Impact of Predation by Ciliate Tetrahymena borealis on Conjugation in Aeromonas salmonicida subsp. salmonicida
Abstract
1. Introduction
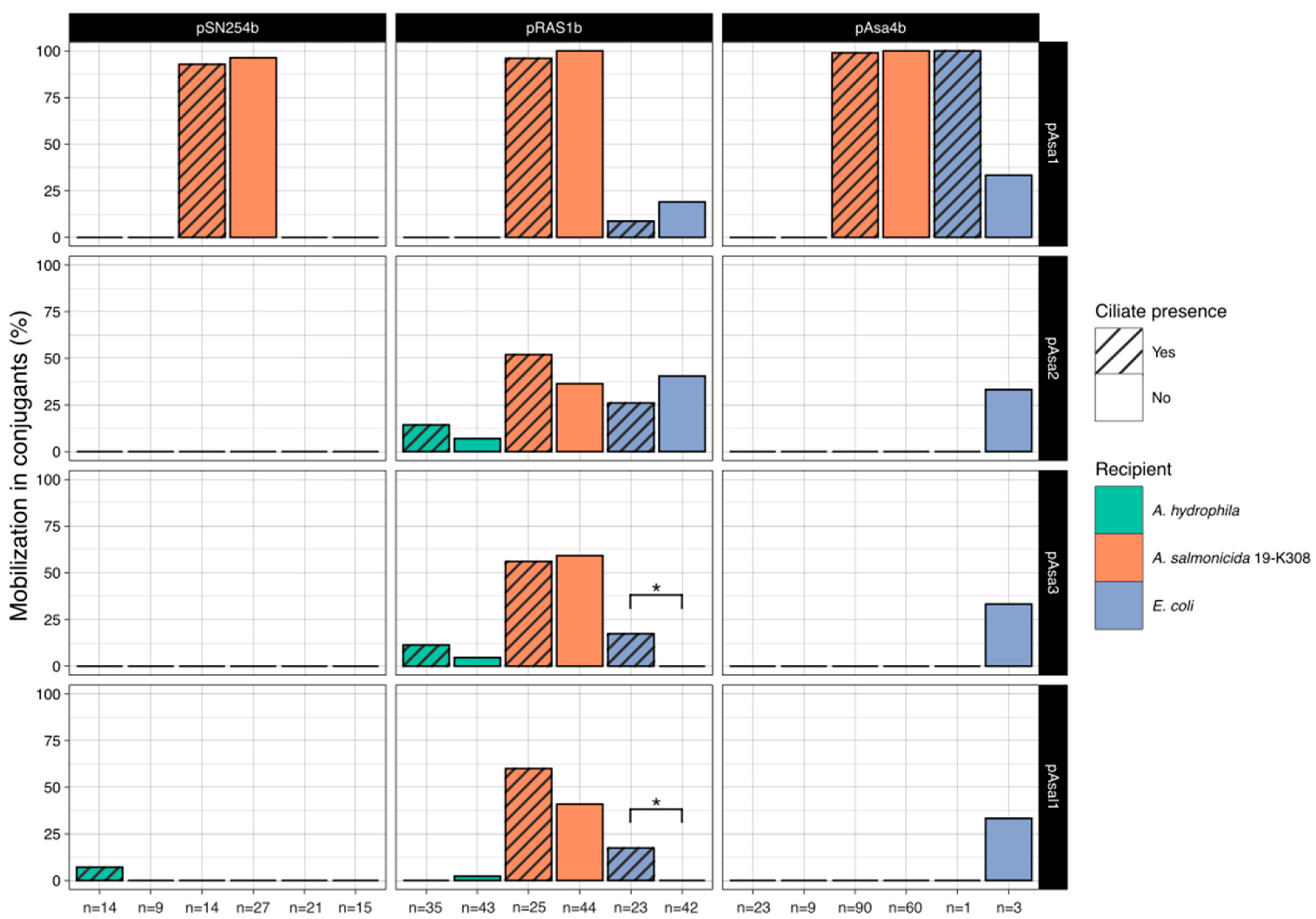
2. Results
3. Discussion
4. Materials and Methods
4.1. Ciliates and Bacteria
4.2. Cocultures and Conjugation Assay
4.3. PCR Genotyping
4.4. DAPI Staining and Microscopy
4.5. Sequence Analysis of Conjugative Plasmids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matz, C.; Kjelleberg, S. Off the Hook—How Bacteria Survive Protozoan Grazing. Trends Microbiol. 2005, 13, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Denoncourt, A.M.; Paquet, V.E.; Charette, S.J. Potential Role of Bacteria Packaging by Protozoa in the Persistence and Transmission of Pathogenic Bacteria. Front. Microbiol. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koubar, M.; Rodier, M.H.; Garduño, R.A.; Frère, J. Passage through Tetrahymena Tropicalis Enhances the Resistance to Stress and the Infectivity of Legionella Pneumophila. FEMS Microbiol. Lett. 2011, 325, 10–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Dong, Y.; Wang, N.; Li, S.; Yang, Y.; Wang, Y.; Awan, F.; Lu, C.; Liu, Y. Tetrahymena thermophila Predation Enhances Environmental Adaptation of the Carp Pathogenic Strain Aeromonas hydrophila NJ-35. Front. Cell Infect. Microbiol. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Örmälä-Odegrip, A.-M.; Ojala, V.; Hiltunen, T.; Zhang, J.; Kh Bamford, J.; Laakso, J. Protist Predation Can Select for Bacteria with Lowered Susceptibility to Infection by Lytic Phages. BMC Evol. Biol. 2015, 15, 81. [Google Scholar] [CrossRef]
- Oguri, S.; Matsuo, J.; Hayashi, Y.; Nakamura, S.; Hanawa, T.; Fukumoto, T.; Mizutani, Y.; Yao, T.; Akizawa, K.; Suzuki, H.; et al. Ciliates Promote the Transfer of the Gene Encoding the Extended-Spectrum b-Lactamase CTX-M-27 between Escherichia coli Strains. J. Antimicrob. Chemother. 2011, 66, 527–530. [Google Scholar] [CrossRef]
- Matsuo, J.; Oguri, S.; Nakamura, S.; Hanawa, T.; Fukumoto, T.; Hayashi, Y.; Kawaguchi, K.; Mizutani, Y.; Yao, T.; Akizawa, K.; et al. Ciliates Rapidly Enhance the Frequency of Conjugation between Escherichia coli Strains through Bacterial Accumulation in Vesicles. Res. Microbiol. 2010, 161, 711–719. [Google Scholar] [CrossRef]
- Okubo, T.; Matushita, M.; Ohara, Y.; Matsuo, J.; Oguri, S.; Fukumoto, T.; Hayasaka, K.; Akizawa, K.; Shibuya, H.; Shimizu, C.; et al. Ciliates Promote the Transfer of a Plasmid Encoding BlaNDM-5 from Escherichia coli, Isolated from a Hospital in Japan, to Other Human Pathogens. Int. J. Antimicrob. Agents 2017, 49, 387–388. [Google Scholar] [CrossRef]
- Matsushita, M.; Okubo, T.; Hasegawa, T.; Matsuo, J.; Watanabe, T.; Iwasaki, S.; Fukumoto, T.; Hayasaka, K.; Akizawa, K.; Shimizu, C.; et al. Tetrahymena Promotes Interactive Transfer of Carbapenemase Gene Encoded in Plasmid between Fecal Escherichia coli and Environmental Aeromonas caviae. Microbiol. Immunol. 2018, 62, 720–728. [Google Scholar] [CrossRef]
- Fenchel, T. Suspension Feeding in Ciliated Protozoa: Feeding Rates and Their Ecological Significance. Microb. Ecol. 1980, 6, 13–25. [Google Scholar] [CrossRef]
- Cairns, J.; Jalasvuori, M.; Ojala, V.; Brockhurst, M.; Hiltunen, T. Conjugation Is Necessary for a Bacterial Plasmid to Survive under Protozoan Predation. Biol. Lett. 2016, 12, 20150953. [Google Scholar] [CrossRef] [PubMed]
- Durocher, A.F.; Denoncourt, A.M.; Paquet, V.E.; Charette, S.J. Evidence That Bacteria Packaging by Tetrahymena Is a Widespread Phenomenon. Microorganisms 2020, 8, 1548. [Google Scholar] [CrossRef] [PubMed]
- Paquet, V.E.; Durocher, A.F.; Charette, S.J. Aeromonas salmonicida Intraspecies Divergence Revealed by the Various Strategies Displayed When Grazed by Tetrahymena pyriformis. FEMS Microbiol. Lett. 2022, 369, fnac067. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish, 6th ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319326740. [Google Scholar]
- Morin, R. L’utilisation Des Antibiotiques Pour Combattre La Furonculose Chez l’omble de Fontaine Génère de l’antibiorésistance Chez Aeromonas salmonicida. L’aquicole 2010, 15, 2–6. [Google Scholar]
- Trudel, M.V.; Vincent, A.T.; Attéré, S.A.; Labbé, M.; Derome, N.; Culley, A.I.; Charette, S.J. Diversity of Antibiotic-Resistance Genes in Canadian Isolates of Aeromonas salmonicida subsp. salmonicida: Dominance of PSN254b and Discovery of PAsa8. Sci. Rep. 2016, 6, 35617. [Google Scholar] [CrossRef]
- Fournier, K.C.; Paquet, V.E.; Attéré, S.A.; Farley, J.; Marquis, H.; Gantelet, H.; Ravaille, C.; Vincent, A.T.; Charette, S.J. Expansion of the PRAS3 Plasmid Family in Aeromonas salmonicida subsp. salmonicida and Growing Evidence of Interspecies Connections for These Plasmids. Antibiotics 2022, 11, 1047. [Google Scholar] [CrossRef]
- Vincent, A.T.; Hosseini, N.; Charette, S.J. The Aeromonas salmonicida Plasmidome: A Model of Modular Evolution and Genetic Diversity. Ann. N. Y. Acad. Sci. 2020, 1488, 16–32. [Google Scholar] [CrossRef]
- Belland, R.J.; Trust, T.J. Aeromonas salmonicida Plasmids: Plasmid-Directed Synthesis of Proteins in Vitro and in Escherichia coli Minicells. Microbiology 1989, 135, 513–524. [Google Scholar] [CrossRef]
- Boyd, J.; Williams, J.; Curtis, B.; Kozera, C.; Singh, R.; Reith, M. Three Small, Cryptic Plasmids from Aeromonas salmonicida subsp. salmonicida A449. Plasmid 2003, 50, 131–144. [Google Scholar] [CrossRef]
- Reith, M.E.; Singh, R.K.; Curtis, B.; Boyd, J.M.; Bouevitch, A.; Kimball, J.; Munholland, J.; Murphy, C.; Sarty, D.; Williams, J.; et al. The Genome of Aeromonas salmonicida subsp. salmonicida A449: Insights into the Evolution of a Fish Pathogen. BMC Genom. 2008, 9, 427. [Google Scholar] [CrossRef]
- Attéré, S.A.; Vincent, A.T.; Trudel, M.V.; Chanut, R.; Charette, S.J. Diversity and Homogeneity among Small Plasmids of Aeromonas salmonicida subsp. salmonicida Linked with Geographical Origin. Front. Microbiol. 2015, 6, 1274. [Google Scholar] [CrossRef]
- Attéré, S.A.; Vincent, A.T.; Paccaud, M.; Frenette, M.; Charette, S.J. The Role for the Small Cryptic Plasmids as Moldable Vectors for Genetic Innovation in Aeromonas salmonicida subsp. salmonicida. Front. Genet. 2017, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Trudel, M.V.; Paquet, V.E.; Boyle, B.; Tanaka, K.H.; Dallaire-Dufresne, S.; Daher, R.K.; Frenette, M.; Derome, N.; Charette, S.J. Detection of Variants of the PRAS3, PAB5S9, and PSN254 Plasmids in Aeromonas salmonicida subsp. salmonicida: Multidrug Resistance, Interspecies Exchanges, and Plasmid Reshaping. Antimicrob. Agents Chemother. 2014, 58, 7367–7374. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, M.-A.; Vincent, A.T.; Schneider, A.; Paquet, V.E.; Frenette, M.; Charette, S.J. One Aeromonas salmonicida subsp. salmonicida Isolate with a PAsa5 Variant Bearing Antibiotic Resistance and a PRAS3 Variant Making a Link with a Swine Pathogen. Sci. Total Environ. 2019, 690, 313–320. [Google Scholar] [CrossRef]
- Vincent, A.T.; Charette, S.J. To Be or Not to Be Mesophilic, That Is the Question for Aeromonas salmonicida. Microorganisms 2022, 10, 240. [Google Scholar] [CrossRef]
- Nanney, D.L.; McCoy, J.W. Characterization of the Species of the Tetrahymena pyriformis Complex. Trans. Am. Microsc. Soc. 1976, 95, 664–682. [Google Scholar] [CrossRef]
- Vaillancourt, K.C.; Paquet, V.E.; Vincent, A.T.; Schneider, A.; Thompson, C.; Laurent, M.; Frenette, M.; Charette, S.J. Draft Genome Sequence of an Aeromonas salmonicida subsp. salmonicida Strain from the Canadian Pacific Coast Bearing a Variant of PRAS1. Microbiol. Resour. Announc. 2021, 10, e00291-21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.H.; Vincent, A.T.; Trudel, M.V.; Paquet, V.E.; Frenette, M.; Charette, S.J. The Mosaic Architecture of Aeromonas salmonicida subsp. salmonicida PAsa4 Plasmid and Its Consequences on Antibiotic Resistance. PeerJ 2016, 2016, e2595. [Google Scholar] [CrossRef]
- Cury, J.; Abby, S.S.; Doppelt-Azeroual, O.; Néron, B.; Rocha, E.P.C. Identifying Conjugative Plasmids and Integrative Conjugative Elements with CONJscan. In Horizontal Gene Transfer—Methods in Molecular Biology; de la Cruz, F., Ed.; Humana Press Inc.: New York, NY, USA, 2019; Volume 2075, pp. 265–283. [Google Scholar]
- Garcillán-Barcia, M.P.; Redondo-Salvo, S.; Vielva, L.; de la Cruz, F. MOBscan: Automated Annotation of MOB Relaxases. Methods Mol. Biol. 2020, 2075, 295–308. [Google Scholar] [CrossRef]
- Vincent, A.T.; Trudel, M.V.; Freschi, L.; Nagar, V.; Gagné-Thivierge, C.; Levesque, R.C.; Charette, S.J. Increasing Genomic Diversity and Evidence of Constrained Lifestyle Evolution Due to Insertion Sequences in Aeromonas salmonicida. BMC Genom. 2016, 17, 44. [Google Scholar] [CrossRef]
- Attéré, S.A.; Gagné-Thivierge, C.; Paquet, V.E.; Leduc, G.R.; Vincent, A.T.; Charette, S.J. Aeromonas salmonicida Isolates from Canada Demonstrate Wide Distribution and Clustering among Mesophilic Strains. Genome 2023, 66, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.N.; Jessee, J.; Bloom, F.R.; Hanahan, D. Differential Plasmid Rescue from Transgenic Mouse DNAs into Escherichia coli Methylation-Restriction Mutants. Proc. Natl. Acad. Sci. USA 1990, 87, 4645–4649. [Google Scholar] [CrossRef]
- Mcintosh, D.; Cunningham, M.; Ji, B.; Fekete, F.A.; Parry, E.M.; Clark, S.E.; Zalinger, Z.B.; Gilg, I.C.; Danner, G.R.; Johnson, K.A.; et al. Transferable, Multiple Antibiotic and Mercury Resistance in Atlantic Canadian Isolates of Aeromonas salmonicida subsp. salmonicida Is Associated with Carriage of an IncA/C Plasmid Similar to the Salmonella enterica Plasmid PSN254. J. Antimicrob. Chemother. 2008, 61, 1221–1228. [Google Scholar] [CrossRef]
- Smillie, C.; Garcillán-Barcia, M.P.; Francia, M.V.; Rocha, E.P.C.; de la Cruz, F. Mobility of Plasmids. Microbiol. Mol. Biol. Rev. 2010, 74, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Lin, X.; Liu, J.; Guo, C.; Gao, S.; Du, H.; Lu, C.; Liu, Y. Identification of Aeromonas hydrophila Genes Preferentially Expressed after Phagocytosis by Tetrahymena and Involvement of Methionine Sulfoxide Reductases. Front. Cell Infect. Microbiol. 2016, 6. [Google Scholar] [CrossRef]
- Bastos Gomes, G.; Jerry, D.R.; Miller, T.L.; Hutson, K.S. Current Status of Parasitic Ciliates Chilodonella spp. (Phyllopharyngea: Chilodonellidae) in Freshwater Fish Aquaculture. J. Fish. Dis. 2017, 40, 703–715. [Google Scholar] [CrossRef]
- Li, M.; Bastos Gomes, G.; Zhao, W.; Hu, G.; Huang, K.; Yoshinaga, T.; Clark, T.G.; Li, W.; Zou, H.; Wu, S.; et al. Cultivation of Fish Ciliate Parasites: Progress and Prospects. Rev. Aquac. 2023, 15, 142–162. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P. Fish Immunity and Parasite Infections: From Innate Immunity to Immunoprophylactic Prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef]
- Pinheiro, M.D.O.; Bols, N.C. Delineating Cellular Interactions between Ciliates and Fish by Co-Culturing Tetrahymena thermophila with Fish Cells. Cell Biol. Int. 2014, 38, 1138–1147. [Google Scholar] [CrossRef]
- Lupo, A.; Coyne, S.; Berendonk, T.U. Origin and Evolution of Antibiotic Resistance: The Common Mechanisms of Emergence and Spread in Water Bodies. Front. Microbiol. 2012, 3, 18. [Google Scholar] [CrossRef]
- De La Cruz, F.; Frost, L.S.; Meyer, R.J.; Zechner, E.L. Conjugative DNA Metabolism in Gram-Negative Bacteria. FEMS Microbiol. Rev. 2010, 34, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowski, R.; Bekel, T.; Goesmann, A.; Krause, L.; Krömeke, H.; Kaiser, O.; Eichler, W.; Pühler, A.; Schlüter, A. Insight into the Plasmid Metagenome of Wastewater Treatment Plant Bacteria Showing Reduced Susceptibility to Antimicrobial Drugs Analysed by the 454-Pyrosequencing Technology. J. Biotechnol. 2008, 136, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Hanley, D.; Bowen, J.; Lee, J.H.; Cole, E.; VerPlank, L.A.; Gaertig, J.; Gorovsky, M.A.; Bruns, P.J. Germline and Somatic Transformation of Mating Tetrahymena thermophila by Particle Bombardment. Genetics 1997, 146, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Page, F.C. An Illustrated Key to Freshwater and Soil Amoebae: With Notes on Cultivation and Ecology; Freshwater Biological Association, Ed.; Freshwater Biological Association: Ambleside, UK, 1976; ISBN 9780900386268. [Google Scholar]
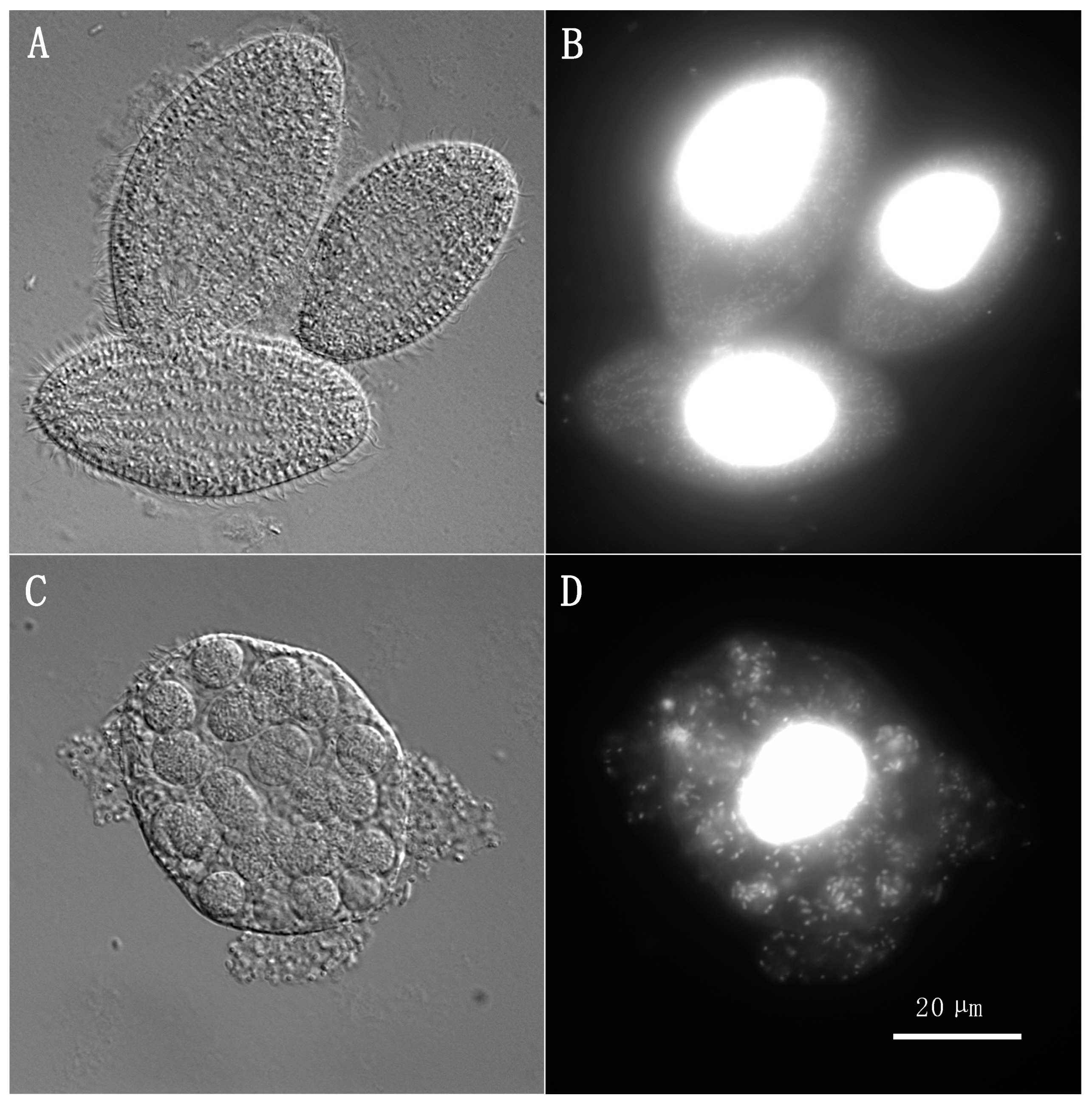
| Plasmid | Recipient | Ratio A 1:104 | Ratio B 1:105 | Ratio C 1:106 | Ratio D 1:107 | Basal Rate Control | Negative Control |
|---|---|---|---|---|---|---|---|
| pSN254b | A. hydrophila | − | − | ++ | +++ | ++ | − |
| A. s. 19-K308 | +++ | − | ++ | ++ | ++ | − | |
| E. coli | +++ | − | +++ | +++ | +++ | − | |
| pRAS1b | A. hydrophila | − | − | ++ | ++ | ++ | − |
| A. s. 19-K308 | − | − | +++ | +++ | +++ | − | |
| E. coli | − | − | +++ | +++ | +++ | − | |
| pAsa4b | A. hydrophila | − | − | + | + | + | − |
| A. s. 19-K308 | − | − | ++ | ++ | ++ | − | |
| E. coli | − | − | + | − | − | − |
| Strain a | Role in Conjugation Assay | Lifestyle | Origin | ARGs Carried by Conjugative Plasmid |
|---|---|---|---|---|
| A. salmonicida subsp. salmonicida 2004-05 MF26 | Donor (plasmid pSN254b) | Psychrophilic | New Brunswick, Canada [24] | tetA, floR, sul1, sul2, blaCMY, aadA, strA, strB |
| A. salmonicida subsp. salmonicida 2004-072 | Donor (plasmid pRAS1b) | Psychrophilic | British Columbia, Canada [28] | tetA, sul1 |
| A. salmonicida subsp. salmonicida SHY13-2627 | Donor (plasmid pAsa4b) | Psychrophilic | Québec, Canada [29] | tetA(E), sul1 |
| A. salmonicida 19-K308 | Recipient | Mesophilic | Québec, Canada [33] | N/A |
| A. hydrophila HER1210 (ATCC 7966) | Recipient | Mesophilic | ATCC/Félix d’Hérelle reference center | N/A |
| E. coli DH5α | Recipient | Mesophilic | [34] | N/A |
| Ratio A 1:104 | Ratio B 1:105 | Ratio C 1:106 | Ratio D 1:107 | Basal Rate Control | Negative Control | |
|---|---|---|---|---|---|---|
| Donor bacteria | A. salmonicida subsp. salmonicida (2004-05 MF26, 2004-072 or SHY13-2627) (5 × 108 CFU) | N/A | ||||
| Recipient bacteria | A. salmonicida 19-K308, A. hydrophila or E. coli (5 × 108 CFU) | |||||
| T. borealis | 105 cells | 104 cells | 103 cells | 102 cells | N/A | |
| Target | Forward Primer Sequence | Reverse Primer Sequence | Reference |
|---|---|---|---|
| pSN254b (blaCMY gene) | GACAGCCTCTTTCTCCACATTTGC | CTGGTCATTGCCTCTTCGTAACTC | This study |
| pRAS1b (backbone) | TGATATGGACAGCCACAAATG | CTTCACCGATCACGTCTTTG | [28] |
| pAsa4b (backbone) | ACTTATAAGACCATCCTGACGGC | TGAGATATCTTTCCAGTCCACACC | [29] |
| pAsa1 (toxin- antitoxin region) | GGACGATTAACCTTCGCATC | GTATCGCCCAACTTCTTCCA | [20] |
| pAsa2 (hypothetical protein gene) | AAAAGAGCGTGCAACCCTAA | GCGATGCTACTTCATTCACC | [20] |
| pAsa3 (toxin- antitoxin region) | TCATGGAGAATGTTCGCAAG | GCCCAATTATCACAGCAACA | [20] |
| pAsal1 (aopP gene) | TAACATGGGTGAGTCAGGA | TGCATGTTTGTAAAAAGTAGGTG | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durocher, A.F.; Paquet, V.E.; St-Laurent, R.E.; Duchaine, C.; Charette, S.J. Impact of Predation by Ciliate Tetrahymena borealis on Conjugation in Aeromonas salmonicida subsp. salmonicida. Antibiotics 2024, 13, 960. https://doi.org/10.3390/antibiotics13100960
Durocher AF, Paquet VE, St-Laurent RE, Duchaine C, Charette SJ. Impact of Predation by Ciliate Tetrahymena borealis on Conjugation in Aeromonas salmonicida subsp. salmonicida. Antibiotics. 2024; 13(10):960. https://doi.org/10.3390/antibiotics13100960
Chicago/Turabian StyleDurocher, Alicia F., Valérie E. Paquet, Rébecca E. St-Laurent, Caroline Duchaine, and Steve J. Charette. 2024. "Impact of Predation by Ciliate Tetrahymena borealis on Conjugation in Aeromonas salmonicida subsp. salmonicida" Antibiotics 13, no. 10: 960. https://doi.org/10.3390/antibiotics13100960
APA StyleDurocher, A. F., Paquet, V. E., St-Laurent, R. E., Duchaine, C., & Charette, S. J. (2024). Impact of Predation by Ciliate Tetrahymena borealis on Conjugation in Aeromonas salmonicida subsp. salmonicida. Antibiotics, 13(10), 960. https://doi.org/10.3390/antibiotics13100960







