Analysis of the Antibiotic-Potentiating Activity, Absorption, Distribution, Metabolism, and Excretion (ADME) and the Molecular Docking Properties of Phytol Against Multi-Drug-Resistant (MDR) Strains
Abstract
1. Introduction
2. Results and Discussion
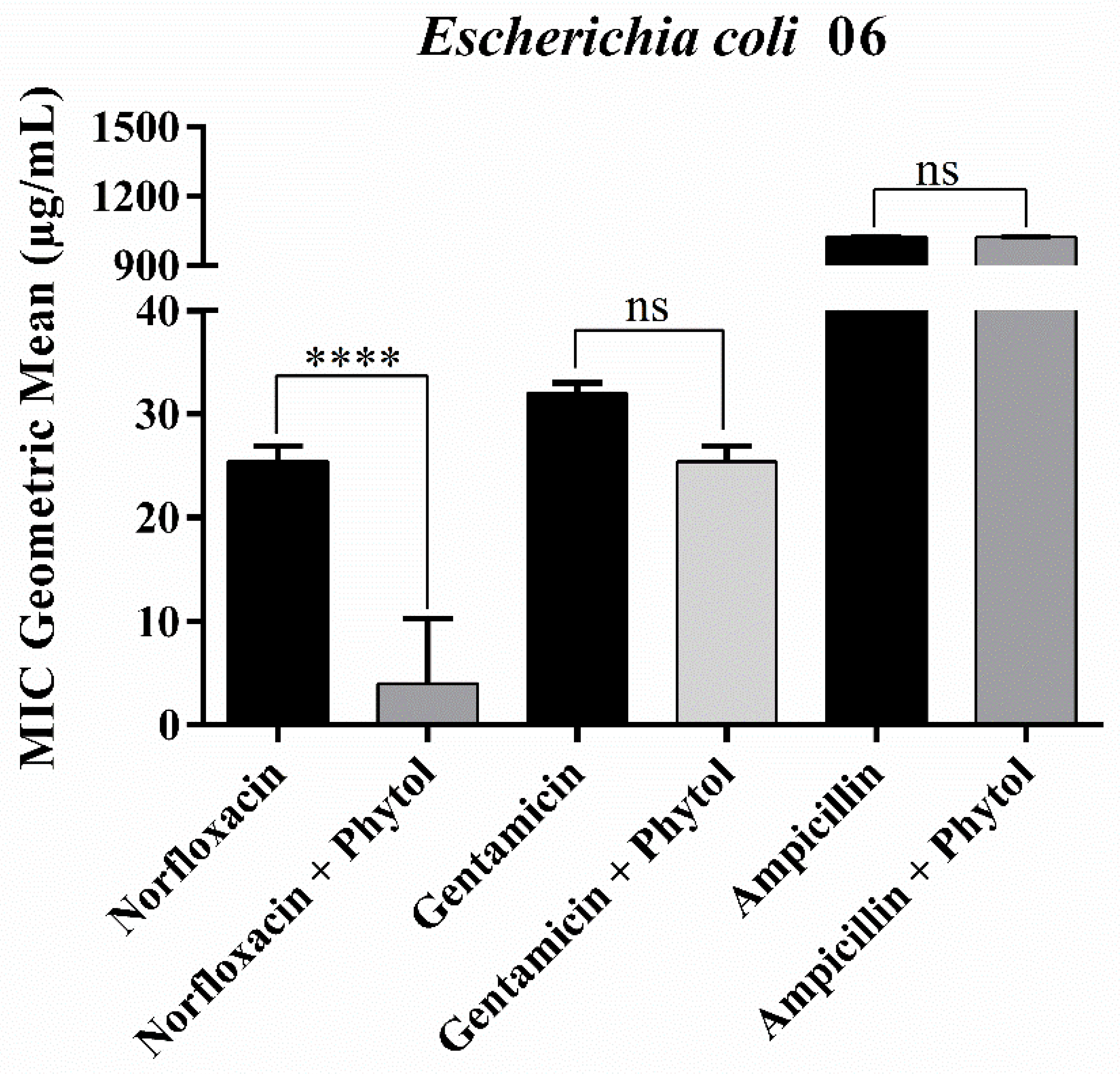
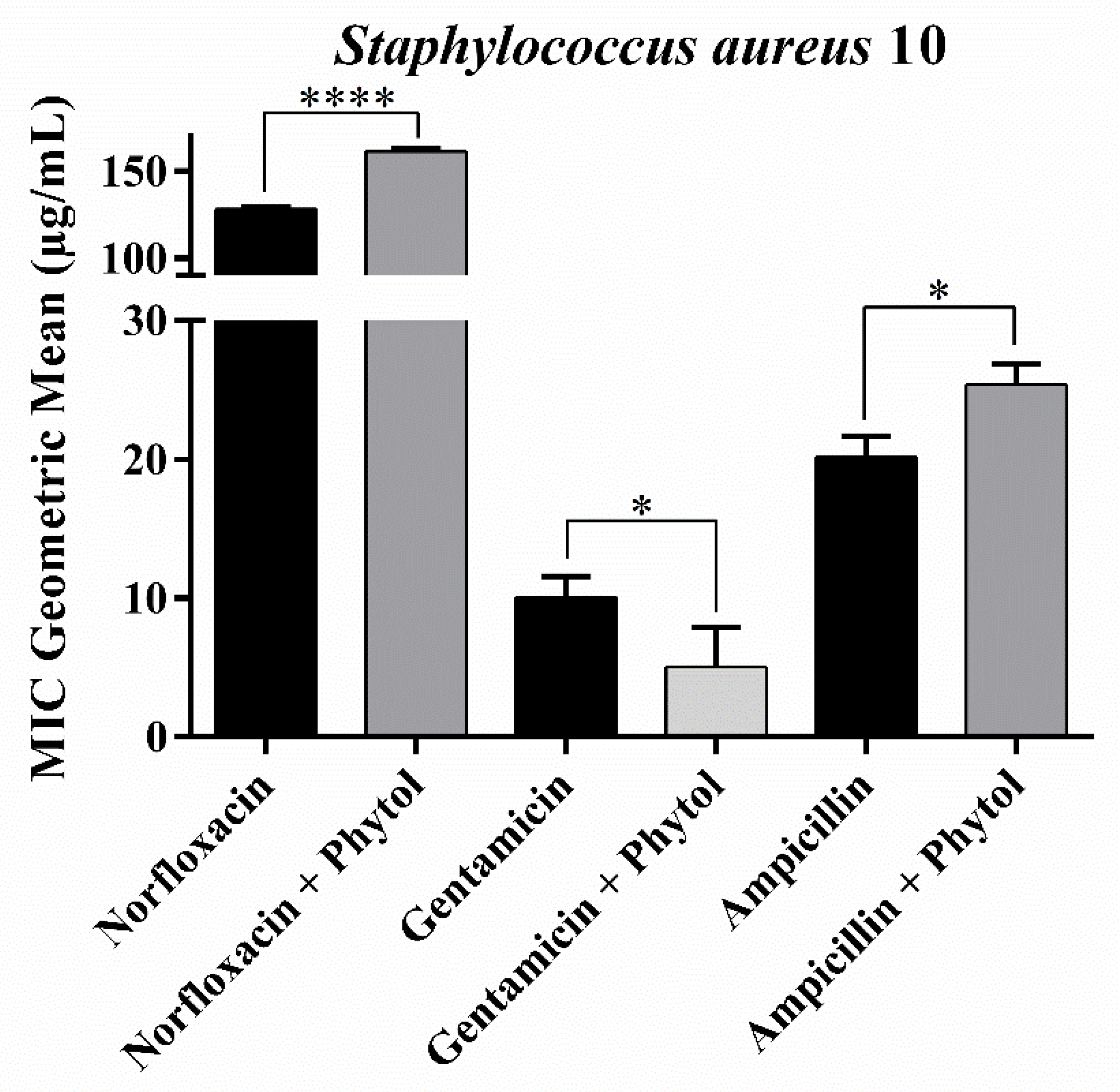
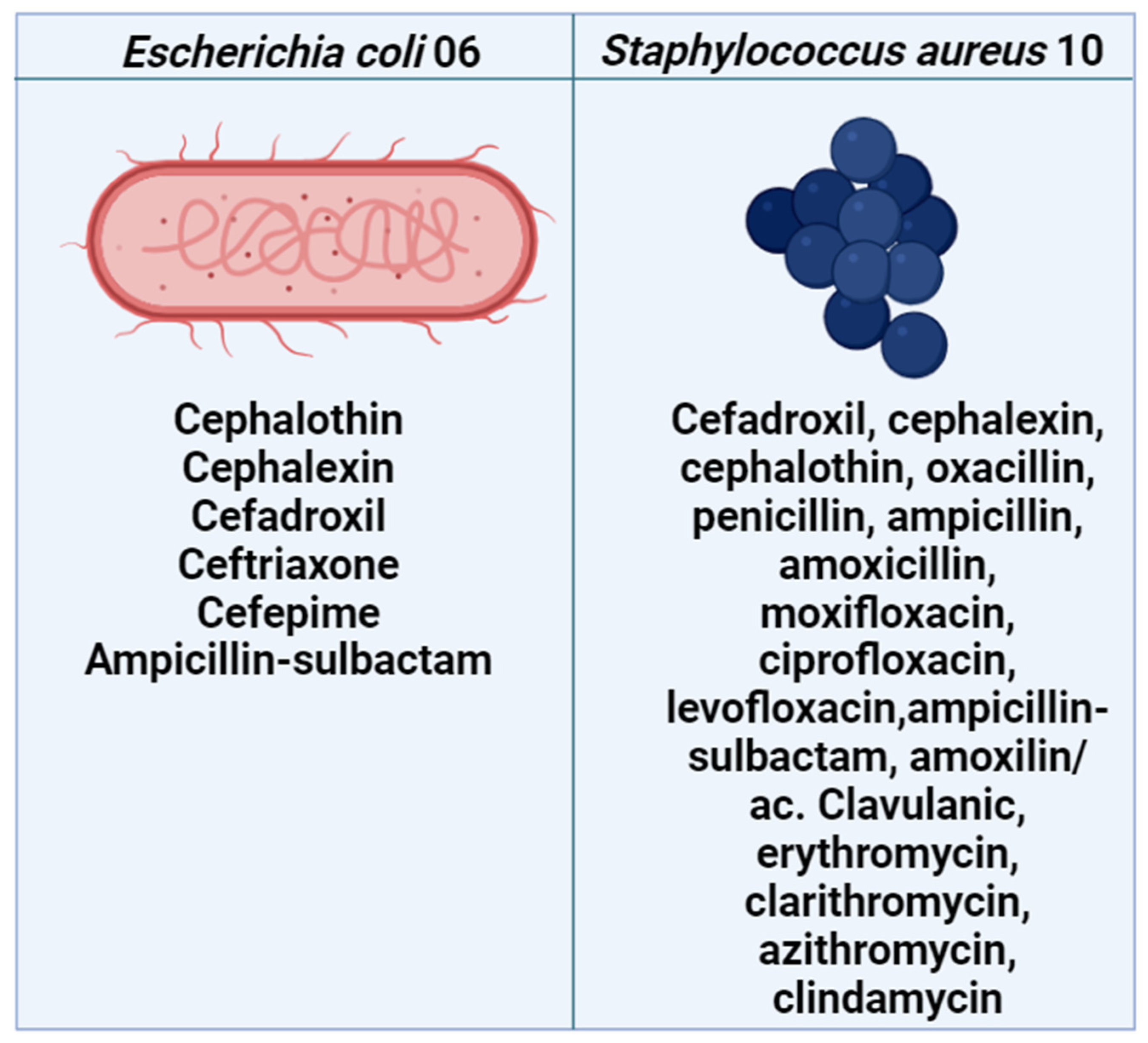
2.1. Antibacterial and Drug-Potentiating Activity
2.2. In Silico ADME Prediction
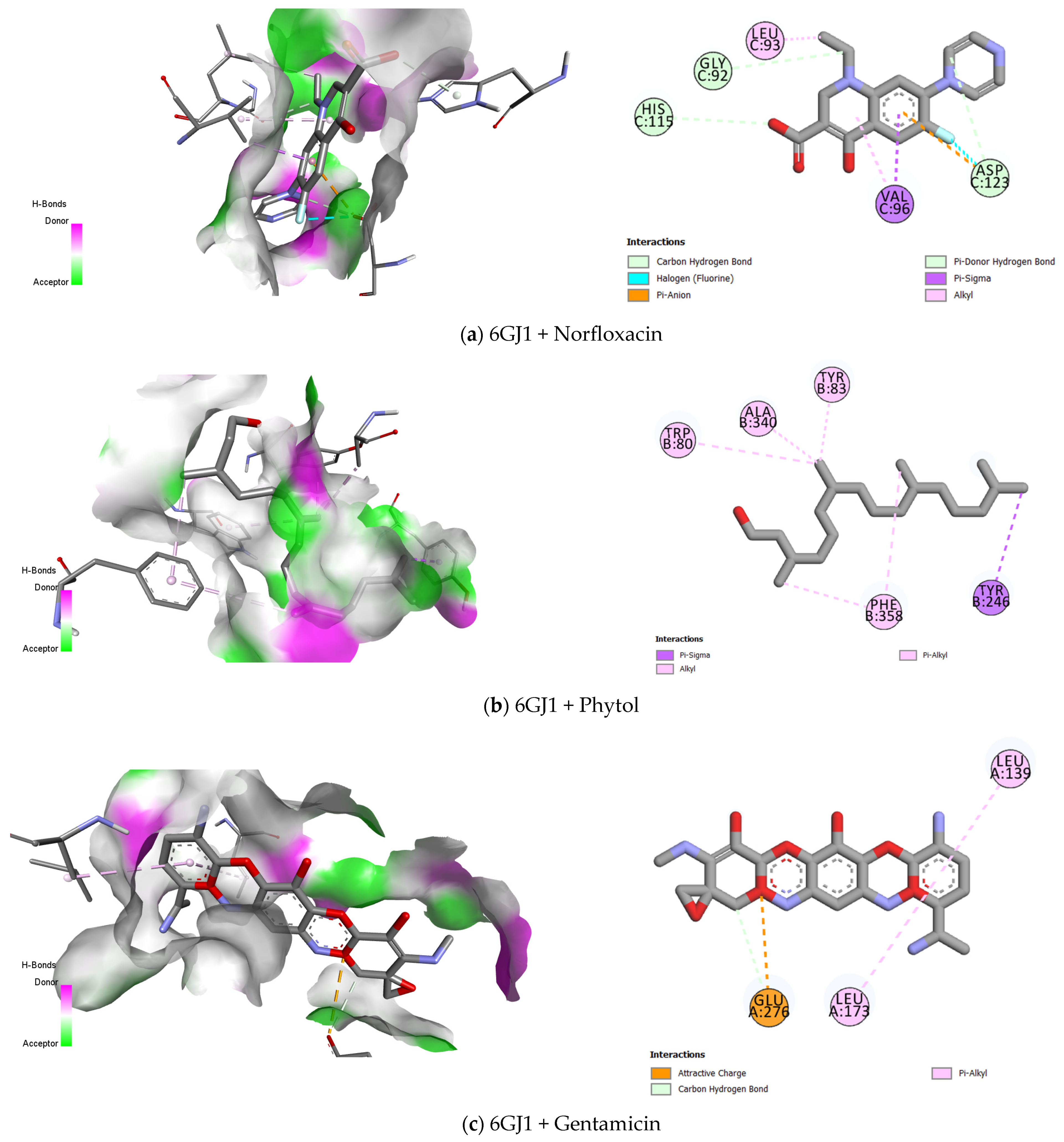
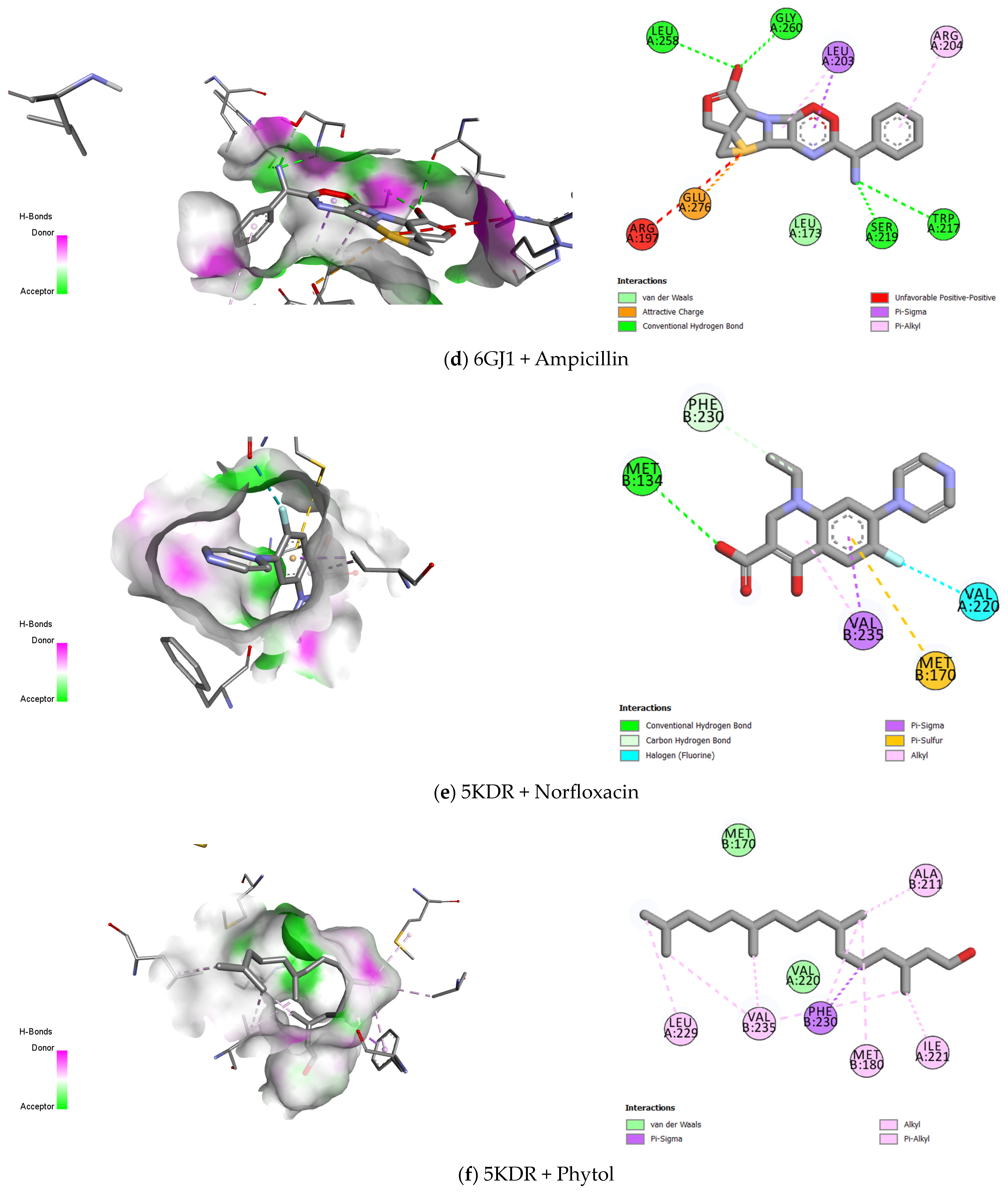
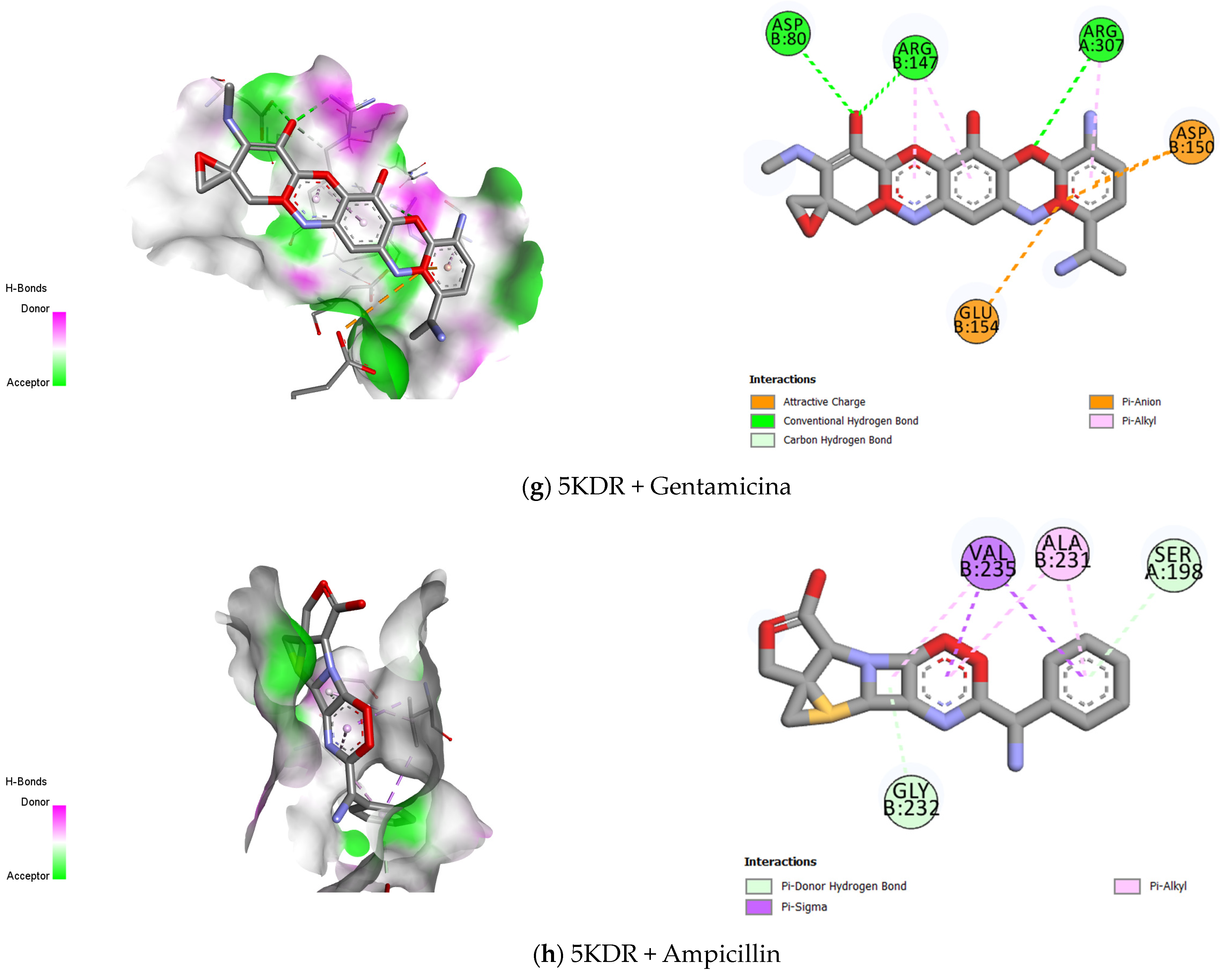
2.3. Molecular Docking
3. Materials and Methods
3.1. Antibiotics and Reagents
3.2. Culture Media and Bacterial Strains
3.3. In Vitro Antibacterial Activity
3.4. Drug-Potentiating Activity
3.5. In Silico ADME Prediction
3.6. Statistical Analysis
3.7. Molecular Docking
3.7.1. Protein of Interest
3.7.2. Binder Treatment
3.7.3. Grid Calculation and Adjustment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kourkouta, L.; Koukourikos, K.; Iliadis, C.; Plati, P.; Dimitriadou, A. History of antibiotics. Sumer. J. Med. Healthc. 2018, 1, 51–55. [Google Scholar]
- Haider, R. Penicillin and the Antibiotics Revolution Global History. Asian J. Pharm. Res. 2023, 13, 55–62. [Google Scholar] [CrossRef]
- Premlatha, M. Microbial resistance to antibiotics. In Bacterial Adaptation to Co-Resistance, 1st ed.; Mandal, S., Paul, D., Eds.; Springer: Singapore, 2019; pp. 61–80. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial Resistance in the Context of the Sustainable Development Goals: A Brief Review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Faïs, T.; Cougnoux, A.; Dalmasso, G.; Laurent, F.; Delmas, J.; Bonnet, R. Antibiotic Activity of Escherichia coli against Multiresistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2016, 60, 6986–6988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Pathogenesis of Staphylococcus aureus Abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Dias, K.J.S.O.; Miranda, G.M.; Bessa, J.R.; Araújo, A.C.J.; Freitas, P.R.; Almeida, R.S.; Paulo, C.L.R.; Araújo Neto, J.B.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as bacterial efflux pump inhibitors: A systematic review. Front. Pharmacol. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Santos, C.C.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; Almeida, A.A.C.; Oliveira, G.A.L.; Costa, J.P.; Sousa, D.P.; Freitas, R.M. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Ryu, K.-R.; Choi, J.-Y.; Chung, S.; Kim, D.-H. Anti-scratching Behavioral Effect of the Essential Oil and Phytol Isolated from Artemisia princeps Pamp. in Mice. Planta Med. 2011, 77, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Bandyopadhyay, P.K. In vivo and in vitro antimicrobial activity of phytol, a diterpene molecule, isolated and characterized from Adhatoda vasica Nees. (Acanthaceae), to control severe bacterial disease of ornamental fish, Carassius auratus, caused by Bacillus licheniformis PKBMS16. Microb. Pathog. 2020, 141, 103977–103987. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, M.C.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, N.-N.; Yao, Z.-J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.-P.; Cao, D.-S. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef]
- Houghton, P.J.; Howes, M.-J.; Lee, C.C.; Steventon, G. Uses and abuses of in vitro tests in ethnopharmacology: Visualizing an elephant. J. Ethnopharmacol. 2007, 110, 391–400. [Google Scholar] [CrossRef]
- McNeil, M.J.; Porter, R.B.; Williams, L.A.; Rainford, L. Chemical composition and antimicrobial activity of the essential oils from Cleome spinosa. Nat. Prod. Commun. 2010, 5, 1301–1306. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Silva, J.C.; Pereira, R.L.S.; Freitas, T.S.; Rocha, J.E.; Macedo, N.S.; Nonato, C.d.F.A.; Linhares, M.L.; Tavares, D.S.A.; Bezerra da Cunha, F.A.; Coutinho, H.D.M.; et al. Evaluation of antibacterial and toxicological activities of essential oil of Ocimum gratissimum L. and its major constituent eugenol. Food Biosci. 2022, 50, 102128. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Ghaneian, M.T.; Nourmoradi, H.; Zarei, A.; Khodarahmi, F. Antimicrobial Activity, Toxicity and Stability of Phytol as a Novel Surface Disinfectant. Environ. Health Eng. Manag. J. 2015, 2, 13–16. [Google Scholar]
- Adeshina, I.; Ayuba, O.; Onoja, I.; Adamu, K.; Ogbu, H. Evaluation of the Anti-Bacterial Activity of Phytol against Erysipelothrix piscisicarius Infection in Nile Tilapia (Oreochromis niloticus). Int. J. Vet. Res. 2021, 1, 9–19. [Google Scholar] [CrossRef]
- Srinivasan, R.; Devaraj, S.; Rajendran, R.; Jayaprakash, R.; Gnanamani, E.; Perumal, P.T. Exploring the Anti-Quorum Sensing and Antibiofilm Efficacy of Phytol against Serratia marcescens-Associated Acute Pyelonephritis Infection in Wistar Rats. Front. Cell. Infect. Microbiol. 2017, 7, 498. [Google Scholar] [CrossRef]
- Eluchie, C.N.; Oyeleke, S.B.; Salihu, A.; Tunde, A.A.; Awolola, G.V. Effect of Phytol on Dehydrogenase Activity of Bacterial Isolates from Grilled Meat. Am. J. Food Sci. Technol. 2016, 4, 1–6. [Google Scholar]
- Inoue, Y.; Hada, T.; Shiraishi, A.; Hirose, K.; Hamashima, H.; Kobayashi, S. Biphasic Effects of Geranylgeraniol, Teprenone, and Phytol on the Growth of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 1770–1774. [Google Scholar] [CrossRef]
- Walters, W.P.; Murcko, A.A.; Murcko, M.A. Recognizing molecules with drug-like properties. Curr. Opin. Chem. Biol. 1999, 3, 384–387. [Google Scholar] [CrossRef]
- Walters, W.P. Going further than Lipinski’s rule in drug design. Expert Opin. Drug Discov. 2012, 7, 99–107. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Islam, A.; Uddin Emon, N.; Islam, Q.S.; Ahsan, M. Chemico-Biological Profiling of Blumea lacera (Burm.f.) DC. (Family: Asteraceae) Provides New Insights as a Potential Source of Antioxidant, Cytotoxic, Antimicrobial, and Antidiarrheal Agents. Evid.-Based Complement. Alternat. Med. 2022, 2022, 2293415. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Islam, T.; Rokonuzzman, M.; Prottay, A.A.S.; Akter, F.; Hossain, M.I.; Chowdhury, R.; Kazi, M.A.; Khalipha, A.B.R.; Coutinho, H.D.M.; et al. Modulatory effects of phytol on the antiemetic property of domperidone, possibly through the D2 receptor interaction pathway: In vivo and in silico studies. 3 Biotech 2023, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Shanmugam, G.; Varghese, E.L.; Haribabu, Y.; Savarimuthu, I. Phytol loaded PLGA nanoparticles ameliorate scopolamine-induced cognitive dysfunction by attenuating cholinesterase activity, oxidative stress and apoptosis in Wistar rat. Nutr. Neurosci. 2022, 25, 485–501. [Google Scholar] [CrossRef]
- Usman, M.A.; Usman, F.I.; Abubakar, M.S.; Salman, A.A.; Adamu, A.; Ibrahim, M.A. Phytol suppresses parasitemia and ameliorates anaemia and oxidative brain damage in mice infected with Plasmodium berghei. Exp. Parasitol. 2021, 224, 108097. [Google Scholar] [CrossRef]
- Lee, W.; Woo, E.-R.; Lee, D.G. Phytol Has Antibacterial Property by Inducing Oxidative Stress Response in Pseudomonas aeruginosa. Free Radic. Res. 2016, 50, 1309–1318. [Google Scholar] [CrossRef]
- Goldstein, E.J.C. Norfloxacin, a Fluoroquinolone Antibacterial Agent. Am. J. Med. 1987, 82, 3–17. [Google Scholar] [CrossRef]
- Araújo, N.J.S.; da Silva, A.R.P.; Costa, M.d.S.; Pereira Silva, C.A.; Freitas, T.S.; Sousa, E.O.; Barbosa Filho, J.M.; Soares de Matos, Y.M.L.; Coutinho, H.D.M.; Andrade-Pinheiro, J.C. Evaluation of the Antibacterial Activity of Hecogenin Acetate and Its Inhibitory Potential of NorA and MepA Efflux Pumps from Staphylococcus aureus. Microb. Pathog. 2023, 174, 1–10. [Google Scholar] [CrossRef]
- da Silva-Júnior, O.S.; Franco, C.d.J.P.; Moraes, A.A.B.; Cruz, J.N.; Costa, K.S.d.; Nascimento, L.D.d.; Andrade, E.H.d.A. In Silico Analyses of Toxicity of the Major Constituents of Essential Oils from Two Ipomoea L. Species. Toxicon 2021, 195, 111–118. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Poma, P.; Tutone, M.; McCubrey, J.A.; Sajeva, M.; Notarbartolo, M. Phytol and Heptacosane Are Possible Tools to Overcome Multidrug Resistance in an In Vitro Model of Acute Myeloid Leukemia. Pharmaceuticals 2022, 15, 356. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Poma, P.; Notarbartolo, M. Natural Inhibitors of P-Glycoprotein in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2023, 24, 4140. [Google Scholar] [CrossRef]
- Sakthivel, R.; Malar, D.S.; Archunan, G.; Devi, K.P. Phytol Ameliorated Benzo(a)pyrene-Induced Lung Carcinogenesis in Swiss Albino Mice via Inhibition of Oxidative Stress and Apoptosis. Environ. Toxicol. 2018, 34, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-N.; Trang, N.M.; Kang, H.; Kim, K.H.; Jeong, G.-S. Phytol Suppresses Osteoclast Differentiation and Oxidative Stress through Nrf2/HO-1 Regulation in RANKL-Induced RAW264.7 Cells. Cells 2022, 11, 3596. [Google Scholar] [CrossRef] [PubMed]
- Mackie, J.T.; Atshaves, B.P.; Ross Payne, H.; McIntosh, A.L.; Schroeder, F.; Kier, A.B. Phytol-Induced Hepatotoxicity in Mice. Toxicol. Pathol. 2009, 37, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Alencar, M.V.O.B.; Islam, M.T.; Ali, E.S.; Santos, J.V.O.; Paz, M.F.C.J.; Sousa, J.M.C.; Dantas, S.M.M.M.; Mishra, S.K.; Cavalcante, A.A.C. Association of Phytol with Toxic and Cytotoxic Activities in an Antitumoral Perspective: A Meta-Analysis and Systemic Review. Anti-Cancer Agents Med. Chem. 2018, 18, 1828–1837. [Google Scholar] [CrossRef]
- Islam, M.T.; Streck, L.; de Alencar, M.V.O.B.; Silva, S.W.C.; da Conceição Machado, K.; da Conceição Machado, K.; Júnior, A.L.G.; Paz, M.F.C.J.; da Mata, A.M.O.F.; de Castro e Sousa, J.M.; et al. Evaluation of Toxic, Cytotoxic and Genotoxic Effects of Phytol and Its Nanoemulsion. Chemosphere 2017, 177, 93–101. [Google Scholar] [CrossRef]
- Bilder, P.; Lightle, S.; Bainbridge, G.; Parce, J.W.; Ala, P.; Xu, Y.; Qi, H.; Viitanen, P.; Benson, T.E. The Structure of the Carboxyltransferase Component of Acetyl-CoA Carboxylase Reveals a Zinc-Binding Motif Unique to the Bacterial Enzyme. Biochemistry 2006, 45, 1712–1722. [Google Scholar] [CrossRef]
- Xiang, S.; Callaghan, M.M.; Watson, K.G.; Tong, L. Crystal Structures of Human and Staphylococcus aureus Pyruvate Carboxylase and Molecular Insights into the Carboxyltransfer Reaction. Nat. Struct. Mol. Biol. 2008, 15, 295–302. [Google Scholar] [CrossRef]
- Freiberg, C.; Brunner, N.A.; Schiffer, G.; Lampe, T.; Pohlmann, J.; Brands, M.; Raabe, M.; Haebich, D.; Ziegelbauer, K. Novel Bacterial Acetyl Coenzyme A Carboxylase Inhibitors with Antibiotic Efficacy In Vivo. Antimicrob. Agents Chemother. 2006, 50, 2707–2712. [Google Scholar] [CrossRef]
- Linda, P.C.; Watson, K.G.; Sokolova, A.; Swenson, L.; Finer-Moore, J.S.; Stroud, R.M. A Symmetrical Tetramer for S. aureus Pyruvate Carboxylase in Complex with Coenzyme A. Structure 2009, 17, 823–832. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Y. QM/MM Study of the Reaction Mechanism of the Carboxyl Transferase Domain of Pyruvate Carboxylase from Staphylococcus aureus. Biochemistry 2014, 53, 4455–4466. [Google Scholar] [CrossRef]
- Silvers, M.A.; Niessen, S.; Hoover, H.S.; Cravatt, B.F.; Wright, D.L.; Ge, H.; Finzel, B.C. Crystal Structure of Carboxyltransferase from Staphylococcus aureus Bound to the Antibacterial Agent Moiramide B. Biochemistry 2016, 55, 4666–4674. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.D.; Felisberto-Rodrigues, C.; Meir, A.; Prevost, M.S.; Redzej, A.; Trokter, M.; Waksman, G. Secretion Systems in Gram-Negative Bacteria: Structural and Mechanistic Insights. Nat. Rev. Microbiol. 2015, 13, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, Y.; Flaugnatti, N.; Durand, E.; Journet, L.; Cascales, E. Biogenesis and Structure of a Type VI Secretion Baseplate. Nat. Microbiol. 2018, 3, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Taillefer, B.; Giraud, J.F.; Cascales, E. No Fitness Cost Entailed by Type VI Secretion System Synthesis, Assembly, Contraction, or Disassembly in Enteroaggregative Escherichia coli. J. Bacteriol. 2023, 205, e0035723. [Google Scholar] [CrossRef]
- Asgari, B.; Hamidian, M.; Hall, R.M. Identification of Virulence Genes Associated with Pathogenicity of Translocating Escherichia coli with Special Reference to the Type 6 Secretion System. Microorganisms 2024, 12, 1851. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Favaro, M.T.P.; Dropa, M.; Nakazato, L.; Brocchi, M. Characterizing the Type 6 Secretion System (T6SS) and Its Role in the Virulence of Avian Pathogenic Escherichia coli Strain APECO18. PeerJ 2021, 9, e12631. [Google Scholar] [CrossRef]
- Merciecca, T.; Reiter, T.; Simonet, M.; Renaud, F.; Verheyde, B.; Weber, M.; Blanchard, A.; Aujoulat, F. Role of Klebsiella pneumoniae Type VI Secretion System (T6SS) in Long-Term Gastrointestinal Colonization. Sci. Rep. 2022, 12, 16968. [Google Scholar] [CrossRef]
- Serapio-Palacios, A.; Barra, A.; Ishii, R.; Levy, M.; Remis, J.P.; Schneidman-Duhovny, D.; Mekalanos, J.J. Type VI Secretion Systems of Pathogenic and Commensal Bacteria Mediate Niche Occupancy in the Gut. Cell Rep. 2022, 39, 110731. [Google Scholar] [CrossRef]
- Cummins, E.A.; Leverenz, M.; McGann, P.; Glick, E.; Crofts, T.; Klim, J. Parallel Loss of Type VI Secretion Systems in Two Multi-Drug-Resistant Escherichia coli Lineages. Microb. Genom. 2023, 9, 001133. [Google Scholar] [CrossRef]
- M100-S16; Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; p. 184.
- Coutinho, H.D.M.; Costa, J.G.M.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the Antibiotic Activity against a Multiresistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.L.; Moreira, A.M.T.; Teles, B.R.S.; Kamdem, J.P.; AlAsmari, A.F.; Alasmari, F.; Khan, M.; Barros, L.M.; Ibrahim, M. Mentha arvensis Oil Exhibits Repellent, Acute Toxic, and Antioxidant Activities in Nauphoeta cinerea. Sci. Rep. 2024, 14, 21599. [Google Scholar] [CrossRef]
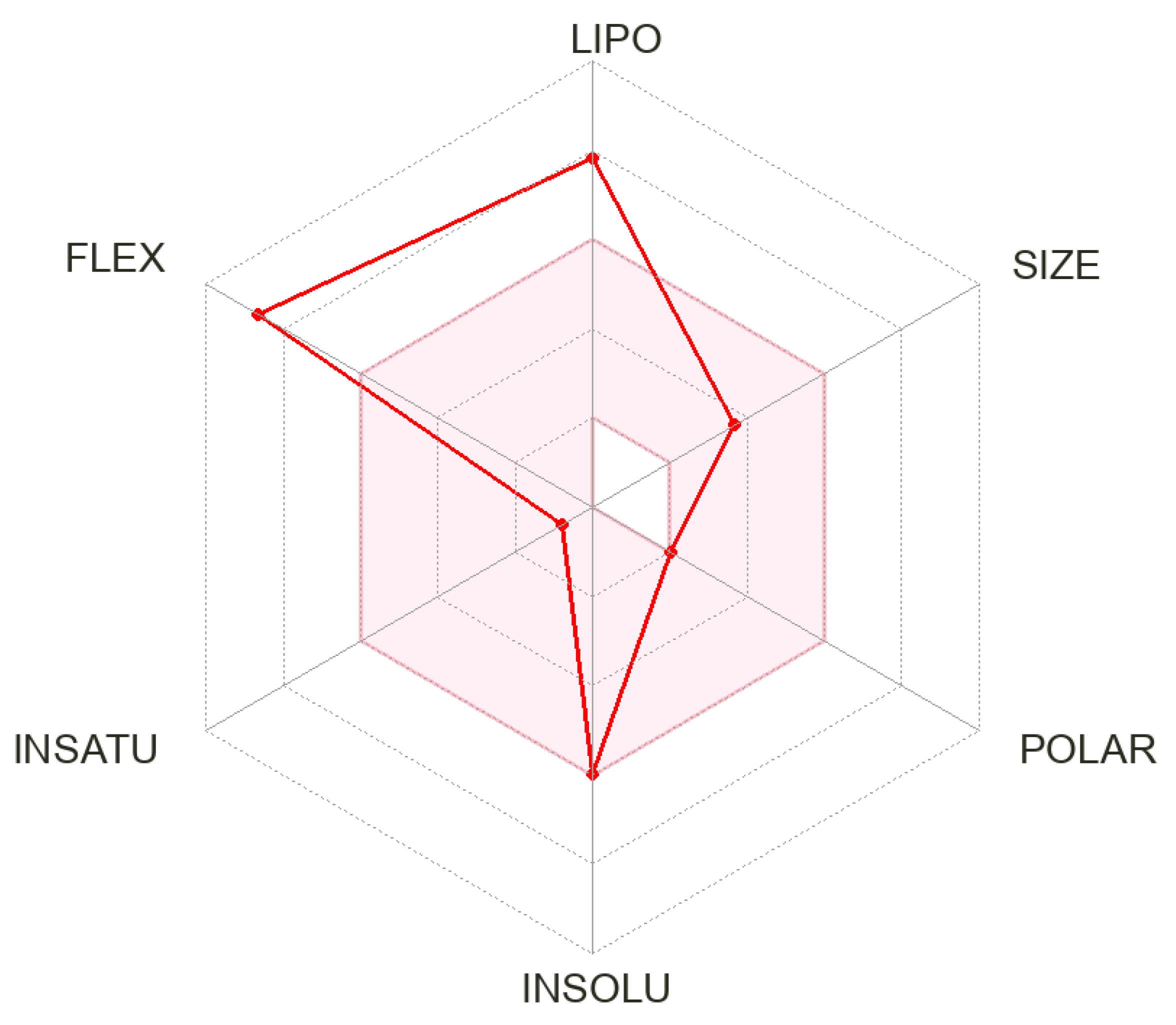
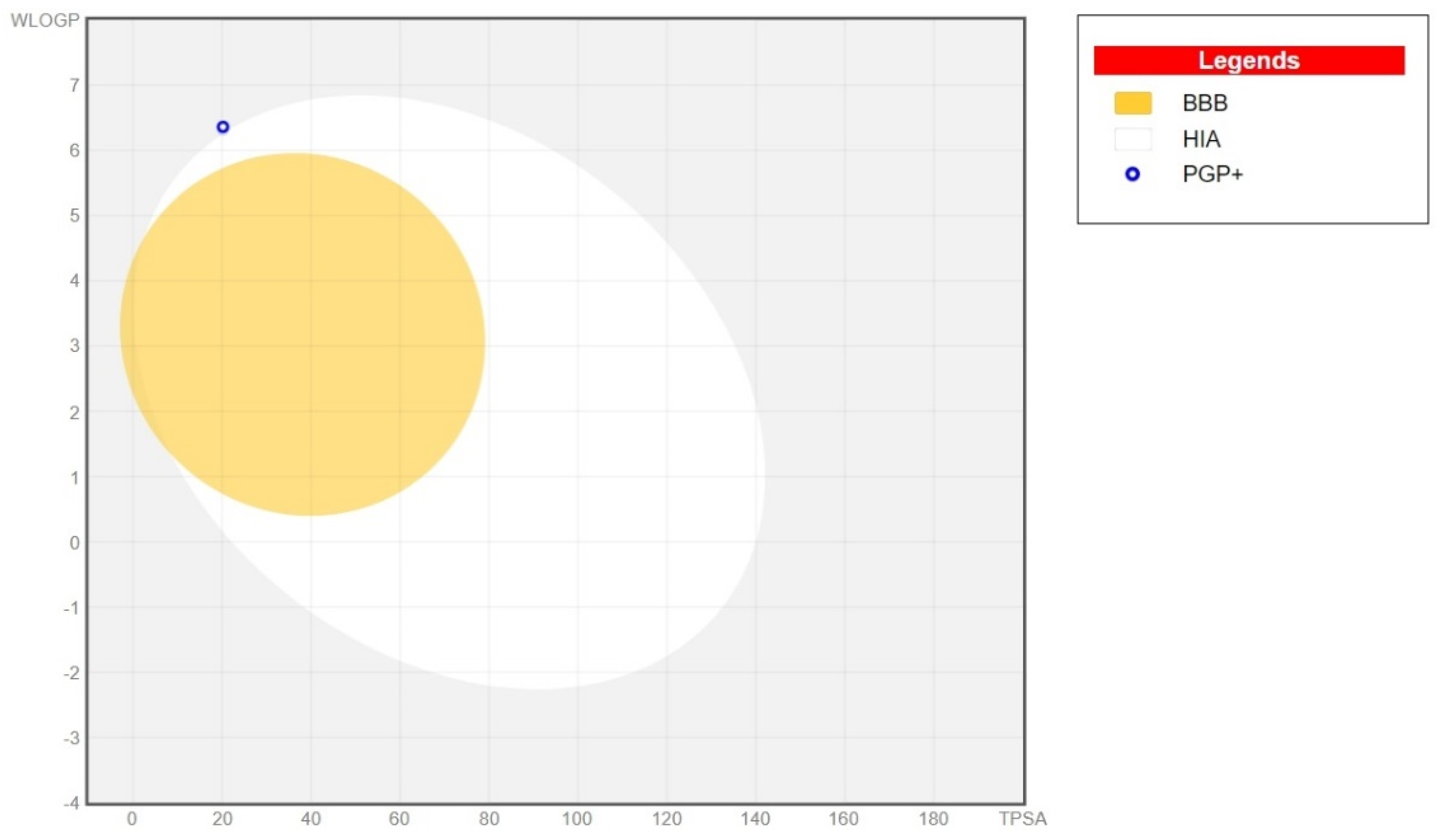
| Physicochemical Properties | |
| MF | C20H40O |
| MW | 296.53 g/mol |
| NRB | 13 |
| NHA | 1 |
| NHD | 1 |
| TPSA | 20.23 Å2 |
| Log Po/w (XLOGP3) | 8.19 |
| Log S (ESOL) | −5.98 |
| Pharmacokinetics | |
| HIA | Low |
| BBB | No |
| P-gp substrate | Yes |
| CYP1A2 inhibitor | No |
| CYP2C19 inhibitor | No |
| CYP2C9 inhibitor | Yes |
| CYP2D6 inhibitor | No |
| CYP3A4 inhibitor | No |
| Log Kp (cm/s) | −2.29 cm/s |
| Druglikeness | |
| Lipinski | Yes (1) |
| Ghose | No (1) |
| Veber | No (1) |
| Egan | No (1) |
| Muegge | No (2) |
| Medicinal Chemistry | |
| PAINS | 0 |
| Brenk | 1: isolated_alkene |
| Leadlikeness | No (2) |
| Synthetic accessibility | 4.30 |
| Protein | Compounds | Biniding Affinites (kcal/mol) | Bond Type |
|---|---|---|---|
| 6GJ1–E. coli | Norfloxacin | −7.673 | Carbon–Hydrogen bond |
| Halogen (Fluorine) | |||
| Pi- Anion | |||
| Pi- Donor Hydrogen Bond | |||
| Pi- Sigma | |||
| Alkyl | |||
| Gentamicin | −9.688 | Attractive charge | |
| Carbon–Hydrogen bond | |||
| Pi- Alkyl | |||
| Ampicillin | −9.105 | van der Waals | |
| Attractive Charge | |||
| Conventional Hydrogen Bond | |||
| Unfavourable Positive–Positive | |||
| Pi- Sigma | |||
| Pi- Alkyl | |||
| Phytol | −5.061 | Pi- Sigma | |
| Pi- Alkyl | |||
| Alkyl | |||
| 5KDR–S. aureus | Norfloxacin | −6.687 | Conventional Hydrogen Bond |
| Carbon–Hydrogen bond | |||
| Halogen (Fluorine) | |||
| Pi- Sigma | |||
| Pi- Sulphur | |||
| Alkyl | |||
| Gentamicin | −7.59 | Attractive Charge | |
| Conventional Hydrogen Bond | |||
| Carbon–Hydrogen bond | |||
| Pi- Anion | |||
| Pi- Alkyl | |||
| Ampicillin | −7.999 | Pi- Donor Hydrogen Bond | |
| Pi- Sigma | |||
| Pi- Alkyl | |||
| Phytol | −5.509 | van der Waals | |
| Pi- Sigma | |||
| Alkyl | |||
| Pi- Alkyl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Bezerra, J.W.; Menezes, S.A.; Silva, J.T.d.C.; de Sousa, S.G.; Alves, D.S.; Alencar, G.G.; Araújo, I.M.; Rodrigues, E.Y.d.S.; Oliveira-Tintino, C.D.d.M.; da Cruz, R.P.; et al. Analysis of the Antibiotic-Potentiating Activity, Absorption, Distribution, Metabolism, and Excretion (ADME) and the Molecular Docking Properties of Phytol Against Multi-Drug-Resistant (MDR) Strains. Antibiotics 2024, 13, 1171. https://doi.org/10.3390/antibiotics13121171
Almeida-Bezerra JW, Menezes SA, Silva JTdC, de Sousa SG, Alves DS, Alencar GG, Araújo IM, Rodrigues EYdS, Oliveira-Tintino CDdM, da Cruz RP, et al. Analysis of the Antibiotic-Potentiating Activity, Absorption, Distribution, Metabolism, and Excretion (ADME) and the Molecular Docking Properties of Phytol Against Multi-Drug-Resistant (MDR) Strains. Antibiotics. 2024; 13(12):1171. https://doi.org/10.3390/antibiotics13121171
Chicago/Turabian StyleAlmeida-Bezerra, José Weverton, Saulo Almeida Menezes, José Thyálisson da Costa Silva, Simone Galdino de Sousa, Daniel Sampaio Alves, Gabriel Gonçalves Alencar, Isaac Moura Araújo, Ewerton Yago de Sousa Rodrigues, Cícera Datiane de Morais Oliveira-Tintino, Rafael Pereira da Cruz, and et al. 2024. "Analysis of the Antibiotic-Potentiating Activity, Absorption, Distribution, Metabolism, and Excretion (ADME) and the Molecular Docking Properties of Phytol Against Multi-Drug-Resistant (MDR) Strains" Antibiotics 13, no. 12: 1171. https://doi.org/10.3390/antibiotics13121171
APA StyleAlmeida-Bezerra, J. W., Menezes, S. A., Silva, J. T. d. C., de Sousa, S. G., Alves, D. S., Alencar, G. G., Araújo, I. M., Rodrigues, E. Y. d. S., Oliveira-Tintino, C. D. d. M., da Cruz, R. P., Rocha, J. E., Tintino, S. R., Barbosa-Filho, J. M., Morais-Braga, M. F. B., de Menezes, I. R. A., Raposo, A., & Coutinho, H. D. M. (2024). Analysis of the Antibiotic-Potentiating Activity, Absorption, Distribution, Metabolism, and Excretion (ADME) and the Molecular Docking Properties of Phytol Against Multi-Drug-Resistant (MDR) Strains. Antibiotics, 13(12), 1171. https://doi.org/10.3390/antibiotics13121171













