Registry-Based Retrospective Cohort Study of Mortality among Adults Admitted to Intensive Care Units in Istanbul with Hospital Acquired Pseudomonas aeruginosa Bloodstream-Infection between 2014–2021
Abstract
1. Introduction
2. Results
2.1. Patient Factors Related to Outcomes and Therapeutic Options
- a higher median CCI score,
- immunocompromised status,
- individuals with a central line,
- hospitalized within the last three months.
2.2. Source of Bacteremia
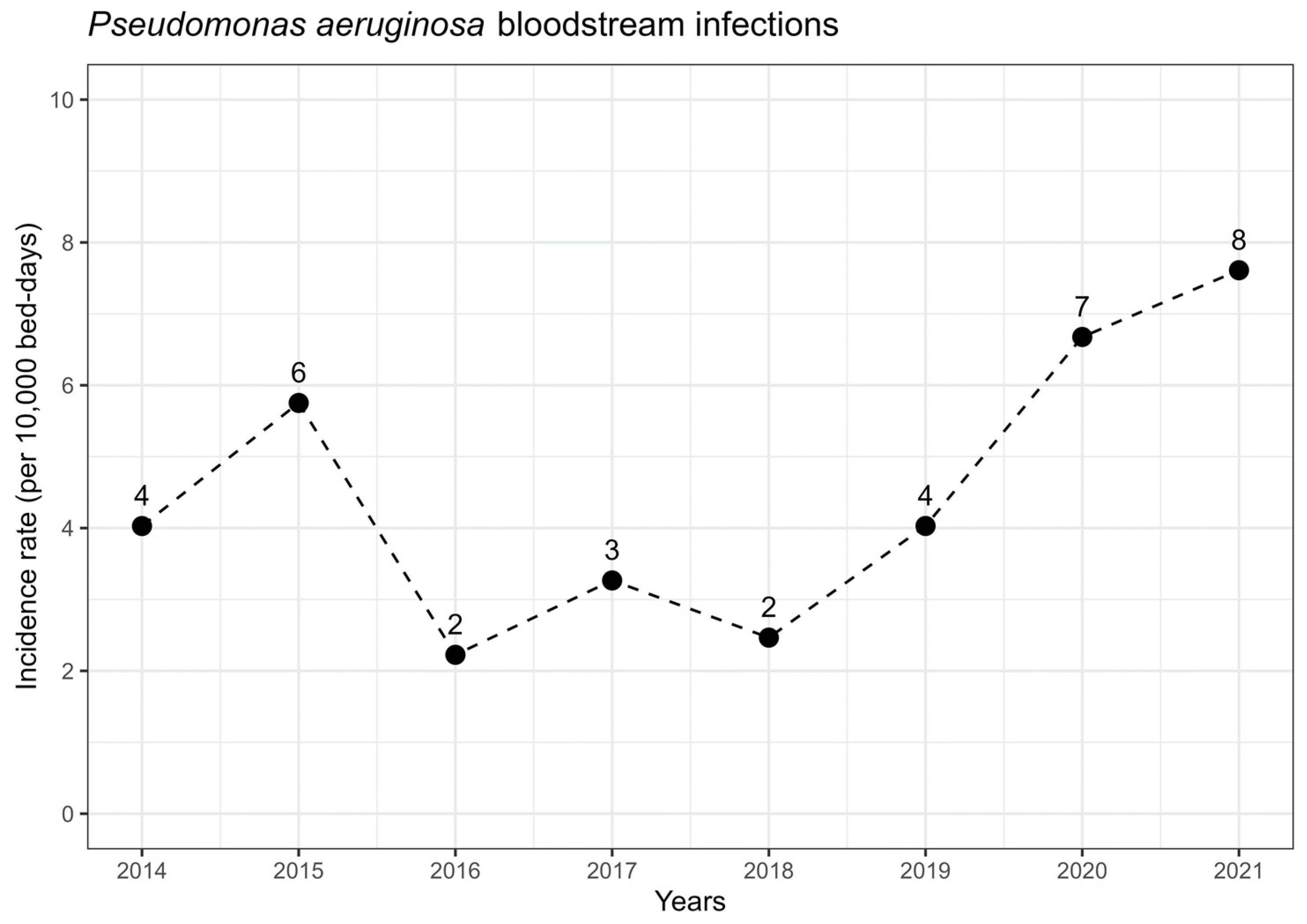
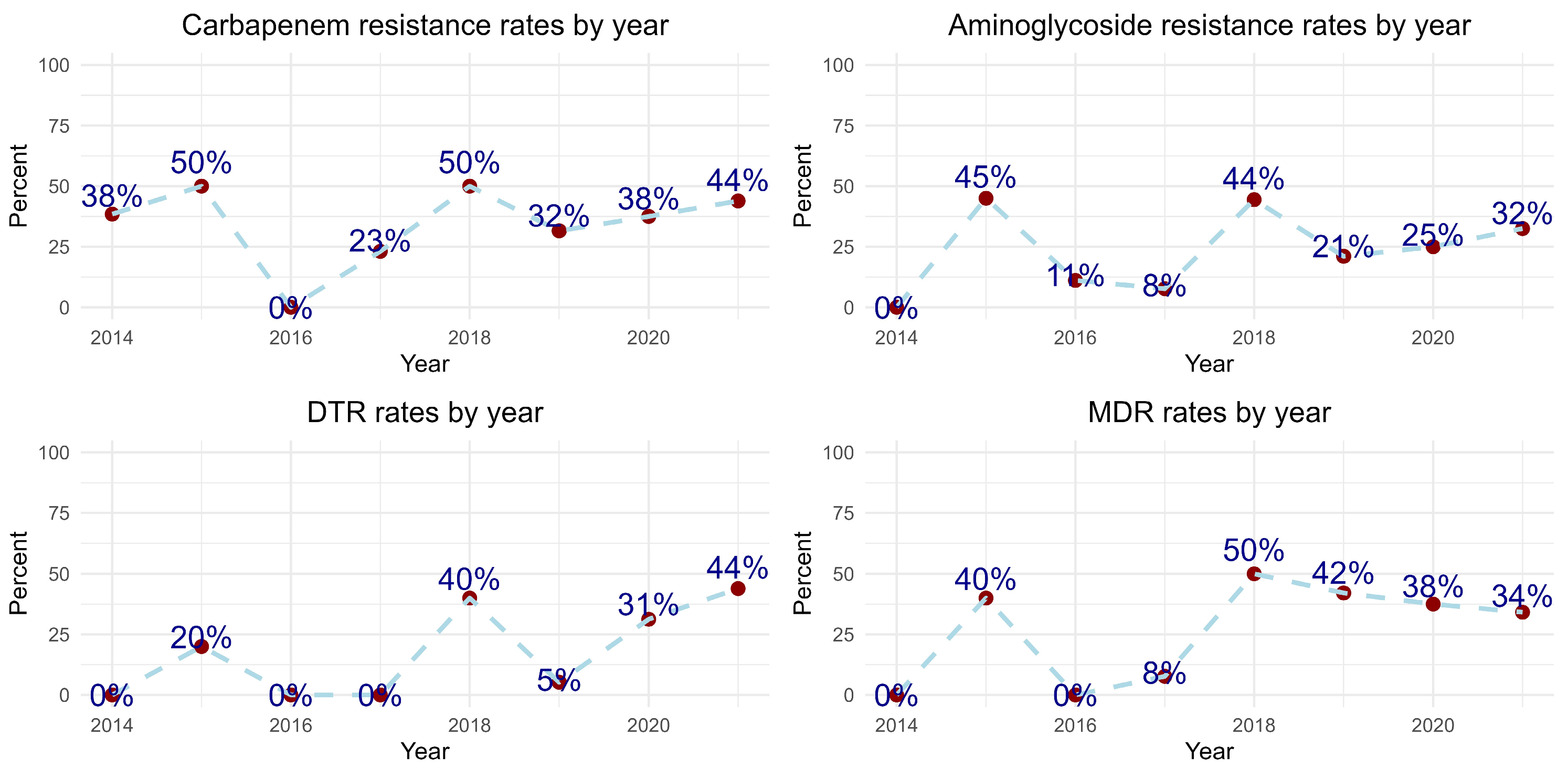
2.3. Microbiologic Factors and Resistance Patterns by Years
3. Discussion
3.1. Strengths of the Study
- The study included data from four third-level hospitals over an eight-year period in Istanbul, the most populated city in Turkey. This provides a large and diverse dataset.
- The study focused specifically on P. aeruginosa bloodstream infections in intensive care units, which is an important area of research due to the severity of these infections.
- The study was conducted in a setting with a high prevalence of multidrug-resistant P. aeruginosa, which is valuable information for clinicians and researchers.
- The study used new definitions of resistance, such as DTR and “Susceptible-Increased Dose” and analyzed resistance epidemiology and therapeutic options using these new definitions. This may lead to more effective treatment strategies in the future.
- Our study innovates by extensively analyzing Pseudomonas aeruginosa bloodstream infections in ICUs, focusing on multidrug-resistant strains, and assessing the impact of new resistance definitions and treatment strategies on mortality. Additionally, it explores the epidemiological shifts during the COVID-19 pandemic, providing basic insights into the evolution of antibiotic resistance and infectious diseases in a critical care setting.
3.2. Limitations of the Study
- The study was retrospective, which means that some data may have been missing or incomplete. This could affect the accuracy of these results.
- Because of the lack of data, some variables could not be included in the multivariable analysis. This may have limited the scope of the study and the conclusions that can be drawn from it.
- We did not include any variables for assessing clinical severity, such as APACHE-II or the Pitt bacteremia index, due to the unavailability of reliable data from paperwork archives during the study period.
4. Materials and Methods
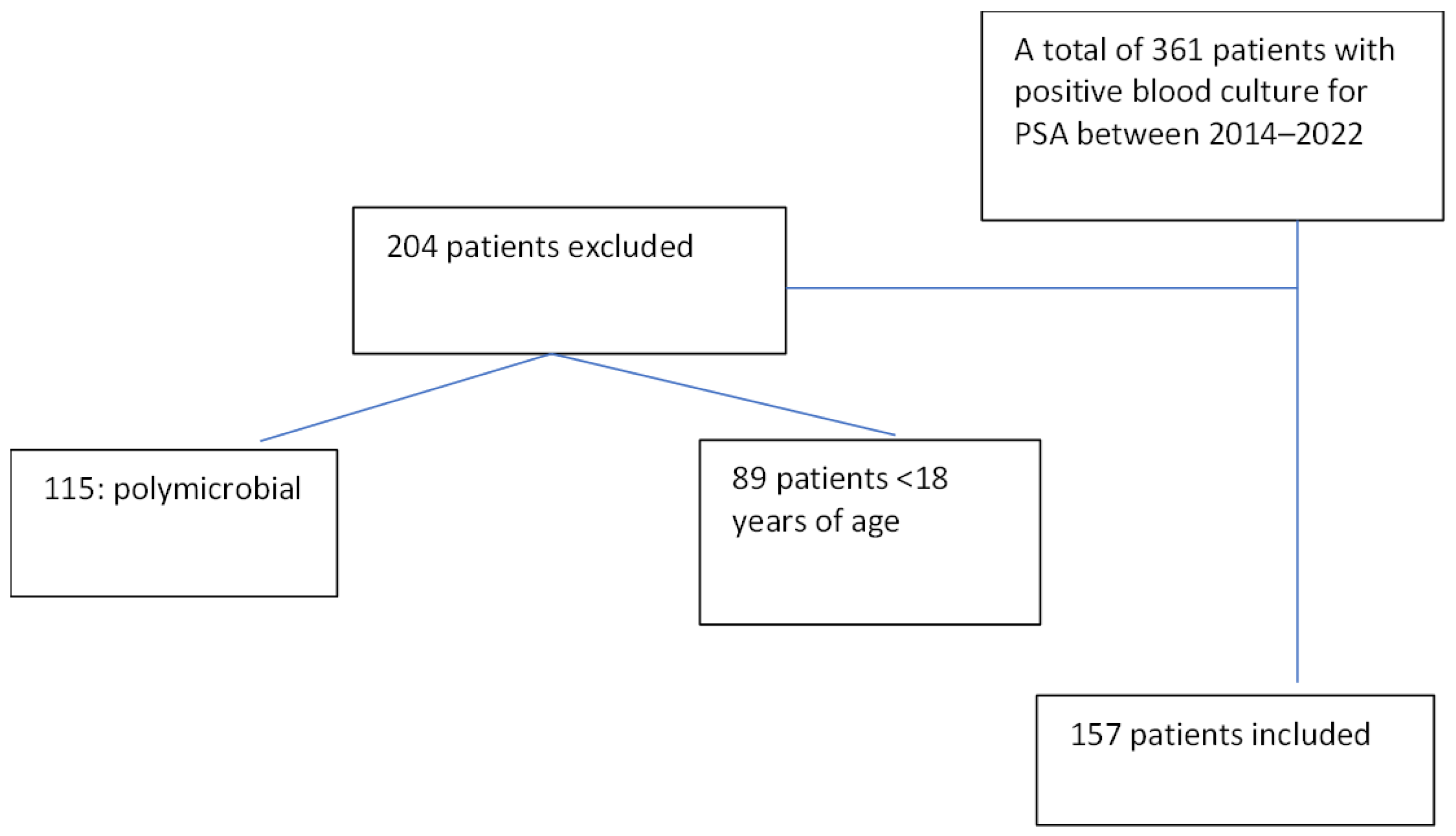
4.1. Study Design and Setting
4.2. Participants and Definitions
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Statement of Authors
References
- Corona, A.; De Santis, V.; Agarossi, A.; Prete, A.; Cattaneo, D.; Tomasini, G.; Bonetti, G.; Patroni, A.; Latronico, N. Antibiotic Therapy Strategies for Treating Gram-Negative Severe Infections in the Critically Ill: A Narrative Review. Antibiotics 2023, 12, 1262. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Al-Orphaly, M.; Hadi, H.A.; Eltayeb, F.K.; Al-Hail, H.; Samuel, B.G.; Sultan, A.A.; Skariah, S. Epidemiology of Multidrug-Resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere 2021, 6, e00202-21. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Wang, M.G.; Liu, Z.Y.; Liao, X.P.; Sun, R.Y.; Li, R.B.; Liu, Y.; Fang, L.X.; Sun, J.; Liu, Y.H.; Zhang, R.M. Retrospective Data Insight into the Global Distribution of Carbapenemase-Producing Pseudomonas aeruginosa. Antibiotics 2021, 10, 548. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-Resistant Pathogens Associated with Adult Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef]
- Surveillance of Antimicrobial Resistance in Europe, 2021 Data: Executive Summary. Available online: https://www.who.int/europe/publications/i/item/9789289058513 (accessed on 19 January 2023).
- Kahlmeter, G. EUCAST Proposes to Change the Definition and Usefulness of the Susceptibility Category ‘Intermediate’. Clin. Microbiol. Infect. 2017, 23, 894–895. [Google Scholar] [CrossRef]
- Meylan, S.; Guery, B. In the Name of Common Sense: EUCAST Breakpoints and Potential Pitfalls. Clin. Microbiol. Infect. 2020, 26, 1593–1594. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-Lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. Aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [CrossRef]
- Peña, C.; Suarez, C.; Ocampo-Sosa, A.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A.; Calbo, E.; Rodríguez-Baño, J.; et al. Effect of Adequate Single-Drug vs Combination Antimicrobial Therapy on Mortality in Pseudomonas aeruginosa Bloodstream Infections: A Post Hoc Analysis of a Prospective Cohort. Clin. Infect. Dis. 2013, 57, 208–216. [Google Scholar] [CrossRef]
- Foster, R.A.; Troficanto, C.; Bookstaver, P.B.; Kohn, J.; Justo, J.A.; Al-Hasan, M.N. Utility of Combination Antimicrobial Therapy in Adults with Bloodstream Infections Due to Enterobacteriaceae and Non-Fermenting Gram-Negative Bacilli Based on In Vitro Analysis at Two Community Hospitals. Antibiotics 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; Handelsman, J.; Maki, D.G. Does Combination Antimicrobial Therapy Reduce Mortality in Gram-Negative Bacteraemia? A Meta-Analysis. Lancet Infect. Dis. 2004, 4, 519–527. [Google Scholar] [CrossRef]
- Paul, M.; Benuri-Silbiger, I.; Soares-Weiser, K.; Leibovici, L. β Lactam Monotherapy versus β Lactam-Aminoglycoside Combination Therapy for Sepsis in Immunocompetent Patients: Systematic Review and Meta-Analysis of Randomised Trials. BMJ 2004, 328, 668. [Google Scholar] [CrossRef] [PubMed]
- Afonso, E.; Conoscenti, E.; Blot, S. Combination Antimicrobial Therapy in Pseudomonas aeruginosa Bacteremia. Eur. J. Pediatr. 2020, 179, 1997–1998. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Losito, A.R.; Raffaelli, F.; Del Giacomo, P.; Tumbarello, M. New Drugs for the Treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: A Narrative Review. Antibiotics 2022, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Chaïbi, K.; Jaureguy, F.; Do Rego, H.; Ruiz, P.; Mory, C.; El Helali, N.; Mrabet, S.; Mizrahi, A.; Zahar, J.R.; Pilmis, B. What to Do with the New Antibiotics? Antibiotics 2023, 12, 654. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Wilson, J.W.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Incidence of Pseudomonas aeruginosa Bacteremia: A Population-Based Study. Am. J. Med. 2008, 121, 702. [Google Scholar] [CrossRef]
- Tam, V.H.; Rogers, C.A.; Chang, K.T.; Weston, J.S.; Caeiro, J.P.; Garey, K.W. Impact of Multidrug-Resistant Pseudomonas aeruginosa Bacteremia on Patient Outcomes. Antimicrob. Agents Chemother. 2010, 54, 3717–3722. [Google Scholar] [CrossRef]
- Vitkauskiene, A.; Skrodeniene, E.; Dambrauskiene, A.; Macas, A.; Sakalauskas, R. Pseudomonas aeruginosa Bacteremia: Resistance to Antibiotics, Risk Factors, and Patient Mortality. Medicina 2010, 46, 490. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zeng, J.; Chang, Y.; Han, S.; Zhao, J.; Fan, Y.; Xiong, Z.; Zou, X.; Wang, C.; et al. Risk Factors for Mortality of Inpatients with Pseudomonas aeruginosa Bacteremia in China: Impact of Resistance Profile in the Mortality. Infect. Drug Resist. 2020, 13, 4115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, H.J.; Moon, S.M.; Park, K.H.; Chong, Y.P.; Kim, M.N.; Kim, S.H.; Lee, S.O.; Kim, Y.S.; Woo, J.H.; et al. Impact of Adequate Empirical Combination Therapy on Mortality from Bacteremic Pseudomonas aeruginosa Pneumonia. BMC Infect. Dis. 2012, 12, 308. [Google Scholar] [CrossRef]
- Cheong, H.S.; Kang, C.I.; Wi, Y.M.; Kim, E.S.; Lee, J.S.; Ko, K.S.; Chung, D.R.; Lee, N.Y.; Song, J.H.; Peck, K.R. Clinical Significance and Predictors of Community-Onset Pseudomonas aeruginosa Bacteremia. Am. J. Med. 2008, 121, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.I.; Kim, S.H.; Kim, H.B.; Park, S.W.; Choe, Y.J.; Oh, M.D.; Kim, E.C.; Choe, K.W. Pseudomonas aeruginosa Bacteremia: Risk Factors for Mortality and Influence of Delayed Receipt of Effective Antimicrobial Therapy on Clinical Outcome. Clin. Infect. Dis. 2003, 37, 745–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.L.; Huang, A.W.; Liu, S.L.; Liu, W.J.; Zhang, N.; Lu, X.Z. Mortality Attributable to Carbapenem-Resistant Pseudomonas aeruginosa Bacteremia: A Meta-Analysis of Cohort Studies. Emerg. Microbes Infect. 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shbaklo, N.; Lupia, T.; De Rosa, F.G.; Corcione, S. Infection Control in the Era of COVID-19: A Narrative Review. Antibiotics 2021, 10, 1244. [Google Scholar] [CrossRef]
- Hawkins, B.K.; Walker, S.D.; Shorman, M.A. Missed Opportunities for Antifungal Stewardship during the COVID-19 Era. Antibiotics 2023, 12, 1352. [Google Scholar] [CrossRef]
- Malik, S.S.; Mundra, S. Increasing Consumption of Antibiotics during the COVID-19 Pandemic: Implications for Patient Health and Emerging Anti-Microbial Resistance. Antibiotics 2022, 12, 45. [Google Scholar] [CrossRef]
- Ng, Q.X.; Ong, N.Y.; Lee, D.Y.X.; Yau, C.E.; Lim, Y.L.; Kwa, A.L.H.; Tan, B.H. Trends in Pseudomonas aeruginosa (P. Aeruginosa) Bacteremia during the COVID-19 Pandemic: A Systematic Review. Antibiotics 2023, 12, 409. [Google Scholar] [CrossRef]
- Sloot, R.; Nsonwu, O.; Chudasama, D.; Rooney, G.; Pearson, C.; Choi, H.; Mason, E.; Springer, A.; Gerver, S.; Brown, C.; et al. Rising Rates of Hospital-Onset Klebsiella spp. and Pseudomonas aeruginosa Bacteraemia in NHS Acute Trusts in England: A Review of National Surveillance Data, August 2020–February 2021. J. Hosp. Infect. 2022, 119, 175–181. [Google Scholar] [CrossRef]
- Ioannou, P.; Alexakis, K.; Maraki, S.; Kofteridis, D.P. Pseudomonas Bacteremia in a Tertiary Hospital and Factors Associated with Mortality. Antibiotics 2023, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; NCZID; DHQP Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection) Device Assoc. Modul. BSI 2022, 1–48. Available online: https://www.cdc.gov/nhsn/psc/bsi/index.html (accessed on 15 May 2022).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.K.; Lyman, J.A. Updated Review of Blood Culture Contamination. Clin. Microbiol. Rev. 2006, 19, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0, 2020, 1–77. Available online: http://www.eucast.org/clinical_breakpoints (accessed on 20 January 2023).
- Clinical & Laboratory Standards Institute: CLSI Guidelines n.d. Available online: https://clsi.org/ (accessed on 20 January 2023).
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Bochenska, M.; Fumagalli, L.; Dowzicky, M. Trends of major antimicrobial resistance phenotypes in enterobacterales and gram-negative non-fermenters from ATLAS and EARS-net surveillance systems: Italian vs. European and global data, 2008–2018. Diagn. Microbiol. Infect. Dis. 2021, 101, 115512. [Google Scholar] [CrossRef]
| Characteristics by Outcome | Characteristics by Directed Therapy Approach | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Overall, N = 157 1 | Survived, N = 87 (55.5%) 1 | Death, N = 70 (44.5%) 1 | p-Value 2 | N | Overall, N = 148 1 | Monotherapy, N = 80 (54%) 1 | Combination, N = 68 (46%) 1 | p-Value 2 |
| Period | 157 | 0.9 | 148 | 0.051 | ||||||
| Period 1 (2014–2019) | 84 (54%) | 47 (56%) | 37 (44%) | 76 (51%) | 47 (59%) | 29 (43%) | ||||
| Period 2 (2020–2021) | 73 (46%) | 40 (55%) | 33 (45%) | 72 (49%) | 33 (41%) | 39 (57%) | ||||
| Patient related factors | ||||||||||
| Age | 157 | 68 (57, 77) | 65 (56, 76) | 68 (58, 78) | 0.4 | 148 | 66 (56, 77) | 68 (56, 76) | 65 (56, 77) | 0.7 |
| Sex male | 157 | 100 (64%) | 55 (55%) | 45 (45%) | 0.9 | 148 | 94 (64%) | 50 (62%) | 44 (65%) | 0.8 |
| CCI | 157 | 4 (2, 6) | 4 (2, 5) | 5 (3, 7) | 0.003 | 148 | 4 (2, 6) | 4 (2, 6) | 4 (2, 6) | >0.9 |
| Mechanical Ventilation | 157 | 127 (81%) | 70 (55%) | 57 (45%) | >0.9 | 148 | 119 (80%) | 63 (79%) | 56 (82%) | 0.7 |
| Immunocompromised | 157 | 29 (18%) | 8 (28%) | 21 (72%) | <0.001 | 148 | 27 (18%) | 16 (20%) | 11 (16%) | 0.5 |
| Cerebrovascular event | 154 | 40 (26%) | 32 (80%) | 8 (20%) | <0.001 | 146 | 39 (27%) | 23 (29%) | 16 (24%) | 0.5 |
| Solid cancer | 157 | 31 (20%) | 10 (32%) | 21 (68%) | 0.004 | 148 | 28 (19%) | 15 (19%) | 13 (19%) | >0.9 |
| Central line | 155 | 91 (59%) | 45 (49%) | 46 (51%) | 0.046 | 146 | 82 (56%) | 43 (54%) | 39 (58%) | 0.6 |
| Admission last 3 month | 102 | 59 (58%) | 27 (46%) | 32 (54%) | 0.008 | 97 | 55 (57%) | 31 (65%) | 24 (49%) | 0.12 |
| Source of bacteremia 3 | 157 | 0.054 | 148 | 0.4 | ||||||
| Primary | 45 (29%) | 21 (47%) | 24 (53%) | 43 (29%) | 23 (53%) | 20 (47%) | ||||
| Secondary | 112(71%) | 66(59%) | 46(41%) | 105(71%) | 57(54%) | 48(45%) | ||||
| Complicated UTI | 13 (8.3%) | 8 (61%) | 5 (39%) | 11 (7.4%) | 8 (72%) | 3 (28%) | ||||
| Pneumonia | 39 (25%) | 20 (51%) | 19 (49%) | 34 (23%) | 19 (56%) | 15 (44%) | ||||
| Hepatobiliary | 9 (5.7%) | 3 (33%) | 6 (67%) | 9 (6.1%) | 7 (78%) | 2 (22%) | ||||
| Catheter Inf | 44 (28%) | 28 (64%) | 16 (36%) | 44 (30%) | 20 (46%) | 24 (54%) | ||||
| Complicated SSTI | 7 (4.5%) | 7 (100%) | 0 (0%) | 7 (4.7%) | 3 (43%) | 4 (57%) | ||||
| Microbiologic factors | ||||||||||
| TZP Resistance | 157 | 60 (38%) | 34 (56%) | 26 (44%) | 0.6 | 148 | 57 (39%) | 19 (33%) | 38 (67%) | <0.001 |
| Carbapenem Resistance | 157 | 59 (38%) | 33 (56%) | 26 (44%) | >0.9 | 148 | 56 (38%) | 22 (39%) | 34 (61%) | 0.005 |
| Aminoglycoside Resistance | 155 | 40 (26%) | 18 (45%) | 22 (55%) | 0.15 | 146 | 39 (27%) | 13 (33%) | 26 (67%) | 0.003 |
| AP Cephalosporin Resistance | 154 | 59 (38%) | 34 (58%) | 25 (42%) | 0.6 | 145 | 57 (39%) | 20 (35%) | 37 (65%) | <0.001 |
| DTR 3 | 157 | 84 (54%) | 43 (52%) | 41 (48%) | 0.3 | 148 | 81 (55%) | 33 (41%) | 48 (59%) | <0.001 |
| MDR 3 | 157 | 48 (31%) | 23 (48%) | 25 (52%) | 0.2 | 148 | 45 (30%) | 15 (33%) | 30 (67%) | <0.001 |
| Control Blood Culture (within 3–7 days) | 64 | 0.002 | 64 | 0.076 | ||||||
| Sterilization achieved | 53 (83%) | 42 (79%) | 11 (21%) | 53 (83%) | 23 (44%) | 30 (56%) | ||||
| Not sterilized | 11 (17%) | 8 (73%) | 3 (27%) | 11 (17%) | 8 (73%) | 3 (27%) | ||||
| Treatment related factors | ||||||||||
| ET duration (days) | 157 | 3.0 (2.0, 6.0) | 4.0 (3.0, 7.0) | 3.0 (1.0, 4.0) | <0.001 | 148 | 3.0 (2.0, 7.0) | 3.0 (2.0, 7.0) | 3.0 (3.0, 5.2) | 0.5 |
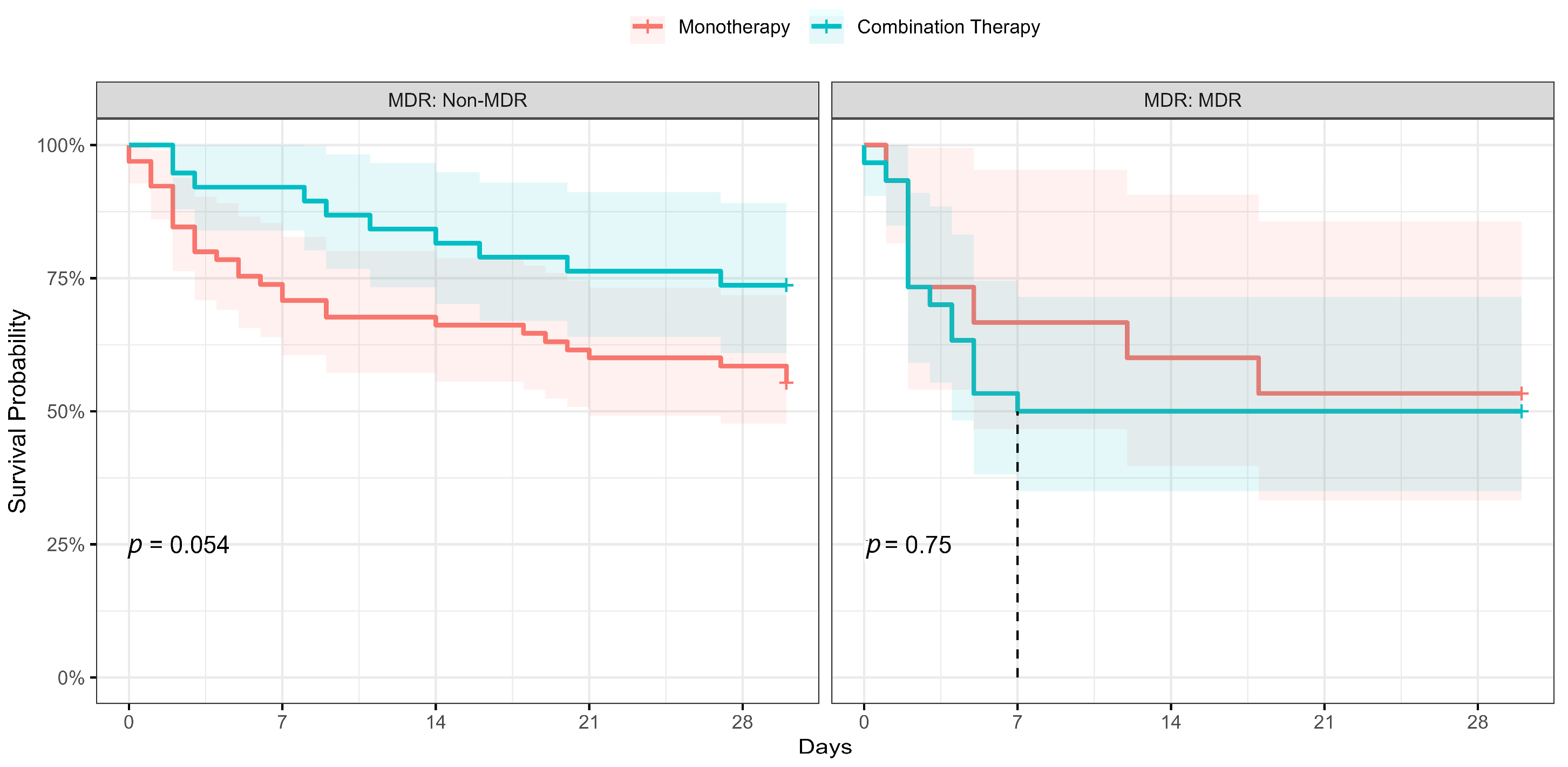
| Combination ET | 136 | 23 (17%) | 15 (63%) | 8 (37%) | 0.4 | 136 | 23 (17%) | 6 (26%) | 17 (74%) | 0.008 |
| ET with TZP (mono/combi) | 145 | 57 (39%) | 34 (60%) | 23 (40%) | 0.4 | 141 | 57 (40%) | 38 (66%) | 19 (34%) | 0.008 |
| ET with CP (mono/combi) | 145 | 69 (48%) | 36 (52%) | 33 (48%) | 0.5 | 141 | 69 (49%) | 30 (43%) | 39 (57%) | 0.024 |
| ET with AG (mono/combi) | 145 | 9 (6.2%) | 6 (66%) | 3 (34%) | 0.7 | 141 | 9 (6.4%) | 1 (11%) | 8 (89%) | 0.013 |
| ET Polymyxins | 145 | 14 (9.7%) | 9 (64%) | 5 (46%) | 0.5 | 141 | 14 (9.9%) | 4 (29%) | 10 (71%) | 0.052 |
| DT duration (days) | 157 | 11 (2, 14) | 14 (12, 15) | 2 (0, 7) | <0.001 | 148 | 12 (3, 14) | 10 (3, 14) | 14 (5, 14) | 0.2 |
| Combination DT | 148 | 68 (46%) | 43 (63%) | 25 (37%) | 0.3 | |||||
| DT TZP | 157 | 34 (22%) | 22 (65%) | 12 (35%) | 0.2 | 148 | 34 (23%) | 29 (85%) | 5 (15%) | <0.001 |
| DT Carbapenem | 157 | 67 (43%) | 43 (73%) | 24 (27%) | 0.057 | 148 | 67 (45%) | 22 (33%) | 45 (67%) | <0.001 |
| DT Aminoglycoside | 157 | 27 (17%) | 22 (81%) | 5 (29%) | 0.003 | 148 | 27 (18%) | 3 (11%) | 24 (89%) | <0.001 |
| DT Polymyxin | 157 | 34 (22%) | 20 (59%) | 14 (41%) | 0.7 | 148 | 34 (23%) | 1 (3%) | 33 (97%) | <0.001 |
| Bacteremia/sepsis related biochemical factors 1 | ||||||||||
| CRP Ratio (72 h/0 h) | 117 | 0.61 (0.43, 0.76) | 0.60 (0.43, 0.75) | 0.62 (0.43, 0.94) | 0.6 | 117 | 0.61 (0.43, 0.76) | 0.60 (0.40, 0.75) | 0.64 (0.45, 0.82) | 0.6 |
| PCT Ratio (72 h/0 h) cutoff ≥ 0.4 | 103 | 39 (38%) | 22 (29%) | 17 (65%) | <0.001 | 103 | 39 (38%) | 22 (56%) | 17 (44%) | 0.4 |
| Neutrophil Count Ratio (24 h/0 h) | 126 | 0.75 (0.64, 0.94) | 0.74 (0.60, 0.90) | 0.77 (0.67, 1.37) | 0.053 | 126 | 0.75 (0.64, 0.94) | 0.77 (0.61, 0.94) | 0.74 (0.65, 0.94) | >0.9 |
| Platelet count < 1003/L | 157 | 31 (20%) | 4 (4.6%) | 27 (39%) | <0.001 | 148 | 26 (18%) | 15 (58%) | 11 (42%) | 0.7 |
| Acute Kidney Injury | 157 | 43 (27%) | 11 (13%) | 32 (46%) | <0.001 | 148 | 38 (26%) | 20 (53%) | 18 (47%) | 0.8 |
| Univariable Cox PH | Multivariable Cox PH | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N (Total) | HR 1 | 95% CI 1 | p-Value | N (Total) | HR 1 | 95% CI 1 | p-Value |
| Age | 157 | 1.01 | 1.0, 1.02 | 0.2 | 148 | 1.00 | 0.98, 1.02 | 0.8 |
| Gender Male | 157 | 1.06 | 0.66 | 1.70 | 0.8 | 0.99 | 0.56, 1.75 | >0.9 |
| Period | 157 | |||||||
| Period 1 | Reference | |||||||
| Period 2 | 1.06 | 0.66, 1.70 | 0.8 | 148 | 2.18 | 1.14, 4.15 | 0.018 | |
| Immunocompromised state | 157 | 2.70 | 1.61, 4.51 | <0.001 | ||||
| Solid Cancer | 157 | 2.23 | 1.34, 3.72 | 0.002 | ||||
| CCI | 157 | 1.17 | 1.08, 1.27 | <0.001 | 148 | 1.18 | 1.05, 1.31 | 0.004 |
| Procalcitonin on blood culture time | 149 | 1.00 | 1.00, 1.00 | 0.7 | ||||
| C reactive protein level on blood culture time | 157 | 1.00 | 1.00, 1.01 | <0.001 | 148 | 1.00 | 1.00, 1.00 | 0.093 |
| Procalcitonin ratio 72nd h/0 h ≥ 0.4 | 103 | 3.90 | 1.74, 8.77 | <0.001 | ||||
| Platelet count < 105/L | 157 | 4.77 | 2.91, 7.81 | <0.001 | 148 | 6.92 | 3.32, 14.4 | <0.001 |
| Acute Kidney Injury | 157 | 3.06 | 1.91, 4.92 | <0.001 | 148 | 2.64 | 1.37, 5.09 | 0.004 |
| MDR | 157 | 1.51 | 0.93, 2.47 | 0.10 | 148 | 2.49 | 1.25, 4.96 | 0.010 |
| DTR | 157 | 1.42 | 0.89, 2.29 | 0.14 | 148 | 0.63 | 0.26, 1.53 | 0.3 |
| BSI Source | 157 | 148 | ||||||
| Secondary Bloodstream Infections (BSIs) | Reference | |||||||
| Primary Bloodstream Infection (BSI) | 1.39 | 0.85, 2.27 | 0.2 | 2.08 | 1.12, 3.86 | 0.020 | ||
| Blood culture eradication after 3 days | 64 | 0.14 | 0.06, 0.35 | <0.001 | ||||
| Empirical therapy duration ≥ 2.5 days | 157 | 0.33 | 0.21, 0.53 | <0.001 | ||||
| Combination therapy | 148 | 0.78 | 0.47, 1.31 | 0.4 | 148 | 0.62 | 0.31, 1.22 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derin, O.; Şahin, M.; Dumlu, R.; Başgönül, S.; Bayrak, A.D.; Arduç, Ş.; Bayram, S.; Mikaliyova, N.; Kantürk, A.; Öncül, A.; et al. Registry-Based Retrospective Cohort Study of Mortality among Adults Admitted to Intensive Care Units in Istanbul with Hospital Acquired Pseudomonas aeruginosa Bloodstream-Infection between 2014–2021. Antibiotics 2024, 13, 90. https://doi.org/10.3390/antibiotics13010090
Derin O, Şahin M, Dumlu R, Başgönül S, Bayrak AD, Arduç Ş, Bayram S, Mikaliyova N, Kantürk A, Öncül A, et al. Registry-Based Retrospective Cohort Study of Mortality among Adults Admitted to Intensive Care Units in Istanbul with Hospital Acquired Pseudomonas aeruginosa Bloodstream-Infection between 2014–2021. Antibiotics. 2024; 13(1):90. https://doi.org/10.3390/antibiotics13010090
Chicago/Turabian StyleDerin, Okan, Meyha Şahin, Rıdvan Dumlu, Sedef Başgönül, Ahmet Doğukan Bayrak, Şevval Arduç, Sümeyye Bayram, Nurlana Mikaliyova, Arzu Kantürk, Ahsen Öncül, and et al. 2024. "Registry-Based Retrospective Cohort Study of Mortality among Adults Admitted to Intensive Care Units in Istanbul with Hospital Acquired Pseudomonas aeruginosa Bloodstream-Infection between 2014–2021" Antibiotics 13, no. 1: 90. https://doi.org/10.3390/antibiotics13010090
APA StyleDerin, O., Şahin, M., Dumlu, R., Başgönül, S., Bayrak, A. D., Arduç, Ş., Bayram, S., Mikaliyova, N., Kantürk, A., Öncül, A., Yıldız Sevgi, D., Gençer, S., Bayraktar, B., Dökmetaş, İ., & Mert, A. (2024). Registry-Based Retrospective Cohort Study of Mortality among Adults Admitted to Intensive Care Units in Istanbul with Hospital Acquired Pseudomonas aeruginosa Bloodstream-Infection between 2014–2021. Antibiotics, 13(1), 90. https://doi.org/10.3390/antibiotics13010090







