Rapid Antibacterial Assessments for Plastic and Textile Materials Against Escherichia coli
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Tests of Non-Porous Samples
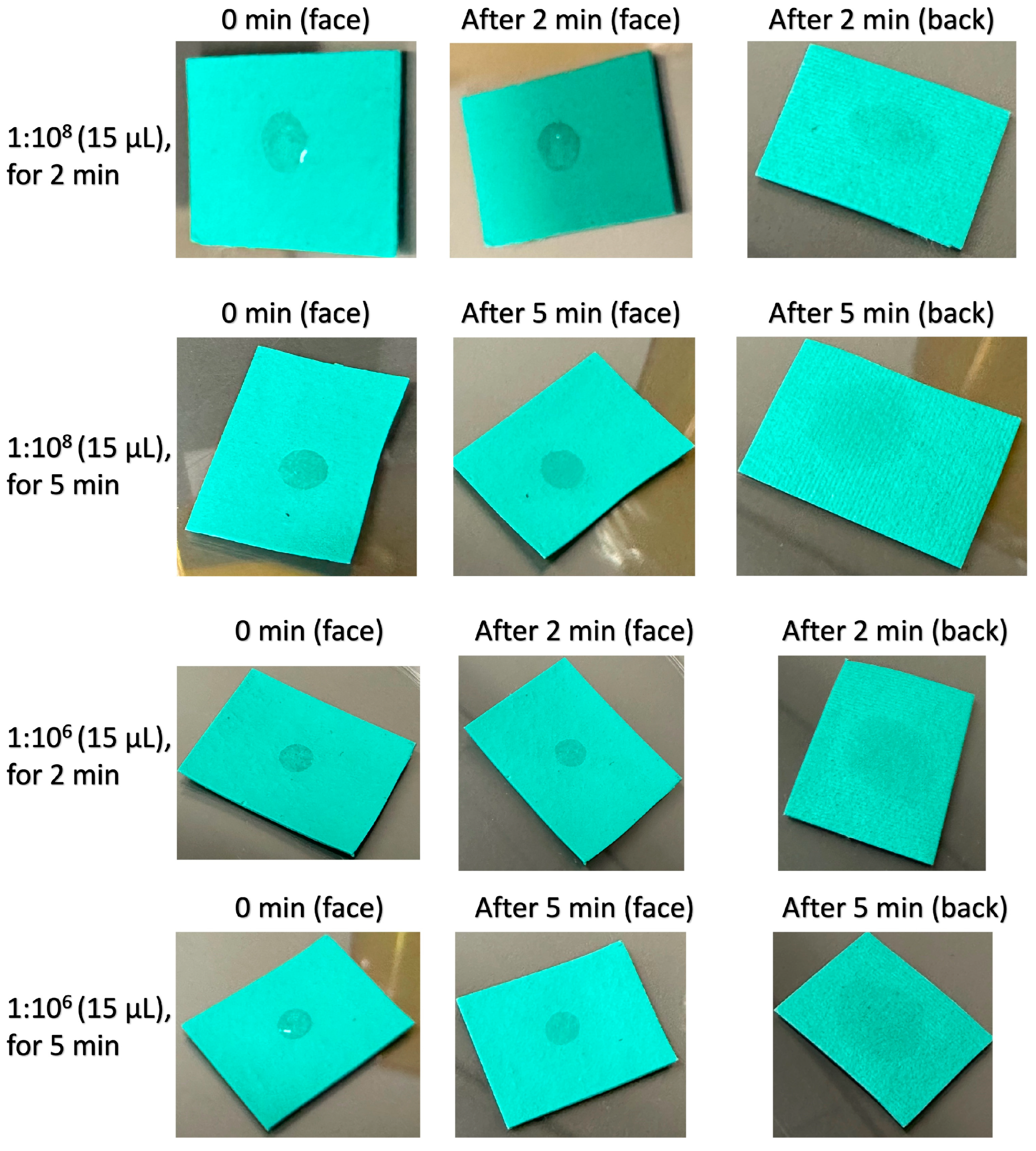
2.2. Antibacterial Tests of Textile Samples
2.3. Case Study About Assessing Highly Hydrophilic Polyurethane Plastic Samples by Rapid Tests
3. Materials and Methods
3.1. Materials
3.2. Antibacterial Assessments of Plastic Samples by ISO 22196:2011
3.3. Antibacterial Assessments of Textile Samples by AATCC TM-100
3.4. Rapid Test for Plastic Samples
3.5. Rapid Test for Textile Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISO 22196:2011 (en); Plastics—Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011.
- AATCC TM100-2019; Test Method for Antibacterial Finishes on Textile Materials: Assessment of. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2019.
- Perez-Gavilan, A.; de Castro, J.V.; Arana, A.; Merino, S.; Retolaza, A.; Alves, S.A.; Francone, A.; Kehagias, N.; Sotomayor-Torres, C.M.; Cocina, D. Antibacterial activity testing methods for hydrophobic patterned surfaces. Sci. Rep. 2021, 11, 6675. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial activity assessment of textiles: Standard methods comparison. Ann. Microbiol. 2011, 61, 493–498. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Bruyand, M.; Mariani-Kurkdjian, P.; Gouali, M.; De Valk, H.; King, L.; Le Hello, S.; Bonacorsi, S.; Loirat, C. Hemolytic uremic syndrome due to shiga toxin-producing Escherichia coli infection. Med. Mal. Infect. 2018, 48, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Krogvold, L.; Henrichsen, T.; Bjerre, A.; Brackman, D.; Dollner, H.; Gudmundsdottir, H.; Syversen, G.; Næss, P.A.; Bangstad, H.J. Clinical aspects of a nationwide epidemic of severe haemolytic uremic syndrome (hus) in children. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Kampmeier, S.; Berger, M.; Mellmann, A.; Karch, H.; Berger, P. The 2011 german enterohemorrhagic Escherichia coli o104: H4 outbreak—The danger is still out there. In Escherichia coli, a Versatile Pathogen; Springer: Cham, Switzerland, 2018; pp. 117–148. [Google Scholar]
- Who Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 October 2024).
- Maheux, A.F.; Dion-Dupont, V.; Bouchard, S.; Bisson, M.-A.; Bergeron, M.G.; Rodriguez, M.J. Comparison of four β-glucuronidase and β-galactosidase-based commercial culture methods used to detect Escherichia coli and total coliforms in water. J. Water Health 2015, 13, 340–352. [Google Scholar] [CrossRef]
- Wei, X.; Wu, Q.; Feng, Y.; Chen, M.; Zhang, S.; Chen, M.; Zhang, J.; Yang, G.; Ding, Y.; Yang, X. Off-on fluorogenic substrate harnessing esipt and aie features for in situ and long-term tracking of β-glucuronidase in Escherichia coli. Sens. Actuators B Chem. 2020, 304, 127242. [Google Scholar] [CrossRef]
- Meighan, P. Validation of the MicroSnap coliform and E. coli test system for enumeration and detection of coliforms and E. coli in a variety of foods. J. AOAC Int. 2014, 97, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Pala, L.; Sirec, T.; Spitz, U. Modified enzyme substrates for the detection of bacteria: A review. Molecules 2020, 25, 3690. [Google Scholar] [CrossRef]
- Nayak, R.S. Comparison of the Novel MicroSnap™ Coliform Test Kit with the 3M™ Petri-films. Master’s Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Renaud, D.; Kelton, D.; LeBlanc, S.; Haley, D.; Jalbert, A.; Duffield, T. Validation of commercial luminometry swabs for total bacteria and coliform counts in colostrum-feeding equipment. J. Dairy Sci. 2017, 100, 9459–9465. [Google Scholar] [CrossRef] [PubMed]
- Owuama, C.I. Determination of minimum inhibitory concentration (mic) and minimum bactericidal concentration (mbc) using a novel dilution tube method. Afr. J. Microbiol. Res. 2017, 11, 977–980. [Google Scholar]
- Hong, K.H.; Kim, S. Objective evaluation of antimicrobial property of textile materials using image analysis. Fibers Polym. 2011, 12, 1048–1053. [Google Scholar] [CrossRef]
- Xia, W.; Wang, H.; Lu, Q.; Tan, Y.; Zhao, P. Study on the effectiveness test of bacterial activity in the antibacterial property vibration test of antibacterial textiles. J. Biobased Mater. Bioenergy 2019, 13, 140–143. [Google Scholar] [CrossRef]
- Campos, M.D.; Zucchi, P.C.; Phung, A.; Leonard, S.N.; Hirsch, E.B. The activity of antimicrobial surfaces varies by testing protocol utilized. PLoS ONE 2016, 11, e0160728. [Google Scholar] [CrossRef]
- Haase, H.; Jordan, L.; Keitel, L.; Keil, C.; Mahltig, B. Comparison of methods for determining the effectiveness of antibacterial functionalized textiles. PLoS ONE 2017, 12, e0188304. [Google Scholar] [CrossRef]
- Hilgenberg, B.; Vossebein, L. Test method dependent efficacy of antibacterial textiles. Tenside Surfact. Det. 2018, 55, 398–403. [Google Scholar] [CrossRef]
- Cunliffe, A.J.; Askew, P.D.; Stephan, I.; Iredale, G.; Cosemans, P.; Simmons, L.M.; Verran, J.; Redfern, J. How do we determine the efficacy of an antibacterial surface? A review of standardised antibacterial material testing methods. Antibiotics 2021, 10, 1069. [Google Scholar]
- Maitz, S.; Poelzl, S.; Dreisiebner, D.; Zarschenas, E.; Kittinger, C. Antimicrobial non-porous surfaces: A comparison of the standards iso 22196: 2011 and the recently published ISO 7581:2023. Front. Microbiol. 2024, 15, 1400265. [Google Scholar] [CrossRef]
- Cai, L.; Wu, D.; Xia, J.; Shi, H.; Kim, H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci. Total Environ. 2019, 671, 1101–1107. [Google Scholar] [CrossRef]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, K.S.; Taylor, M.; Castillo, A.; Cisneros-Zevallos, L.; Oh, J.K. Influence of surface roughness, nanostructure, and wetting on bacterial adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef] [PubMed]
- Khezripour, A.R.; Souri, D.; Tavafi, H.; Ghabooli, M. Serial dilution bioassay for the detection of antibacterial potential of znse quantum dots and their fourier transform infra-red spectroscopy. Measurement 2019, 148, 106939. [Google Scholar] [CrossRef]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the technical performance of leather, artificial leather, and trendy alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]
- Sudha, T.B.; Thanikaivelan, P.; Aaron, K.P.; Krishnaraj, K.; Chandrasekaran, B. Comfort, chemical, mechanical, and structural properties of natural and synthetic leathers used for apparel. J. Appl. Polym. Sci. 2009, 114, 1761–1767. [Google Scholar] [CrossRef]
- Roh, E.K.; Oh, K.W. The subjective hand and preferences evaluation of artificial leather by use. Fash. Text. Res. J. 2017, 19, 79–89. [Google Scholar] [CrossRef]
- MicroSnap™–Coliform and E. coli. Available online: https://hardydiagnostics.com/media/assets/product/documents/MS1CEC_MS2ECOLI_MS2COLIFORM%20IFU.pdf (accessed on 28 February 2022).
- Comparing Total Viable Counts from Traditional Plates with MicroSnap® Values. Available online: https://www.hygiena.com/documents/66466/microsnap-vs-plating-bespoke-standard-curve.pdf (accessed on 28 February 2022).
| Plastic Substrate | Sample Type | CFU/mL of E. coli at Contact Hours | |
|---|---|---|---|
| 0 h | 24 h | ||
| Silicone rubber | Non-antimicrobial | 8.00 × 108 | 2.47 × 109 |
| Antimicrobial | 0 | 0 | |
| High-density polyethylene | Non-antimicrobial | 6.00 × 108 | 1.52 × 109 |
| Antimicrobial | 0 | 0 | |
| 3D-printed rigid plastic | Non-antimicrobial | 1.11 × 109 | 1.80 × 109 |
| Antimicrobial | 0 | 0 | |
| 3D-printed tough plastic | Non-antimicrobial | 1.12 × 109 | 1.84 × 109 |
| Antimicrobial | 0 | 0 | |
| Concentration of E. coli Solution Inoculated | |||||
|---|---|---|---|---|---|
| Plastic Substrate | Swabbed Sample | 1:107 | 1:108 | ||
| RLU | CFU/Swab | RLU | CFU/Swab | ||
| Silicone rubber | Direct pipetting | 696 | >>1.00 × 104 | 1525 | >>1.00 × 104 |
| Swabbing | 1251 | >>1.00 × 104 | 1670 | >>1.00 × 104 | |
| Non-antimicrobial | 1763 | >>1.00 × 104 | 2926 | >>1.00 × 104 | |
| Antimicrobial | 1 | <5 | 5 | <15 | |
| High-density polyethylene | Direct pipetting | 1747 | >>1.00 × 104 | 1402 | >>1.00 × 104 |
| Swabbing | 2735 | >>1.00 × 104 | 715 | >>1.00 × 104 | |
| Non-antimicrobial | 2113 | >>1.00 × 104 | 753 | >>1.00 × 104 | |
| Antimicrobial | 0.3 | <5 | 0 | <1 | |
| 3D-printed rigid plastic | Direct pipetting | 440 | >>1.00 × 104 | 1746 | >>1.00 × 104 |
| Swabbing | 1357 | >>1.00 × 104 | 3048 | >>1.00 × 104 | |
| Non-antimicrobial | 1244 | >>1.00 × 104 | 3404 | >>1.00 × 104 | |
| Antimicrobial | 0 | <1 | 5 | <35 | |
| 3D-printed tough plastic | Direct pipetting | 1584 | >>1.00 × 104 | 209 | >>1.00 × 104 |
| Swabbing | 269 | >>1.00 × 104 | 1445 | >>1.00 × 104 | |
| Non-antimicrobial | 1989 | >>1.00 × 104 | 2224 | >>1.00 × 104 | |
| Antimicrobial | 0 | <1 | 0 | <1 | |
| CAA % (w/w) on Cotton Fabrics | CFU/mL of E. coli Collected at Different Hours | |
|---|---|---|
| 0 h | 24 h | |
| 0 | 9.08 × 108 | 3.50 × 109 |
| 0.5 | 0 | 0 |
| 1.5 | 0 | 0 |
| Concentration of E. coli Solution | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:108 | 1:107 | 1:106 | |||||||
| Volume of E. coli Solution (µL) | 20.1 | 15.1 | 10.1 | 30.1 | 20.1 | 10.1 | 30.1 | 20.1 | 10.1 |
| Swab Sample | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) |
| Direct pipetting to saline solution # | 5551 (>>1.00 × 104) | 4007 (>>1.00 × 104) | 4491 (>>1.00 × 104) | 3500 (>>1.00 × 104) | 4747 (>>1.00 × 104) | 4245 (>>1.00 × 104) | 1386 (>>1.00 × 104) | 730 (>>1.00 × 104) | 180 (<5100) |
| Pristine cotton fabric | 4602 (>>1.00 × 104) | 3855 (>>1.00 × 104) | 3532 (>>1.00 × 104) | 3355 (>>1.00 × 104) | 3113 (>>1.00 × 104) | 1887 (>>1.00 × 104) | 548 (>>1.00 × 104) | 15 (<140) | 1 (<5) |
| Cotton fabric with 0.5% CAA | 0 (<1) | 2 (10) | 0 (<1) | 0 (<1) | 1 (<5) | 3 (<15) | 0 (<1) | 0 (<1) | 0 (<1) |
| Cotton fabric with 1.5% CAA | 0 (<1) | 0 (<1) | 1 (<5) | 0 (<1) | 1 (<5) | 1 (<5) | 0 (<1) | 0 (<1) | 0 (<1) |
| Concentration of E. coli Solution (Volume of Inoculum) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:108 (20.1 µL) | 1:107 (30.1 µL) | 1:106 (30.1 µL) | |||||||
| Dilution Factor | 1 | 10−1 | 10−2 | 1 | 10−1 | 10−2 | 1 | 10−1 | 10−2 |
| Swab Sample | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) |
| Direct pipetting to saline solution # | 5255 (>>1.00 × 104) | 4167 (>>1.00 × 104) | 32 (<450) | 3862 (>>1.00 × 104) | 513 (>>1.00 × 104) | 3 (<15) | 133 (<3230) | 0 (<1) | 0 (<1) |
| Pristine cotton fabric | 3206 (>>1.00 × 104) | 2652 (>>1.00 × 104) | 20 (<200) | 3803 (>>1.00 × 104) | 34 (<480) | 2 (<10) | 4 (<20) | 1 (<5) | 2 (<10) |
| Cotton fabric with 0.5% CAA | 0 (<1) | 2 (<10) | 2 (<10) | 1 (<5) | 1 (<5) | 1 (<5) | 2 (<10) | 1 (<5) | 1 (<5) |
| Cotton fabric with 1.5% CAA | 1 (<5) | 0 (<1) | 0 (<5) | 1 (<5) | 3 (<15) | 1 (<5) | 0 (<1) | 0 (<1) | 0 (<1) |
| Concentration and Volume of E. coli Solution | Rapid Test for Plastics | Rapid Test for Textiles # | ||
|---|---|---|---|---|
| 1:107 (30.1 µL) | 1:108 (20.1 µL) | 1:107 (30.1 µL) | 1:108 (20.1 µL) | |
| RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | RLU (CFU/Swab) | |
| Direct pipetting @ | 893 (>>1.00 × 104) | 2386 (>>1.00 × 104) | 3608 (>>1.00 × 104) | 3203 (>>1.00 × 104) |
| Swabbing | 1607 (>>1.00 × 104) | 5076 (>>1.00 × 104) | N.A. | N.A. |
| PU with 0% CAA | 2 (<10) | 1 (<5) | 2190 (>>1.00 × 104) | 4754 (>>1.00 × 104) |
| PU with 1% CAA | 1 (<5) | 0 (<1) | 0 (<1) | 5 (<30) |
| PU with 2% CAA | 2 (<10) | 0 (<1) | 1.3 (<10) | 3.7 (<20) |
| CAA% (w/w) on PU Leathers | CFU/mL of E. coli Collected at Different Hours | |
|---|---|---|
| 0 h | 24 h | |
| 0 | 4.20 × 108 | 6.40 × 108 |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| Volume of E. coli Inoculum (µL) | Volume of 0.85% (w/v) Saline Solution (mL) |
|---|---|
| 30.1 | 2.98 |
| 20.1 | 2.99 |
| 15.1 | 2.99 |
| 10.1 | 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luk, A.M.Y.; Luk, A.M.H.; Chiou, J.A.; Ho, M.-Y.; Ngai, C.-M.; Kan, C.-W. Rapid Antibacterial Assessments for Plastic and Textile Materials Against Escherichia coli. Antibiotics 2024, 13, 1156. https://doi.org/10.3390/antibiotics13121156
Luk AMY, Luk AMH, Chiou JA, Ho M-Y, Ngai C-M, Kan C-W. Rapid Antibacterial Assessments for Plastic and Textile Materials Against Escherichia coli. Antibiotics. 2024; 13(12):1156. https://doi.org/10.3390/antibiotics13121156
Chicago/Turabian StyleLuk, Anson M. Y., Adrian M. H. Luk, Jiachi Amber Chiou, Man-Yi Ho, Chi-Man Ngai, and Chi-Wai Kan. 2024. "Rapid Antibacterial Assessments for Plastic and Textile Materials Against Escherichia coli" Antibiotics 13, no. 12: 1156. https://doi.org/10.3390/antibiotics13121156
APA StyleLuk, A. M. Y., Luk, A. M. H., Chiou, J. A., Ho, M.-Y., Ngai, C.-M., & Kan, C.-W. (2024). Rapid Antibacterial Assessments for Plastic and Textile Materials Against Escherichia coli. Antibiotics, 13(12), 1156. https://doi.org/10.3390/antibiotics13121156







