Serotype Distribution and Antimicrobial Resistance of Salmonella Isolates from Poultry Sources in China
Abstract
1. Introduction
2. Results
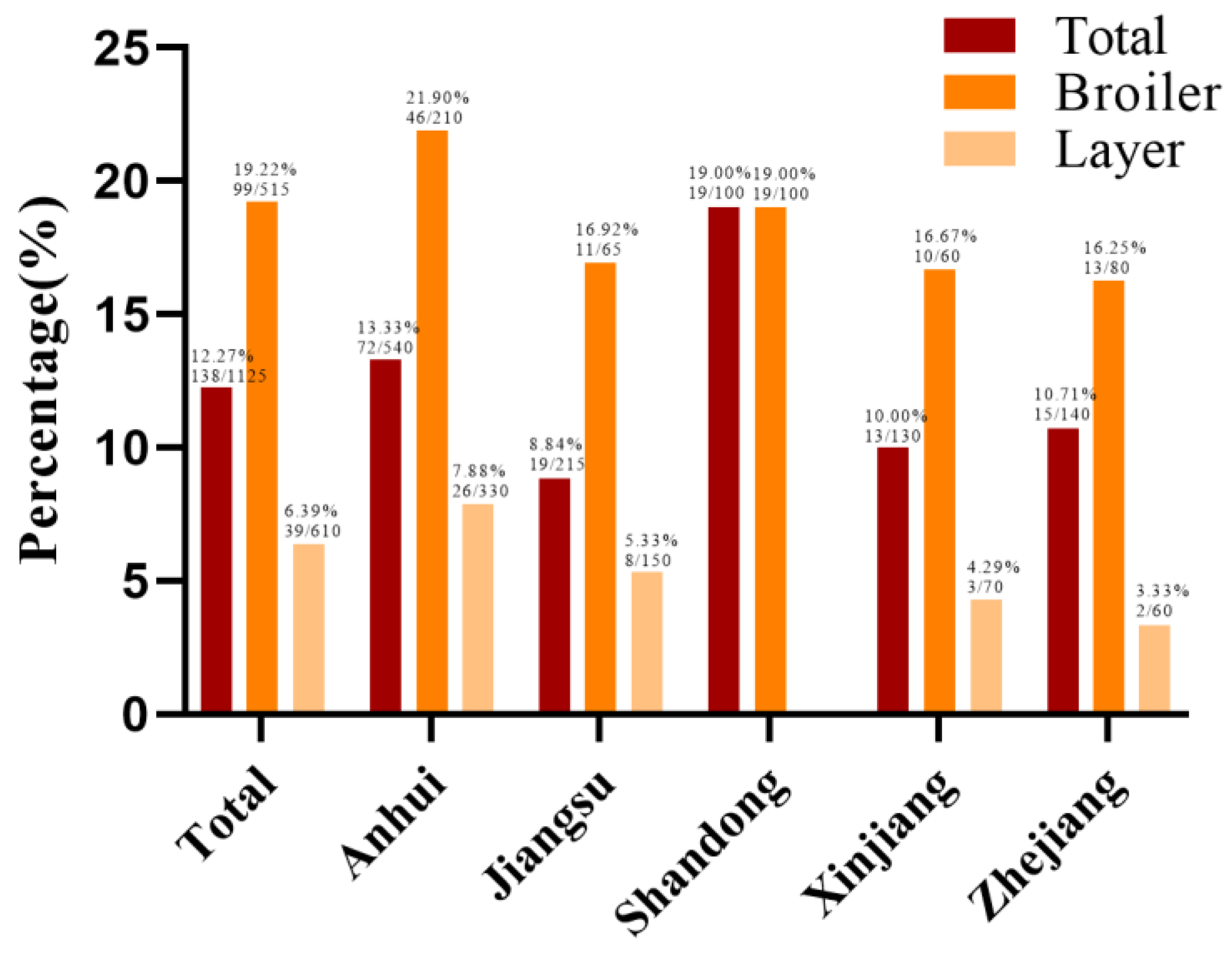
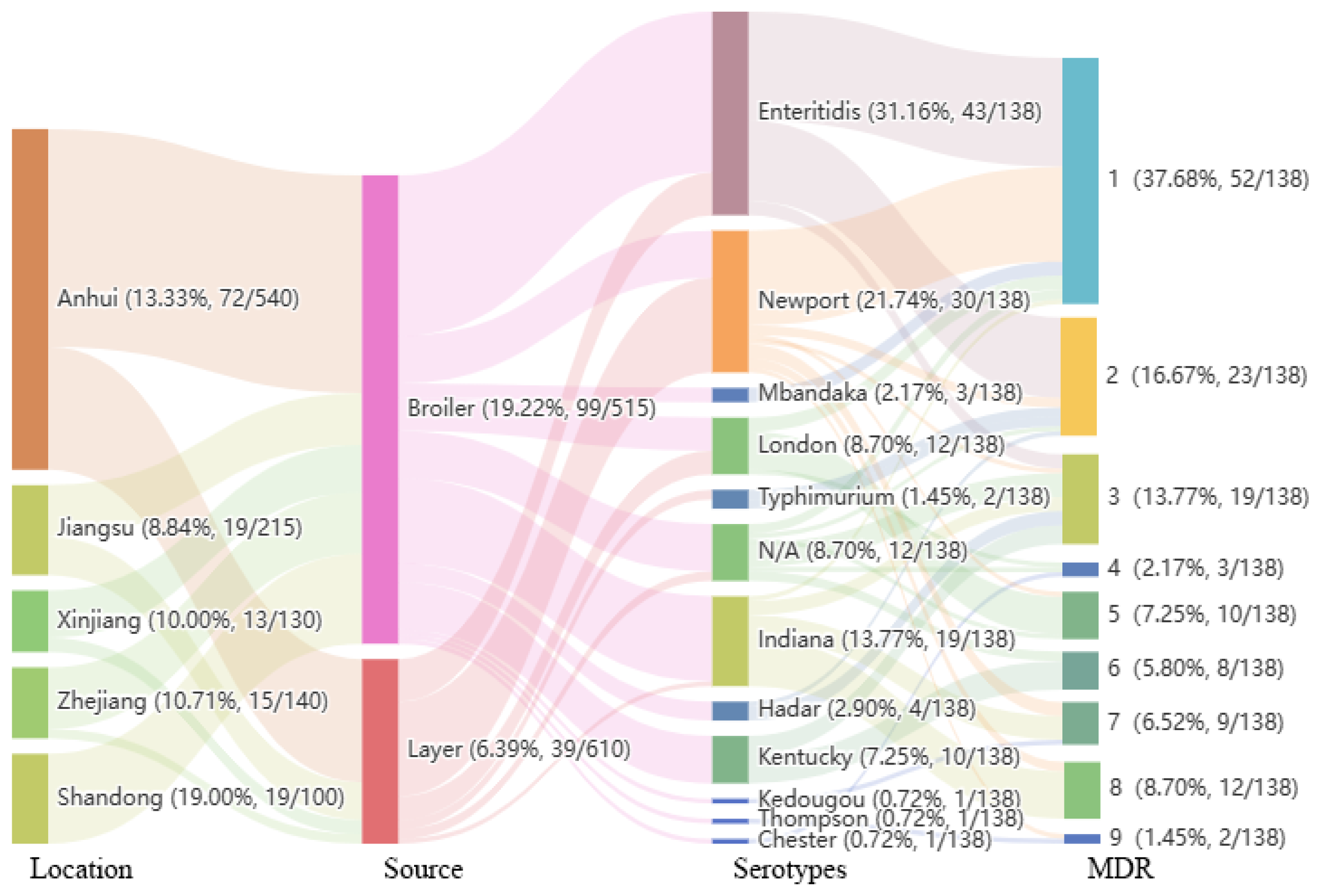
2.1. Isolation and Serotypes
2.2. Antibiotic Resistance and MDR Profiles
2.3. Analysis of Antibiotic Resistance Genes
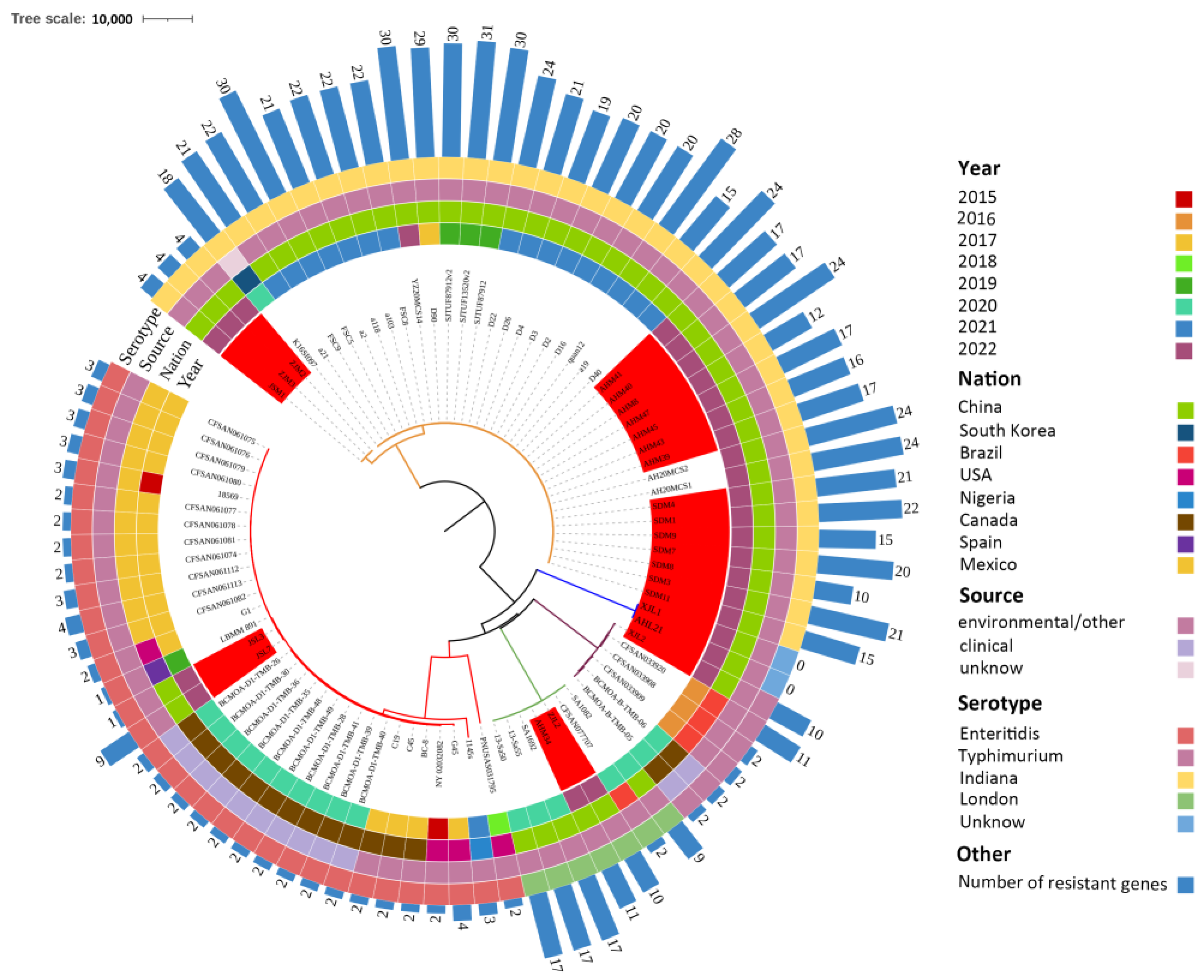
2.4. WGS Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation and Identification of Bacteria
4.3. Serotyping
4.4. Identification of Minimum Inhibitory Concentrations (MICs)
4.5. Detection of Drug Resistance Genes
4.6. Whole-Genome Sequencing
4.7. Data Analysis and Usability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar] [PubMed]
- Bresee, J.S.; Marcus, R.; Venezia, R.A.; Keene, W.E.; Morse, D.; Thanassi, M.; Brunett, P.; Bulens, S.; Beard, R.S.; Dauphin, L.A.; et al. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J. Infect. Dis. 2012, 205, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.L.; Fang, W.H.; Yue, M. Antibiotic Resistance in Typhimurium Isolates Recovered from the Food Chain through National Antimicrobial Resistance Monitoring System between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef]
- Zakaria, Z.; Hassan, L.; Ahmad, N.; Husin, S.A.; Ali, R.M.; Sharif, Z.; Sohaimi, N.M.; Garba, B. Discerning the Antimicrobial Resistance, Virulence, and Phylogenetic Relatedness of Salmonella Isolates Across the Human, Poultry, and Food Materials Sources in Malaysia. Front. Microbiol. 2021, 12, 652642. [Google Scholar] [CrossRef]
- Voss-Rech, D.; Potter, L.; Vaz, C.S.; Pereira, D.I.; Sangioni, L.A.; Vargas, A.C.; de Avila Botton, S. Antimicrobial Resistance in Nontyphoidal Salmonella Isolated from Human and Poultry-Related Samples in Brazil: 20-Year Meta-Analysis. Foodborne Pathog. Dis. 2017, 14, 116–124. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, L.; Hu, Y.; Dottorini, T.; Fanning, S.; Xu, J.; Li, F. Epidemiological Study on Prevalence, Serovar Diversity, Multidrug Resistance, and CTX-M-Type Extended-Spectrum beta-Lactamases of Salmonella spp. from Patients with Diarrhea, Food of Animal Origin, and Pets in Several Provinces of China. Antimicrob. Agents Chemother. 2020, 64, 00092-20. [Google Scholar] [CrossRef]
- Centers for Disease C Prevention. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 U.S. sites, 1996-2012. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 283–287. [Google Scholar]
- Liu, Q.; Chen, W.; Elbediwi, M.; Pan, H.; Wang, L.; Zhou, C.; Zhao, B.; Xu, X.; Li, D.; Yan, X.; et al. Characterization of Salmonella Resistome and Plasmidome in Pork Production System in Jiangsu, China. Front. Vet. Sci. 2020, 7, 617. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, D.; Hao, W.; Sun, R.; Sun, J.; Liu, Y.; Liao, X. Prevalence, antibiotic resistance, virulence genes and molecular characteristics of Salmonella isolated from ducks and wild geese in China. Food Microbiol. 2024, 118, 104423. [Google Scholar] [CrossRef]
- Shen, X.H.; Yin, L.; Zhang, A.Y.; Zhao, R.H.; Yin, D.D.; Wang, J.R.; Dai, Y.; Hou, H.Y.; Pan, X.C.; Hu, X.M.; et al. Prevalence and Characterization of Isolated from Chickens in Anhui, China. Pathogens 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Barnett, R. Typhoid fever. Lancet 2016, 388, 2467. [Google Scholar] [CrossRef] [PubMed]
- Colin, P. International Symposium on Salmonella and salmonellosis. Food Microbiol. 2018, 71, 1. [Google Scholar] [CrossRef]
- Littman, R.J. The plague of Athens: Epidemiology and paleopathology. Mt. Sinai J. Med. 2009, 76, 456–467. [Google Scholar] [CrossRef]
- Medalla, F.; Gu, W.; Mahon, B.E.; Judd, M.; Folster, J.; Griffin, P.M.; Hoekstra, R.M. Estimated Incidence of Antimicrobial Drug-Resistant Nontyphoidal Salmonella Infections, United States, 2004–2012. Emerg. Infect. Dis. 2016, 23, 29–37. [Google Scholar] [CrossRef]
- Peng, M.; Salaheen, S.; Buchanan, R.L.; Biswas, D. Alterations of Salmonella enterica Serovar Typhimurium Antibiotic Resistance under Environmental Pressure. Appl. Environ. Microbiol. 2018, 84, e01173-18. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhang, Y.; Zhang, J.; Xue, C.; Yan, J.; Li, X.; Zheng, X.; Dong, R.; Bai, J.; Su, Y.; et al. Analysis of antibiotic-induced drug resistance of Salmonella enteritidis and its biofilm formation mechanism. Bioengineered 2021, 12, 10254–10263. [Google Scholar] [CrossRef]
- Kagambega, A.; McMillan, E.A.; Bouda, S.C.; Hiott, L.M.; Ramadan, H.; Soro, D.K.; Sharma, P.; Gupta, S.K.; Barro, N.; Jackson, C.R.; et al. Resistance Genes, Plasmids, Multilocus Sequence Typing (MLST), and Phenotypic Resistance of Non-Typhoidal Salmonella (NTS) Isolated from Slaughtered Chickens in Burkina Faso. Antibiotics 2022, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, S.W.; Chang, Y.J.; Su, M.L.; Xie, Y.T.; Sun, S.H. Occurrence and Characterization of Isolated from Large-Scale Breeder Farms in Shandong Province, China. BioMed Res. Int. 2019, 2019, 8159567. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, Y.; Ye, C.; Yang, L.; Wang, T.; Chang, W. Prevalence and Characteristics of Salmonella Isolated from Free-Range Chickens in Shandong Province, China. BioMed Res. Int. 2016, 2016, 8183931. [Google Scholar] [CrossRef]
- Zhu, Y.; Lai, H.; Zou, L.; Yin, S.; Wang, C.; Han, X.; Xia, X.; Hu, K.; He, L.; Zhou, K.; et al. Antimicrobial resistance and resistance genes in Salmonella strains isolated from broiler chickens along the slaughtering process in China. Int. J. Food Microbiol. 2017, 259, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Lynne, A.M.; Nayak, R. Salmonella challenges: Prevalence in swine and poultry and potential pathogenicity of such isolates. J. Anim. Sci. 2008, 86 (Suppl. 14), E149–E162. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.H.; Hao, H.H.; Dai, M.H.; Wang, Y.L.; Ahmad, I.; Liu, Z.L.; Yuan, Z.H. Serotypes and antimicrobial susceptibility of spp. isolated from farm animals in China. Front. Microbiol. 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Elbediwi, M.; Nambiar, R.B.; Yang, H.; Lin, J.; Yue, M. Genomic Characterization of Antimicrobial-Resistant Salmonella enterica in Duck, Chicken, and Pig Farms and Retail Markets in Eastern China. Microbiol. Spectr. 2022, 10, e0125722. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Zhang, W.B.; Zhang, K.; Zhang, Y.; Wang, Z.Y.; Zhang, W.; Li, Y.; Li, Q.C. Characterization of serotypes prevalent in asymptomatic people and patients. BMC Infect. Dis. 2021, 21, 632. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.Q.; Xie, M.Y.; Qu, Z.N.; Zhao, S.J.; Wang, J.W.; Wang, Y.; He, T.; Wang, H.Y.; Zuo, Z.C.; Wu, C.M. Prevalence and antimicrobial resistance of isolated from an integrated broiler chicken supply chain in Qingdao, China. Food Control 2016, 62, 270–276. [Google Scholar] [CrossRef]
- Elsayed, M.M.; El-Basrey, Y.F.H.; El-Baz, A.H.; Dowidar, H.A.; Shami, A.; Al-Saeed, F.A.; Alsamghan, A.; Salem, H.M.; Alhazmi, W.A.; El-Tarabily, K.A.; et al. Ecological prevalence, genetic diversity, and multidrug resistance of Salmonella enteritidis recovered from broiler and layer chicken farms. Poult. Sci. 2024, 103, 103320. [Google Scholar] [CrossRef]
- Liu, C.X.; Yao, K.Y.; Ren, D.X.; Xiao, Y.P. Prevalence and characterization of from meat in slaughterhouses in Hangzhou, China. Int. J. Food Microbiol. 2022, 371, 109649. [Google Scholar] [CrossRef]
- Cai, Y.; Tao, J.; Jiao, Y.; Fei, X.; Zhou, L.; Wang, Y.; Zheng, H.; Pan, Z.; Jiao, X. Phenotypic characteristics and genotypic correlation between Salmonella isolates from a slaughterhouse and retail markets in Yangzhou, China. Int. J. Food Microbiol. 2016, 222, 56–64. [Google Scholar] [CrossRef]
- Dessie, H.K.; Bae, D.H.; Lee, Y.J. Characterization of integrons and their cassettes in and isolates from poultry in Korea. Poult. Sci. 2013, 92, 3036–3043. [Google Scholar] [CrossRef]
- Miranda, J.M.; Rodriguez, J.A.; Galan-Vidal, C.A. Simultaneous determination of tetracyclines in poultry muscle by capillary zone electrophoresis. J. Chromatogr. A 2009, 1216, 3366–3371. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Cardo, M.; Soares, P.; Cara d’Anjo, M.; Leite, A. Multi-Drug and beta-Lactam Resistance in Escherichia coli and Food-Borne Pathogens from Animals and Food in Portugal, 2014–2019. Antibiotics 2022, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Drauch, V.; Kornschober, C.; Palmieri, N.; Hess, M.; Hess, C. Infection dynamics of Infantis strains displaying different genetic backgrounds—With or without pESI-like plasmid—Vary considerably. Emerg. Microbes Infect. 2021, 10, 1471–1480. [Google Scholar] [CrossRef]
- Song, Y.; Wang, F.K.; Liu, Y.; Song, Y.Y.; Zhang, L.; Zhang, F.Y.; Gu, X.X.; Sun, S.H. Occurrence and Characterization of Isolated from Chicken Breeder Flocks in Nine Chinese Provinces. Front. Vet. Sci. 2020, 7, 479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, G.; Tang, W.; Song, X.; Zhao, X.; Wang, C.; Li, Y.; Zou, M. Antimicrobial resistance and genomic characteristics of Salmonella from broilers in Shandong Province. Front. Vet. Sci. 2023, 10, 1292401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, X.M.; Ed-Dra, A.; Zhou, X.; Jia, C.H.; Müller, A.; Liu, Y.Q.; Kehrenberg, C.; Yue, M. Genome-Based Assessment of Antimicrobial Resistance and Virulence Potential of Isolates of Non-Pullorum/Gallinarum Serovars Recovered from Dead Poultry in China. Microbiol. Spectr. 2022, 10, e0096522. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, H.; Bo, Y.; Li, Y.; Zhang, Y.; Liu, Y.; Zhang, J.; Jiang, L.; Chen, G.; Zhang, X. Prevalence and antimicrobial resistance of Salmonella enterica subspecies enterica serovar Enteritidis isolated from broiler chickens in Shandong Province, China, 2013–2018. Poult. Sci. 2021, 100, 1016–1023. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, M.; Zhang, Q.; Zhao, C.; Zhang, Y.; Li, L.; Qi, J.; Luo, Y.; Zhou, D.; Liu, Y. Characterization of integrons and antimicrobial resistance in Salmonella from broilers in Shandong, China. Poult. Sci. 2020, 99, 7046–7054. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, Y.G.; Yang, L.H.; Qin, J.X.; Guo, M.Q.; Lu, Y.X.; Chen, H.Y.; Zhuang, Y.; Zhang, J.H.; Zhang, H.; et al. Molecular and Conventional Analysis of Acute Diarrheal Isolates Identifies Epidemiological Trends, Antibiotic Resistance and Virulence Profiles of Common Enteropathogens in Shanghai. Front. Microbiol. 2018, 9, 164. [Google Scholar] [CrossRef]
- Li, R.C.; Lai, J.; Wang, Y.; Liu, S.L.; Li, Y.; Liu, K.Y.; Shen, J.Z.; Wu, C.M. Prevalence and characterization of species isolated from pigs, ducks and chickens in Sichuan Province, China. Int. J. Food Microbiol. 2013, 163, 14–18. [Google Scholar] [CrossRef]
- Liu, W.B.; Chen, J.; Huang, Y.Y.; Liu, B.; Shi, X.M. Serotype, genotype, and antimicrobial susceptibility profiles of Salmonella from chicken farms in Shanghai. J. Food Prot. 2010, 73, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, S.; Fuche, F.J.; Sears, K.T.; Jones, J.A.; Levine, M.M.; Simon, R.; Tennant, S.M. Refinement of a Live Attenuated Salmonella enterica Serovar Newport Vaccine with Improved Safety. Vaccines 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.X.; Chen, J.; Chen, L.L.; Qi, X.J.; Zhang, R.H. Epidemiology of Foodborne Disease Outbreaks Caused by Nontyphoidal in Zhejiang Province, China, 2010–2019. Foodborne Pathog. Dis. 2021, 18, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Lari, M.; Hosseinzadeh, S.; Manzari, M.; Khaledian, S. Survey of Salmonella in commercial broiler farms in Shiraz, southern Iran. Prev. Vet. Med. 2022, 198, 105550. [Google Scholar] [CrossRef]
- Ramtahal, M.A.; Amoako, D.G.; Akebe, A.L.K.; Somboro, A.M.; Bester, L.A.; Essack, S.Y. A Public Health Insight into in Poultry in Africa: A Review of the Past Decade: 2010–2020. Microb. Drug Resist. 2022, 28, 710–733. [Google Scholar] [CrossRef]
- Baker, M.; Zhang, X.; Maciel-Guerra, A.; Babaarslan, K.; Dong, Y.; Wang, W.; Hu, Y.; Renney, D.; Liu, L.; Li, H.; et al. Convergence of resistance and evolutionary responses in Escherichia coli and Salmonella enterica co-inhabiting chicken farms in China. Nat. Commun. 2024, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.Y.; Strawn, L.K.; Zheng, J.; Reed, E.A.; Rideout, S.L. Diversity and Dynamics of in Water Sources, Poultry Litters, and Field Soils Amended With Poultry Litter in a Major Agricultural Area of Virginia. Front. Microbiol. 2019, 10, 2868. [Google Scholar] [CrossRef]
- Truitt, L.N.; Vazquez, K.M.; Pfuntner, R.C.; Rideout, S.L.; Havelaar, A.H.; Strawn, L.K. Microbial Quality of Agricultural Water Used in Produce Preharvest Production on the Eastern Shore of Virginia. J. Food Prot. 2018, 81, 1661–1672. [Google Scholar] [CrossRef]
- Willmann, M.; El-Hadidi, M.; Huson, D.H.; Schütz, M.; Weidenmaier, C.; Autenrieth, I.B.; Peter, S. Antibiotic Selection Pressure Determination through Sequence-Based Metagenomics. Antimicrob. Agents Chemother. 2015, 59, 7335–7345. [Google Scholar] [CrossRef]
- Dantas, G.; Sommer, M.O.A. Context matters—The complex interplay between resistome genotypes and resistance phenotypes. Curr. Opin. Microbiol. 2012, 15, 577–582. [Google Scholar] [CrossRef]
- Gomi, R.; Matsuda, T.; Matsumura, Y.; Yamamoto, M.; Tanaka, M.; Ichiyama, S.; Yoneda, M. Whole-Genome Analysis of Antimicrobial-Resistant and Extraintestinal Pathogenic in River Water. Appl. Environ. Microbiol. 2017, 83, e02703. [Google Scholar] [CrossRef]
- Kosikowska, U.; Stec, J.; Andrzejczuk, S.; Mendrycka, M.; Pietras-Ozga, D.; Stepien-Pysniak, D. Plasmid-Mediated Fluoroquinolone Resistance Genes in Quinolone-Susceptible spp. Phenotypes Isolated from Recreational Surface Freshwater Reservoir. Front. Cell. Infect. Microbiol. 2022, 12, 885360. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Olsen, J.E.; Thomsen, L.E. Identification of Genes Essential for Antibiotic-Induced Up-Regulation of Plasmid-Transfer-Genes in Cephalosporin Resistant. Front. Microbiol. 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, B.J.; Rosso, M.L.; Schwan, T.G.; Carniel, E. High-frequency conjugative transfer of antibiotic resistance genes to Yersinia pestis in the flea midgut. Mol. Microbiol. 2002, 46, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sobisch, L.Y.; Rogowski, K.M.; Fuchs, J.; Schmieder, W.; Vaishampayan, A.; Oles, P.; Novikova, N.; Grohmann, E. Biofilm Forming Antibiotic Resistant Gram-Positive Pathogens Isolated from Surfaces on the International Space Station. Front. Microbiol. 2019, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Chen, B.; Lei, C.; Yu, Y.; Xu, N.; Zhang, Q.; Wang, T.; Gao, W.; Lu, T.; et al. Xenobiotic pollution affects transcription of antibiotic resistance and virulence factors in aquatic microcosms. Environ. Pollut. 2022, 306, 119396. [Google Scholar] [CrossRef]
- Gong, J.S.; Zeng, X.M.; Zhang, P.; Zhang, D.; Wang, C.M.; Lin, J. Characterization of the emerging multidrug-resistant serovar Indiana strains in China. Emerg. Microbes Infect. 2019, 8, 29–39. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, T.; Guo, M.J.; Zhang, C.C.; Bo, Z.Y.; Wu, Y.T.; Chao, G.X. The large plasmid carried class 1 integrons mediated multidrug resistance of foodborne Indiana. Front. Microbiol. 2022, 13, 991326. [Google Scholar] [CrossRef]
- Ghosh, S.; Biswas, S.; Mukherjee, S.; Pal, A.; Saxena, A.; Sundar, S.; Dujardin, J.C.; Das, S.; Roy, S.; Mukhopadhyay, R.; et al. A Novel Bioimpedance-Based Detection of Miltefosine Susceptibility Among Clinical Isolates of the Indian Subcontinent Exhibiting Resistance to Multiple Drugs. Front. Cell. Infect. Microbiol. 2021, 11, 768830. [Google Scholar] [CrossRef]
- McLellan, H.; Harvey, S.E.; Steinbrenner, J.; Armstrong, M.R.; He, Q.; Clewes, R.; Pritchard, L.; Wang, W.; Wang, S.; Nussbaumer, T.; et al. Exploiting breakdown in nonhost effector-target interactions to boost host disease resistance. Proc. Natl. Acad. Sci. USA 2022, 119, e2114064119. [Google Scholar] [CrossRef]
- Cossi, M.V.C.; Polveiro, R.C.; Yamatogi, R.S.; Camargo, A.C.; Nero, L.A. Multi-locus sequence typing, antimicrobials resistance and virulence profiles of Salmonella enterica isolated from bovine carcasses in Minas Gerais state, Brazil. Braz. J. Microbiol. 2024, 55, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Rahn, K.; De Grandis, S.A.; Clarke, R.C.; McEwen, S.A.; Galan, J.E.; Ginocchio, C.; Curtiss, R., III; Gyles, C.L. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell. Probes 1992, 6, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liu, T.; Lee, M.D.; Hofacre, C.L.; Maier, M.; White, D.G.; Ayers, S.; Wang, L.; Berghaus, R.; Maurer, J.J. Rapid screening of Salmonella enterica serovars Enteritidis, Hadar, Heidelberg and Typhimurium using a serologically-correlative allelotyping PCR targeting the O and H antigen alleles. BMC Microbiol. 2008, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- The Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
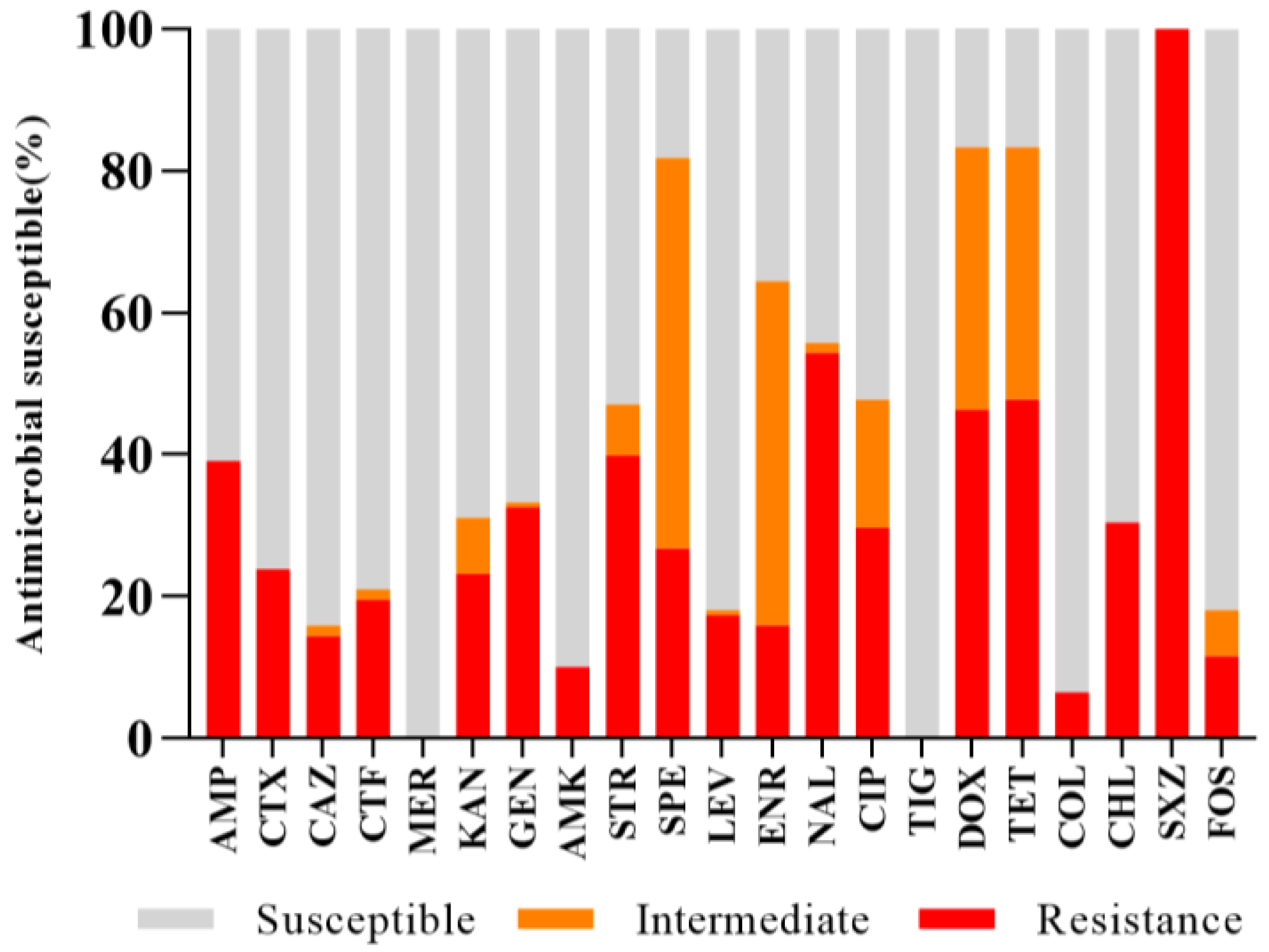


| Antibiotic Category | Antibiotics | MIC Value (mg/L) | No. of Resistant Isolates (%) | |
|---|---|---|---|---|
| MIC50 | MIC90 | |||
| β-lactams | AMP | 2 | >128 | 54 (39.13) |
| CTX | 0.03 | >128 | 33 (23.91) | |
| CAZ | 0.5 | 64 | 20 (14.49) | |
| CTF | 0.5 | >64 | 27 (19.57) | |
| MER | 0.125 | 0.125 | 0 | |
| Aminoglycosides | KAN | 2 | >64 | 32 (23.19) |
| GEN | 1 | >128 | 45 (32.61) | |
| AMK | 2 | 4 | 14 (10.14) | |
| STR | 8 | >128 | 55 (39.86) | |
| SPE | 32 | >128 | 37 (26.81) | |
| Quinolones | LEV | 0.5 | 16 | 24 (17.39) |
| ENR | 0.5 | 16 | 22 (15.94) | |
| NAL | 32 | >1024 | 75 (54.35) | |
| CIP | 0.5 | >32 | 41 (29.71) | |
| Tetracyclines | TIG | 0.5 | 1 | 0 |
| DOX | 8 | 32 | 64 (46.38) | |
| TET | 8 | >64 | 66 (47.83) | |
| Polypeptides | COL | 0.25 | 2 | 9 (6.52) |
| Sulfonamides | SXZ | >1024 | >1024 | 138 (100) |
| Amide alcohols | CHL | 4 | >128 | 42 (30.43) |
| FOS | 16 | 256 | 16 (11.59) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, X.; Hao, J.; Kong, H.; Zhao, L.; Li, M.; Zou, M.; Liu, G. Serotype Distribution and Antimicrobial Resistance of Salmonella Isolates from Poultry Sources in China. Antibiotics 2024, 13, 959. https://doi.org/10.3390/antibiotics13100959
Wang C, Wang X, Hao J, Kong H, Zhao L, Li M, Zou M, Liu G. Serotype Distribution and Antimicrobial Resistance of Salmonella Isolates from Poultry Sources in China. Antibiotics. 2024; 13(10):959. https://doi.org/10.3390/antibiotics13100959
Chicago/Turabian StyleWang, Chu, Xianwen Wang, Juyuan Hao, He Kong, Liyuan Zhao, Mingzhen Li, Ming Zou, and Gang Liu. 2024. "Serotype Distribution and Antimicrobial Resistance of Salmonella Isolates from Poultry Sources in China" Antibiotics 13, no. 10: 959. https://doi.org/10.3390/antibiotics13100959
APA StyleWang, C., Wang, X., Hao, J., Kong, H., Zhao, L., Li, M., Zou, M., & Liu, G. (2024). Serotype Distribution and Antimicrobial Resistance of Salmonella Isolates from Poultry Sources in China. Antibiotics, 13(10), 959. https://doi.org/10.3390/antibiotics13100959






