Study on the Efficacy and Safety of Tedizolid in Japanese Patients
Abstract
1. Introduction
2. Results
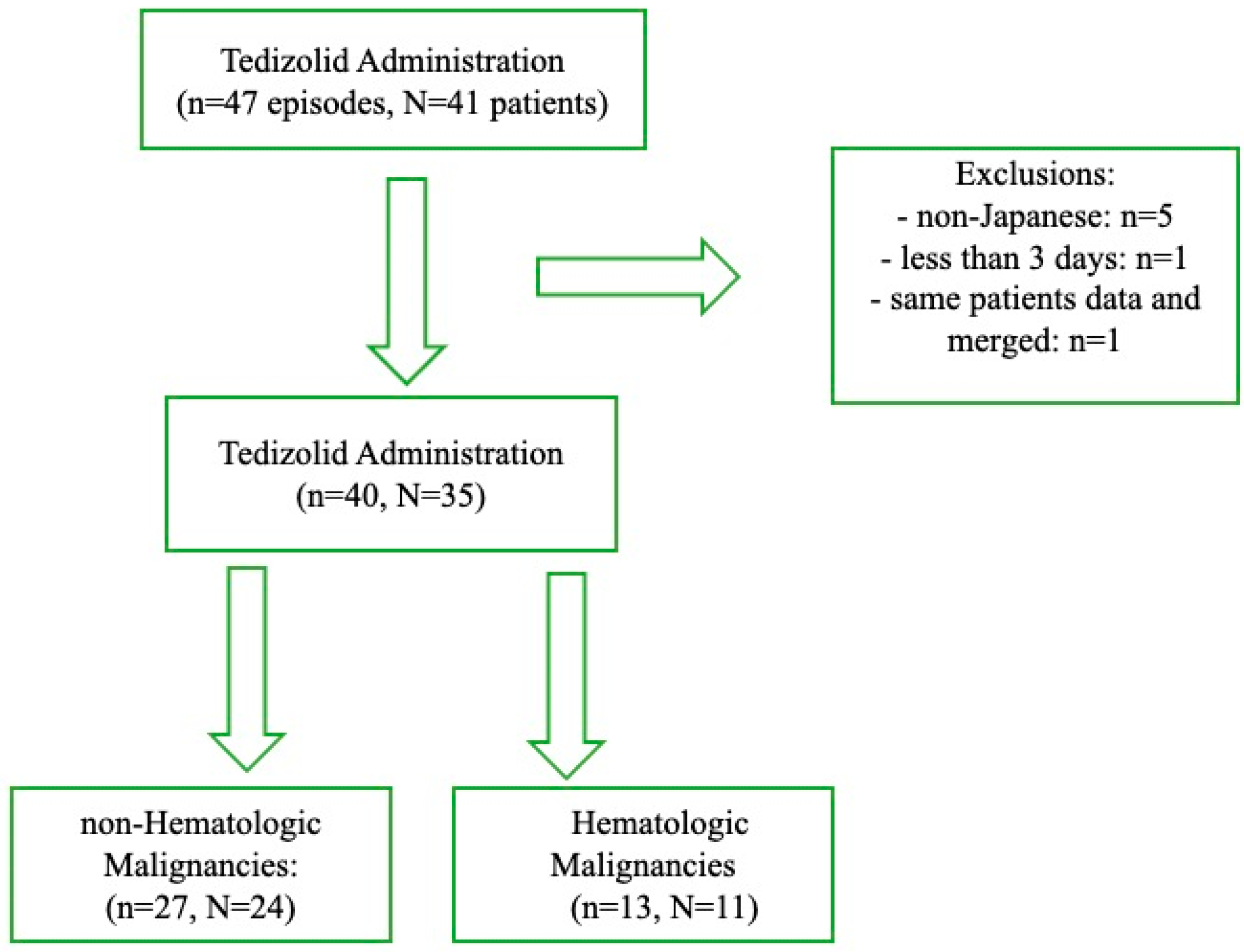
2.1. Patient Selection and Grouping
2.2. Baseline Characteristics
2.3. Laboratory Data at Admission
2.4. Sites of Infection and Pathogens
2.5. Laboratory Data at the End of Treatment and Follow-Up
2.6. Treatment Outcomes
2.7. Platelet Count Transitions and Treatment Discontinuation
2.8. Comparative Analysis: HM vs. Non-HM Groups
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Outcomes
4.3. Data Collection
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Kisgen, J.J.; Mansour, H.; Unger, N.R.; Childs, L.M. Tedizolid: A new oxazolidinone antimicrobial. Am. J. Health Syst. Pharm. 2014, 71, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Milioudi, A.; Wicha, S.G. Pharmacokinetics and Pharmacodynamics of Tedizolid. Clin. Pharmacokinet. 2022, 61, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.J.; Fang, E.; Corey, G.R.; Das, A.F.; De Anda, C.; Prokocimer, P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2014, 14, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Fang, E.; Minassian, S.L.; Prokocimer, P.G. Platelet profile in patients with acute bacterial skin and skin structure infections receiving tedizolid or linezolid: Findings from the Phase 3 ESTABLISH clinical trials. Antimicrob. Agents Chemother. 2014, 58, 7198–7204. [Google Scholar] [CrossRef] [PubMed]
- Prokocimer, P.; De Anda, C.; Fang, E.; Mehra, P.; Das, A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: The ESTABLISH-1 randomized trial. JAMA 2013, 309, 559–569. [Google Scholar] [CrossRef]
- Mikamo, H.; Takesue, Y.; Iwamoto, Y.; Tanigawa, T.; Kato, M.; Tanimura, Y.; Kohno, S. Efficacy, safety and pharmacokinetics of tedizolid versus linezolid in patients with skin and soft tissue infections in Japan—Results of a randomised, multicentre phase 3 study. J. Infect. Chemother. 2018, 24, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Takane, H.; Ogawa, K.; Isagawa, S.; Hirota, T.; Higuchi, S.; Horii, T.; Otsubo, K.; Ieiri, I. Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, in Japanese patients. Antimicrob. Agents Chemother. 2011, 55, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Hiraki, Y.; Matsumoto, K.; Mizoguchi, A.; Kobayashi, T.; Sadoh, S.; Morita, K.; Kamimura, H.; Karube, Y. Thrombocytopenia and anemia caused by a persistent high linezolid concentration in patients with renal dysfunction. J. Infect. Chemother. 2011, 17, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gerson, S.L.; Kaplan, S.L.; Bruss, J.B.; Le, V.; Arellano, F.M.; Hafkin, B.; Kuter, D.J. Hematologic effects of linezolid: Summary of clinical experience. Antimicrob. Agents Chemother. 2002, 46, 2723–2726. [Google Scholar] [CrossRef] [PubMed]
- Rucker, J.C.; Hamilton, S.R.; Bardenstein, D.; Isada, C.M.; Lee, M.S. Linezolid-associated toxic optic neuropathy. Neurology 2006, 66, 595–598. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P.; Cojutti, P.; Del Pin, B.; Zamparini, E.; Furlanut, M. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J. Antimicrob. Chemother. 2012, 67, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J.; Committee, E.G. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, v111–v118. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Roquilly, A.; Croce, M.; Rodriguez Gonzalez, D.; Fujimi, S.; Butterton, J.R.; Broyde, N.; Popejoy, M.W.; Kim, J.Y.; De Anda, C. A Phase 3, Randomized, Double-Blind Study Comparing Tedizolid Phosphate and Linezolid for Treatment of Ventilated Gram-Positive Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia. Clin. Infect. Dis. 2021, 73, e710–e718. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Nakajima, K.; Ichiki, K.; Ishikawa, K.; Yamada, K.; Tsuchida, T.; Otani, N.; Takahashi, Y.; Ishihara, M.; Takubo, S.; et al. Correction of thrombocytopenia caused by linezolid with scheduled sequential tedizolid use in patients with vertebral osteomyelitis by antibiotic resistant Gram-positive organisms. J. Infect. Chemother. 2022, 28, 1023–1028. [Google Scholar] [CrossRef]
- Morrisette, T.; Molina, K.C.; Da Silva, B.; Mueller, S.W.; Damioli, L.; Krsak, M.; Miller, M.A.; Fish, D.N. Real-World Use of Tedizolid Phosphate for 28 Days or More: A Case Series Describing Tolerability and Clinical Success. Open Forum Infect. Dis. 2022, 9, ofac028. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.; McKee, E.E.; Das, D.; Tulkens, P.M.; Hosako, H.; Fiedler-Kelly, J.; Passarell, J.; Radovsky, A.; Prokocimer, P. Nonclinical and pharmacokinetic assessments to evaluate the potential of tedizolid and linezolid to affect mitochondrial function. Antimicrob. Agents Chemother. 2015, 59, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Hegge, N.; Molitor, E.; Brossart, P.; Hahn-Ast, C. Comparison of Empiric Antibiotic Escalation Therapy with Vancomycin (VAN) versus Linezolid (LIN) in Patients with Febrile Neutropenia. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022032. [Google Scholar] [CrossRef] [PubMed]
- Jaksic, B.; Martinelli, G.; Perez-Oteyza, J.; Hartman, C.S.; Leonard, L.B.; Tack, K.J. Efficacy and safety of linezolid compared with vancomycin in a randomized, double-blind study of febrile neutropenic patients with cancer. Clin. Infect. Dis. 2006, 42, 597–607. [Google Scholar] [CrossRef] [PubMed]
| n = 40 Episodes | |
|---|---|
| Median Age (years) (IQR) | 69.0 (24.5) |
| Male, n (%) | 26 (65.0) |
| Median BMI (kg/m2) (IQR) | 23.1 (6.31) |
| Median Duration of TZD Administration (day) (IQR) | 13.5 (46.8) [4–203] |
| [Min–Max] ≥ 14 days, n (%), ≥28 days, n (%) | 19 (47.5)/15 (37.5) |
| Switch from anti-MRSA antibiotics, n (%) | 33 (82.5) |
| Switch from LZD, n (%) | 17 (42.5) |
| Concurrent SSRI use, n (%) | 1 (2.50) |
| Hypertension, n (%) | 29 (72.5) |
| Heart failure, n (%) | 19 (47.5) |
| Diabetes, n (%) | 22 (55.0) |
| COPD/HIV, n (%) | 0 (0.00)/0 (0.00) |
| Myocardial infarction, n (%) | 2 (5.00) |
| Collagen disease, n (%) | 1 (2.50) |
| Liver cirrhosis/Liver disease, n (%) | 9 (22.5) |
| Malignancy, n (%) | 32 (80.0) |
| Chronic renal failure, n (%)/Dialysis, n (%) | 15 (37.5)/6 (15.0) |
| Cerebrovascular disease, n (%) | 6 (15.0) |
| Site of Infection | n = 40 Episodes |
|---|---|
| Septic Arthritis, n (%) | 4 (10.0) |
| Vertebral Osteomyelitis, n (%) | 4 (10.0) |
| Osteomyelitis, n (%) | 6 (15.0) |
| SSTI/Subcutaneous and Intramuscular Abscess, n (%) | 8 (20.0)/8 (20.0) |
| Intravascular Infection, n (%) | 4 (10.0) |
| CRBSI, n (%) | 2 (5.00) |
| Pacemaker Lead Infections + Prosthetic Vascular Infections, n (%) | 1 (2.50) |
| Thrombophlebitis, n (%) | 1 (2.50) |
| Pneumoniae, n (%) | 7 (17.5) |
| FN, n (%) | 4 (10.0) |
| SSI, n (%) | 5 (12.5) |
| Nosocomial Meningitis/CNS Infection/Epidural Abscess, n (%) | 1 (2.50)/1 (2.50)/1 (2.50) |
| Pathogen | |
| Documented infection/bacteremia | 28 (70.0)/14 (35.0) |
| MRSA infection/MRSA bacteremia, n (%) | 20 (50.0)/7 (17.5) |
| MRCNS infection/MRCNS bacteremia, n (%) | 4 (10.0)/4 (10.0) |
| E. faecium infection/E. faecium bacteremia, n (%) | 2 (5.00)/2 (5.00) |
| Corynebacterium sp. infection/Corynebacterium sp. bacteremia, n (%) | 2 (5.00)/1 (2.50) |
| n = 40 Episodes | |
|---|---|
| Clinical cure, n (%) | 29 (72.5) |
| Microbiological eradication, n (%) | 12 (100) |
| 30-day mortality, n (%) | 8 (20.0) |
| 90-day mortality, n (%) | 10 (25.0) |
| Infection-related mortality, n (%) | 6 (15.0) |
| ICU admission, n (%) | 7 (17.5) |
| Mechanical ventilation | 5 (12.5) |
| Vasopressor use, n (%) | 7 (17.5) |
| Tedizolid allergy, n (%) | 0 (0.00) |
| Endophthalmitis, n (%) | 0 (0.00) |
| Clostridioides difficile colitis, n (%) | 0 (0.00) |
| Gastrointestinal bleeding, n (%) | 0 (0.00) |
| Cerebral hemorrhage, n (%) | 0 (0.00) |
| RCC transfusion, n (%) | 9 (22.5) |
| PLT transfusion, n (%) | 6 (15.0) |
| G-CSF use, n (%) | 4 (10) |
| Treatment discontinuation, n (%) | 1 (2.50) (hepatic dysfunction) |
| Non-Hematologic Malignancies: (n = 27) | Hematologic Malignancies: (n = 13) | p | |
|---|---|---|---|
| Median age (year) (IQR) | 72.0 (22.0) | 69.0 (27.0) | 0.716 |
| Male, n (%) | 17 (63.0) | 9 (69.2) | 1 |
| Median BMI (kg/m2) (IQR) | 23.2 (5.10) | 21.1 (8.60) | 0.93 |
| Median days of Tedizolid administration (IQR) [Minimum–Maximum] | 19.0 (66.0) [4–203] | 12.0 (9.00) [4–28] | 0.001 |
| Switch from other anti-MRSA agents, n (%) | 20 (74.1) | 13 (100) | 0.074 |
| Switch from Linezolid, n (%) | 9 (33.3) | 7 (53.8) | 0.631 |
| Hypertension, n (%) | 17 (63.0) | 12 (92.3) | 0.068 |
| Cardiac failure, n (%) | 8 (29.6) | 11 (84.6) | 0.001 |
| Diabetes, n (%) | 12 (44.4) | 10 (76.9) | 0.111 |
| COPD/HIV, n (%) | 0 (0.00) | 0 (0.00) | |
| Myocardial infarction, n (%) | 2 (7.40) | 0 (0.00) | 1 |
| Connective tissue disease, n (%) | 1 (3.70) | 0 (0.00) | 1 |
| Liver cirrhosis/Liver disease, n (%) | 3 (11.1) | 6 (46.2) | 0.038 |
| Malignancy, n (%) | 19 (70.4) | 13 (100.0) | 0.037 |
| Chronic renal failure, n (%) | 11 (40.7) | 4 (30.8) | 0.73 |
| Dialysis, n (%) | 3 (11.1) | 3 (23.1) | 0.37 |
| Cerebrovascular disease, n (%) | 3 (11.1) | 3 (23.1) | 0.37 |
| Non-Hematologic Malignancies: (n = 27) | Hematologic Malignancies: (n = 13) | p | |
|---|---|---|---|
| Clinical cure, n (%) | 24 (88.9) | 5 (38.5) | 0.003 |
| Microbiological eradication, n (%) | 5/5 (100) | 7/7 (100) | |
| 30-day mortality, n (%) | 1 (3.70) | 7 (53.8) | <0.01 |
| 90-day mortality, n (%) | 1 (3.70) | 9 (69.2) | <0.01 |
| Infection-related mortality | 1 (3.70) | 5 (38.5) | |
| ICU admission, n (%) | 0 (0.00) | 7 (53.8) | <0.01 |
| Mechanical ventilation, n (%) | 0 (0.00) | 5 (38.5) | 0.002 |
| Vasopressor use, n (%) | 0 (0.00) | 7 (53.8) | <0.01 |
| RCC transfusion, n (%) | 3 (11.1) | 6 (46.2) | 0.038 |
| PLT transfusion, n (%) | 2 (7.41) | 4 (30.8) | 0.075 |
| G-CSF use, n (%) | 0 (0.0) | 4 (30.8) | 0.008 |
| Site of Infection | Non-Hematologic Malignancies: (n = 27) | Hematologic Malignancies: (n = 13) |
|---|---|---|
| Septic Arthritis, n (%) | 3 (11.1) | 1 (7.69) |
| Vertebral Osteomyelitis, n (%) | 4 (14.8) | 0 (0.00) |
| Osteomyelitis, n (%) | 6 (22.2) | 0 (0.00) |
| SSTI/Subcutaneous and Intramuscular Abscess, n (%) | 12 (44.4) | 2 (15.4) |
| Intravascular Infection, n (%) | 3 (11.1) | 11 (84.6) |
| CRBSI, n (%) | 2 (7.41) | 0 (0.00) |
| Pacemaker Lead Infections + Prosthetic Vascular Infections, n (%) | 1 (3.70) | 0 (0.00) |
| Thrombophlebitis, n (%) | 0 (0.00) | 1 (7.69) |
| Pneumoniae, n (%) | 6 (22.2) | 1 (7.69) |
| FN, n (%) | 0 (0.00) | 4 (30.8) |
| SSI, n (%) | 4 (14.8) | 1 (7.69) |
| Nosocomial Meningitis/CNS Infection/Epidural Abscess, n (%) | 0 (0.00) | 1 (7.69) |
| Pathogen | Non-Hematologic Malignancies: (n = 27) | Hematologic Malignancies: (n = 13) |
| MRSA infection/MRSA bacteremia, n (%) | 17 (63.0)/7 (25.9) | 3 (23.0)/0 (0.00) |
| MRCNS infection/MRCNS bacteremia, n (%) | 2 (7.41)/2 (7.41) | 2 (15.4)/2 (15.4) |
| E. faecium infection/E. faecium bacteremia, n (%) | 0 (0.00)/0 (0.00) | 2 (15.4)/1 (7.69) |
| Corynebacterium sp. infection/Corynebacterium sp. bacteremia, n (%) | 2 (7.41)/0 (0.00) | 2 (15.4)/2 (15.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, K.; Tsuda, Y.; Mori, N. Study on the Efficacy and Safety of Tedizolid in Japanese Patients. Antibiotics 2024, 13, 1237. https://doi.org/10.3390/antibiotics13121237
Ishikawa K, Tsuda Y, Mori N. Study on the Efficacy and Safety of Tedizolid in Japanese Patients. Antibiotics. 2024; 13(12):1237. https://doi.org/10.3390/antibiotics13121237
Chicago/Turabian StyleIshikawa, Kazuhiro, Yasumasa Tsuda, and Nobuyoshi Mori. 2024. "Study on the Efficacy and Safety of Tedizolid in Japanese Patients" Antibiotics 13, no. 12: 1237. https://doi.org/10.3390/antibiotics13121237
APA StyleIshikawa, K., Tsuda, Y., & Mori, N. (2024). Study on the Efficacy and Safety of Tedizolid in Japanese Patients. Antibiotics, 13(12), 1237. https://doi.org/10.3390/antibiotics13121237






