor these tools effectively to the Kenyan landscape, specific prerequisites must be addressed, including adapting diagnostic equipment to harsh conditions, championing low-tech, cost-effective, and easily maintainable equipment, ensuring accessible and affordable training, and embracing reverse innovation for practical application in low-resource environments [].
The following section (Table 2) presents key findings and corresponding recommendations derived from the study’s analysis, offering a comprehensive overview of factors contributing to AMR, associated challenges, and proposed interventions to guide the way forward.

Table 2.
Summarized findings and recommendations to address AMR.
6. Limitations
This study has several limitations that should be considered when interpreting the findings. Firstly, the reliance on a systematic scoping review and single-expert interview may result in an incomplete understanding of the complex and diverse dynamics of AMR in Kenya. The translation of policy into practice and the ever-evolving nature of the country’s healthcare system suggests that the findings may not fully reflect the real-world implementation of AMR interventions. Lastly, while efforts were made to ensure rigor, it is important to acknowledge that the available data and sources may be subject to biases and limitations, which could potentially impact the accuracy of the study’s conclusions.
7. Materials and Methods
This study employed a two-fold approach to investigate Kenya’s efforts against antimicrobial resistance (AMR). Firstly, a comprehensive systematic scoping review integrated qualitative and quantitative data to identify key aspects of Kenya’s progress in addressing AMR, initially focusing on its drivers. The second methodology involved consulting an expert in the Kenyan healthcare system to validate the findings and support the conclusions of this study. Finally, the research explores effective strategies to curb AMR.
7.1. Scoping Review
The scoping review protocol followed the framework proposed by Arksey and O’Malley [], guiding the study through five primary stages. The systematic scoping review also adhered to the PRISMA guidelines for Scoping Reviews (PRISMA-ScR), ensuring methodological rigor and transparency throughout the study [].
7.1.1. Identifying Relevant Studies
Extensive literature searches were conducted using various databases, with a focus on electronic databases to identify relevant articles. To ensure broad coverage and minimize potential gaps, a combination of databases was used: PubMed, Embase (Ovid) and Google Scholar. The following terms and Boolean operators were used: (AMR* or Antimicrobial Resistance* or Bacterial resistance* or Drug resistance*).MP. AND (Surveillance* or Diagnostics* or Epidemiology* or Stewardship* or Awareness*).mp. AND Kenya. MP. The results were refined to include only randomized controlled trials, reviews, or systematic reviews. Additionally, the Google Scholar database was used to screen for grey and unindexed literature [].
Moreover, relevant government and policy reports, including texts and reports from reputable sources such as the WHO, GLASS, and the Ministry of Health (MOH), were also considered for inclusion in the study. This is particularly relevant because, in a number of low- and middle-income countries (LMICs), government and policy reports often serve as the primary and essential source of information on specific issues [].
7.1.2. Study Selection
The titles and abstracts of all the references were carefully examined to efficiently manage the screening process and eliminate duplicates. This initial screening identified potentially relevant articles for further full-text reviews, aligned with the research question. For government and policy reports without available abstracts, the author reviewed executive summaries or similar documents []. Articles selected for full-text review were individually assessed by the author to determine their alignment with the inclusion criteria. To ensure the identification of relevant studies, ‘reverse snowballing’ was used [].
The following inclusion criteria were specified in this review: (1) article types encompassed cohort, RCT, case–control, case report, case series, reviews, editorials, and policy reports; (2) the publication date was restricted to articles published from 2001 onwards, justified by the need to capture contemporary trends and developments in AMR within the specified timeframe; (3) the review focused on analyzing a variable of interest related to AMR; and (4) language restrictions dictated that only originally English and Swahili language papers were considered for inclusion.
7.1.3. Charting the Data
Data extracted from the full texts were systematically organized and recorded in a chart, including essential details such as the study title, author(s), location of the evaluation, method of evaluation, AMR indicators assessed, or specific aspects evaluated, and a summary of the key findings of the study. These dimensions were based on [,], with refinements incorporated during the review process.
7.1.4. Summarizing and Reporting the Results
To illustrate the review process and document study exclusions with the associated reasons, a PRISMA diagram was utilized. This approach aims to identify commonalities and variations among the evaluations, highlighting significant data considerations.
7.2. Expert Opinion
As a complement to the analysis, a complimentary interview was conducted with a prominent expert in the Kenyan AMR realm to gather valuable insights and opinions on the study’s findings. The expert was sought after because of their extensive experience in addressing AMR across various public, private, and faith-based sectors. Their diverse expertise made them a valuable resource to provide insights from multiple perspectives on the challenges and approaches related to antimicrobial resistance. The interview was conducted online and lasted for approximately 30 min. Before the interview, the expert was provided with a detailed description of the study, ensuring a clear understanding of its objectives and scope. Written informed consent was obtained from the expert before proceeding with the interview.
8. Conclusions
In conclusion, the current status of AMR in Kenya presents a scenario of increasing resistance to common first-line drugs and a rise in life-threatening infections caused by drug-resistant pathogens. Despite this, Kenya has made commendable efforts to tackle AMR, through the development of an exceptional policy framework outshining numerous regional counterparts. The detailed NAP and establishment of collaborative partnerships involving both government and civil society organizations underscores the nation’s commitment. Notable initiatives, such as AMR stewardship committees, bolstered laboratory capabilities, and education and awareness programs have shown promising results.
However, the situation becomes concerning when examining policy implementation and effectiveness. This study highlights the complex nature of Kenya’s AMR landscape, in which superb policies coexist with uncertain outcomes in practice. This is mirrored in this study’s exploration, which underscores a sense of fragmentation. However, the trajectory displays a promising dedication to addressing AMR. Sustaining progress and mitigating the impact of AMR will require the establishment of robust data gathering systems, exemplified by further studies such as this, that will serve as the cornerstone for informed policymaking. By systematically recording data on AMR trends and interventions, Kenya can empower evidence-based decision-making, driving impactful reforms, ultimately paving the way for the advancement of the health and well-being of the Kenyan populace.
Author Contributions
A.S.: conceptualization; methodology; validation; writing—original draft; writing—review and editing. P.P.M.T.: conceptualization; methodology; supervision; writing—review and editing; formal analysis. J.A.: validation; supervision; formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data stated in this review are available in the References cited.
Conflicts of Interest
The authors declare no competing or financial interests. The authors declare that they have no known competing financial interests or personal relationships that could appear to have influenced the work reported in this paper.
References
- World Health Organization. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 27 November 2023).
- Rosenblatt-Farrell, N. The landscape of antibiotic resistance. Environ. Health Perspect. 2009, 117, A244–A250. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Devang Dicakar, D.; Jhugroo, C.; Vellappally, S.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Disease-a-Month 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Catena, F.; Coccolini, F.; Hardcastle, T.C.; Roques, C.; Salameh, P. Drivers of antibiotic resistance transmission in low- and middle-income countries from a “one health” perspective—A review. Antibiotics 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, A.; Reid, S.; Assefa, Y. A review of National Action Plans on antimicrobial resistance: Strengths and weaknesses. Antimicrob. Resist. Infect. Control 2022, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. Global Antimicrobial Resistance and Use Surveillance System (GLASS). 2023. Available online: https://iris.who.int/bitstream/handle/10665/364996/9789240062702-eng.pdf?sequence=1 (accessed on 27 November 2023).
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of antimicrobial resistance in low- and middle-income countries: A scattered picture. Antimicrob. Resist. Infect. Control 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Gulumbe, B.H.; Haruna, U.A.; Almazan, J.; Ibrahim, I.H.; Faggo, A.A.; Bazata, A.Y. Combating the menace of antimicrobial resistance in Africa: A review on Stewardship, surveillance and Diagnostic Strategies. Biol. Proced. Online 2022, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Levy Hara, G.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Rolfe, R.; Kwobah, C.; Muro, F.; Ruwanpathirana, A.; Lyamuya, F.; Bodinayake, C.; Nagahawatte, A.; Piyasiri, B.; Sheng, T.; Bollinger, J.; et al. Barriers to implementing antimicrobial stewardship programs in three low- and middle-income country tertiary care settings: Findings from a multi-site qualitative study. Antimicrob. Resist. Infect. Control 2021, 10, 60. [Google Scholar] [CrossRef]
- World Health Organization. Kenya National Action Plan on Antimicrobial Resistance: Review of Progress in the Human Health Sector. Antimicrobial Resistance Policy Information and Action Brief Series. 2022. Available online: https://iris.who.int/bitstream/handle/10665/364530/9789240062689-eng.pdf?sequence=1 (accessed on 28 November 2023).
- OneHealthTrust.ResistanceMap—Antibiotic-Resistance. 2015. Available online: https://resistancemap.cddep.org/AntibioticResistance.php (accessed on 28 November 2023).
- Ita, T.; Luvsansharav, U.O.; Smith, R.M.; Mugoh, R.; Ayodo, C.; Oduor, B.; Jepleting, M.; Oguta, W.; Ouma, C.; Juma, J.; et al. Prevalence of colonization with multidrug-resistant bacteria in communities and hospitals in Kenya. Sci. Rep. 2022, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Global Antibiotic Resistance Partnership—Kenya Working Group. Situation Analysis and Recommendations: Antibiotic Use and Resistance in Kenya. Center for Disease Dynamics, Economics & Policy. 2011. Available online: https://onehealthtrust.org/wp-content/uploads/2017/06/kenya_full_report_web_15.pdf (accessed on 28 November 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.P.; Hafner, T.; Twesigye, G.; Ndiaye, A.; Kiggundu, R.; Mekonnen, N.; Kusu, N.; Berthe, S.; Lusaya, E.P.; Acho, A.; et al. Strengthening multisectoral coordination on antimicrobial resistance: A landscape analysis of efforts in 11 countries. J. Pharm. Policy Pract. 2021, 28, 14. [Google Scholar] [CrossRef] [PubMed]
- Moirongo, R.M.; Aglanu, L.M.; Lamshöft, M.; Adero, B.O.; Yator, S.; Anyona, S.; May, J.; Lorenz, E.; Eibach, D. Laboratory-based surveillance of antimicrobial resistance in regions of Kenya: An assessment of capacities, practices, and barriers by means of multi-facility survey. Front. Public Health 2022, 10, 1003178. [Google Scholar] [CrossRef]
- Omulo, S.; Thumbi, S.M.; Lockwood, S.; Verani, J.R.; Bigogo, G.; Masyongo, G.; Call, D.R. Evidence of superficial knowledge regarding antibiotics and their use: Results of two cross-sectional surveys in an urban informal settlement in Kenya. PLoS ONE 2017, 12, e0185827. [Google Scholar] [CrossRef]
- Mbugua, S.M.; Njoroge, G.; Kijogi, C.; Kamita, M.; Kimani, R.; Mwaura, P.; Aidi, B.W.; Gitaka, J. Exploring perspectives on antimicrobial stewardship: A qualitative study of health managers in Kenya. Glob. Health Res. Policy 2020, 5, 49. [Google Scholar] [CrossRef]
- Kimani, T.; Kiambi, S.; Eckford, S.; Njuguna, J.; Makonnen, Y.; Rugalema, G.; Morzaria, S.P.; Lubroths, J.; Fasina, F.O. Expanding beyond zoonoses: The benefits of a national One Health coordination mechanism to address antimicrobial resistance and other shared health threats at the human-animal-environment interface in Kenya. Rev. Sci. Tech. Off. Int. Des Epizoot. 2019, 38, 155–171. [Google Scholar] [CrossRef]
- Matee, M.; Mshana, S.E.; Mtebe, M.; Komba, E.V.; Moremi, N.; Lutamwa, J.; Kapona, O.; Sekamatte, M.; Mboera, L.E. Mapping and gap analysis on antimicrobial resistance surveillance systems in Kenya, Tanzania, Uganda and Zambia. Bull. Natl. Res. Cent. 2023, 47, 12. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Lum Niba, L.; et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Othieno, J.; Njagi, O.; Azegele, A. Opportunities and challenges in antimicrobial resistance behavior change communication. One Health 2020, 11, 100171. [Google Scholar] [CrossRef]
- Kariuki, S.; Kering, K.; Wairimu, C.; Onsare, R.; Mbae, C. Antimicrobial resistance rates and surveillance in Sub-Saharan Africa: Where are we now? Infect. Drug Resist. 2022, 15, 3589–3609. [Google Scholar] [CrossRef] [PubMed]
- Acam, J.; Kuodi, P.; Medhin, G.; Makonnen, E. Antimicrobial prescription patterns in East Africa: A systematic review. Syst. Rev. 2023, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Otieno, P.A.; Campbell, S.; Maley, S.; Arunga, T.O.; Okumu, M.O. A Systematic Review of Pharmacist-Led antimicrobial stewardship programs in Sub-Saharan Africa. Int. J. Clin. Pract. 2022, 2022, 3639943. [Google Scholar] [CrossRef] [PubMed]
- Murila, B.L.; Nyamu, D.G.; Kinuthia, R.; Njogu, P.M. Rational use of antibiotics and covariates of clinical outcomes in patients admitted to intensive care units of a tertiary hospital in Kenya. Hosp. Pract. 2022, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kamere, N.; Garwe, S.T.; Akinwotu, O.O.; Tuck, C.; Krockow, E.M.; Yadav, S.; Ganiyu Olawale, A.; Diyaolu, A.H.; Munkombwe, D.; Muringu, E.; et al. Scoping review of national antimicrobial stewardship activities in eight African countries and adaptable recommendations. Antibiotics 2022, 11, 1149. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J. Socioeconomic enablers for contagion: Factors impelling the Antimicrobial Resistance epidemic. Antibiotics 2019, 8, 86. [Google Scholar] [CrossRef]
- Cruz, M. Quality-Assured Pharmacies Improving Healthcare for People with Low Incomes in Kenya. 2021. Available online: https://www.businesscalltoaction.org/news/quality-assured-pharmacies-Improving-healthcare-for-people-with-low-incomes-in-Kenya (accessed on 28 November 2023).
- Baptista, P.; Mignano, K.; World Bank Group. Bringing Safe, Quality Medicine to All Goodlife Pharmacy: A Health Hub for East Africa. International Finance Corporation. 2018. Available online: https://www.ifc.org/content/dam/ifc/doclink/2018/goodlife-pharmacy.pdf (accessed on 28 November 2023).
- Koech, L.C.; Irungu, B.; Ng’ang’a, M.; Ondicho, J.; Keter, L. Quality and brands of amoxicillin formulations in Nairobi, Kenya. BioMed Res. Int. 2020, 2020, 7091278. [Google Scholar] [CrossRef]
- Moturi, A.K.; Alegana, V.A.; Mumo, E.; Snow, R.W.; Okiro, E.A.; Macharia, P.M. Geographic accessibility to public and private health facilities in Kenya in 2021: An updated geocoded inventory and spatial analysis. Front. Public Health 2022, 3, 10. [Google Scholar] [CrossRef]
- Walcott-Bryant, A.; Ogallo, W.; Remy, S.L.; Tryon, K.; Shena, W.; Bosker-Kibacha, M. Addressing care continuity and quality challenges in the management of hypertension: Case study of the private health care sector in Kenya. J. Med. Internet Res. 2021, 23, e18899. [Google Scholar] [CrossRef] [PubMed]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Proc. R. Soc. Biol. 2018, 285, 20180332. [Google Scholar] [CrossRef] [PubMed]
- Alila, P.O.; Atieno, R. Agricultural Policy in Kenya: Issues and Processes. Institute for Development Studies. 2023. Available online: https://www.fao.org/fileadmin/user_upload/fsn/docs/Ag_policy_Kenya.pdf (accessed on 28 November 2023).
- Mehdi, Y.; Létourneau-Montminy, M.P.; Gaucher, M.L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Kaur Brar, S.; Cote, C.; Avalos Ramirez, A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Marshall, B.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Ministry of Health. Kenya National Action Plan on Antimicrobial Resistance: Review of Progress in the Human Health Sector. Government of Kenya. 2017. Available online: https://www.health.go.ke/wp-content/uploads/2020/02/Kenya-National-Action-Plan-on-Antimicrobial-Resistance-Review-of-Progress-in-the-Human-Health-Sector.pdf (accessed on 28 November 2023).
- Fitzgibbon, J.E.; Wallis, C.L. Laboratory challenges Conducting international clinical research in Resource-Limited settings. J. Acquir. Immune Defic. Syndr. 2014, 65 (Suppl. S1), S36–S39. [Google Scholar] [CrossRef]
- Munyua, P.M.; Njenga, M.K.; Osoro, E.M.; Onyango, C.O.; Bitek, A.O.; Mwatondo, A.; Muturi, M.L.; Musee, N.; Bigogo, G.; Otiang, E.; et al. Successes and challenges of the One Health approach in Kenya over the last decade. BMC Public Health 2019, 19, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Chang, Y.F.; Chen, S.; Guo, X.; Zhou, X.N.; et al. Global antimicrobial resistance: A system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty 2022, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Canva. Modern Steps Project Management Process Infographic Graph. 2023. Available online: https://www.canva.com/design/DAF7o29Vm1s/NVHknSHounp_bUn33qQl8Q/edit (accessed on 28 November 2023).
- Ministry of Health of Kenya. Regional Meeting in Nairobi Aims to Combat Antimicrobial Resistance (AMR) Threat | Ministry of Health. Retrieved February 19, 2024. Available online: https://www.health.go.ke/regional-meeting-nairobi-aims-combat-antimicrobial-resistance-amr-threat (accessed on 28 November 2023).
- Mbaye, R.; Gebeyehu, R.; Hossmann, S.; Mbarga, N.; Bih-Neh, E.; Eteki, L.; Thelma, O.A.; Oyerinde, A.; Kiti, G.; Mburu, Y.; et al. Who is telling the story? A systematic review of authorship for infectious disease research conducted in Africa, 1980–2016. BMJ Glob. Health 2019, 4, e001855. [Google Scholar] [CrossRef]
- Kokutse, F. Lead Authors from Low-Income Countries on the Decrease. University World News. 2022. Available online: https://www.universityworldnews.com/post.php?story=20220628095022532 (accessed on 1 December 2023).
- Unicef. WASH and Infection Prevention and Control in Health Care Facilities Guidance Note. 2020. Available online: https://www.unicef.org/media/66386/file/WASH-COVID-19-infection-prevention-and-control-in-health-care-facilities-2020.pdf (accessed on 1 December 2023).
- Müller, C. Foreign Aid and the Challenges in Kenya. 2022. Available online: https://ieakenya.or.ke/blog/foreign-aid-and-the-challenges-in-kenya/ (accessed on 1 December 2023).
- Lancaster, C. Aid effectiveness in Africa: The unfinished agenda. J. Afr. Econ. 1999, 8, 487–503. [Google Scholar] [CrossRef]
- BRAC. BRAC Poultry Rearing. 2017. Available online: https://www.brac.net/brac-enteprises/item/885-brac-poultry (accessed on 1 December 2023).
- Saleque, M. Small Scale Poultry Farming Is a Key to Improving Livelihoods: BRAC Experience in Africa. ResearchGate. 2015. Available online: https://www.researchgate.net/publication/318122927_Small_scale_poultry_farming_is_a_key_to_improving_livelihoods_BRAC_experience_in_Africa (accessed on 1 December 2023).
- Ombelet, S.; Ronat, J.B.; Walsh, T.; Yansouni, C.P.; Cox, J.; Vlieghe, E.; Martiny, D.; Semret, M.; Vandenberg, O.; Jacobs, J.; et al. Clinical bacteriology in low-resource settings: Today’s solutions. Lancet Infect. Dis. 2018, 18, e248–e258. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-SCR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Corlett, R.T. Trouble with the Gray Literature. Biotropica 2010, 43, 3–5. [Google Scholar] [CrossRef]
- Godin, K.; Stapleton, J.; Kirkpatrick, S.I.; Hanning, R.M.; Leatherdale, S.T. Applying systematic review search methods to the grey literature: A case study examining guidelines for school-based breakfast programs in Canada. Syst. Rev. 2015, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Horsley, T.; Dingwall, O.; Sampson, M. Checking reference lists to find additional studies for systematic reviews. Cochrane Libr. 2011, 8, 1465–1858. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, M.; Sharma, A.; Vong, S. Developing a situation analysis tool to assess containment of antimicrobial resistance in South East Asia. BMJ 2017, 358, j3760. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).