Abstract
The increasing prevalence of antimicrobial resistance and the limited availability of new antimicrobial agents have created an urgent need for new approaches to combat these issues. One such approach involves reevaluating the use of old antibiotics to ensure their appropriate usage and maximize their effectiveness, as older antibiotics could help alleviate the burden on newer agents. An example of such an antibiotic is chloramphenicol (CHL), which is rarely used due to its hematological toxicity. In the current study, we employed a previously published transposon mutant library in MG1655/pTF2::blaCTX-M-1, containing over 315,000 unique transposon insertions, to identify the genetic factors that play an important role during growth in the presence of CHL. The list of conditionally essential genes, collectively referred to as the secondary resistome (SR), included 67 genes. To validate our findings, we conducted gene knockout experiments on six genes: arcA, hfq, acrZ, cls, mdfA, and nlpI. Deleting these genes resulted in increased susceptibility to CHL as demonstrated by MIC estimations and growth experiments, suggesting that targeting the products encoded from these genes may reduce the dose of CHL needed for treatment and hence reduce the toxicity associated with CHL treatment. Thus, the gene products are indicated as targets for antibiotic adjuvants to favor the use of CHL in modern medicine.
1. Introduction
Antimicrobial resistance (AMR) has emerged as a significant health issue, affecting both humans and animals and representing a substantial threat. According to estimates from 2019, bacterial AMR was associated with approximately 4.95 million deaths [1]. Within this figure, about 1.27 million deaths were directly attributed to bacterial AMR with Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa being the bacteria most linked to resistance with an estimation of 929,000 fatalities worldwide [1]. Hence, urgent action is necessary to tackle these antimicrobial-resistant bacteria.
One approach to address the problem of AMR involves revitalizing old and forgotten antibiotics that are not routinely used to treat bacterial infections due to their toxicity. Chloramphenicol (CHL), which is known for its bacteriostatic activity, is an example of an old and forgotten antibiotic. This antibiotic was originally obtained from Streptomyces venezuelae [2]. It interferes with protein synthesis by binding to peptidyl transferase, an enzyme that catalyzes the peptide bond formation at the 50S ribosomal subunit of 70S ribosomes [3]. Following its introduction, CHL became extensively utilized in both human and veterinary medicine [4,5]. However, the use of CHL has been widely reduced due to its association with hematological toxicity and incidences of serious side effects, such as aplastic anemia [4,6]. The reason behind these adverse drug reactions is assumed to be related to CHL’s interaction with mitochondrial ribosomes, which structurally resemble bacterial 70S ribosomes [5,6]. However, CHL is still employed in human medicine, though mainly in treating superficial bacterial infections like conjunctivitis and otitis externa. It is also utilized in treating a few life-threatening infections when no alternative antibiotics are available [7,8,9].
Extra-intestinal pathogenic E. coli (ExPEC) has recently been identified as the most common Gram-negative pathogen in humans [10]. Furthermore, the prevalence of extended-spectrum β-lactamase (ESBL)-producing ExPEC strains has increased significantly in recent years [10]. Since ExPEC strains are involved in a wide variety of extra-intestinal infections, including meningitis, urinary tract infections (UTIs), and septicemia [11], they have become a major challenge for clinical therapy. Thus, it is critical to combat these pathogens. CHL has been shown to be effective in treating a variety of infections, including meningitis and UTIs [5,12], due to its ability to be readily distributed to many body compartments and tissues. Besides antimicrobial resistance genes, a set of non-essential genes, unrelated to the resistance mechanism per se, is normally essential for the full expression of resistance. This set of genes has been denoted as the secondary resistome (SR) based on a study of genes affecting colistin resistance in Klebsiella pneumoniae [13]. The SR to CHL treatment in E. coli is currently unknown, and the aim of the current study was to determine this in a CTX-M-1-producing strain of E. coli during exposure to CHL at a ½ minimum inhibitory concentration (MIC). Additionally, this study aimed to explore whether deleting genes with a significant impact on fitness would improve the effectiveness of CHL, thereby enhancing treatment efficiency and safety.
2. Materials and Methods
2.1. Bacterial Strains and Antimicrobial Susceptibility Testing
The bacterial wild-type (WT) strain, mutant strains, and plasmids used in the current study are listed in Table 1. Difco™ lysogeny broth (LB) as well as Lennox (Becton, Dickinson, Albertslund, Denmark) and LB agar plates (Becton, Dickinson, Albertslund, Denmark) were used for bacterial growth. Bacterial strains were grown overnight at 37 °C except for the strains harboring the temperature-sensitive plasmid, pKD46, which were grown at 30 °C. The media were supplemented with 20 mg/L cefotaxime (CTX); 50 mg/L kanamycin (KAN); 20 mg/L gentamicin (GEN); and 1–50 mg/L CHL (Sigma, Copenhagen, Denmark) when appropriate. The MIC of CHL for the WT strain and mutants under study was determined via broth microdilution method following Clinical and Laboratory Standards Institute (CLSI)’s guidelines [14].

Table 1.
E. coli strains and plasmids used in this study.
2.2. TraDIS Library, CHL Exposure, and Sequencing
A previously published transposon mutant library in MG1655/pTF2::blaCTX-M-1, containing over 315,000 unique transposon insertions [19], was employed to identify conditionally essential genes to CHL treatment. One ml aliquots of the input library carrying approximately 2 × 109 mutants were prepared. From that, 100 μL was inoculated into a falcon tube containing 9.9 mL Mueller–Hinton Broth-II (MHB-II) (Becton, Dickinson, Albertslund, Denmark), supplemented with 2 mg/L, which corresponds to ½ MIC of CHL for MG1655/pTF2. Two biological replicates were performed. The genomic DNA of the output libraries was extracted after incubation at 37 °C for 24 h using GenElute™ Bacterial Genomic DNA kit (Sigma-Aldrich, Soeborg, Denmark) following manufacturer’s instructions. For qualification and quantification of DNA samples, ratios of 260/280 and 260/230 were measured using NanoDrop (Thermo Fisher Scientific, Roskilde, Denmark), and the concentrations were estimated using Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific) (Table S1). To perform transposon-directed insertion site sequencing (TraDIS), DNA ranging from 2 to 4 μg was fragmented into approximately 300 bp fragments through mechanical shearing using Covaris M220 (Covaris, Woburn, MA, USA). Subsequently, each library was prepared for sequencing following the previously described protocols [20,21]. The quality and quantity of the PCR amplified fragments were evaluated via bioanalyzer (Agilent Technologies) and qPCR (Roche, Hvidovre, Denmark) [20,21]. The fragmented libraries were then pooled and sequenced on a MiSeq machine using a MiSeq reagent kit V2 (50 cycles) (Illumina) following the transposon sequencing recipe previously described [21].
2.3. Bioinformatic Analysis of TraDIS Data
The sequencing data were analyzed as previously described [19,20,21] using the Bio::TraDIS pipeline (https://github.com/sanger-pathogens/Bio-Tradis) (accessed on 27 June 2022). The generated files were then mapped to MG1655 reference genome (LR881938) using SMALT short-read mapper (https://www.sanger.ac.uk/tool/smalt-0/) (accessed on 27 June 2022), resulting in an accurate estimation of the insertion site of the transposon across the genome and unique insertion sites (UISs) (Table 2). The next analysis step was performed using the tradis_comparisons.R script, which revealed the log2 fold change (log2FC) of read counts and q value for each gene between the control without CHL and the test samples. The SR genes were defined as genes with a log2FC ≤ −2 and q value ≤ 0.01 for risk of false discovery. The full output data from R scripts showing the logFC and q value of each gene are described in Table S2. The raw sequence reads of this study were deposited in the European Nucleotide Archive (ENA) under the accession number PRJEB52919. STRING [22] analysis was used to determine the interactions between SR genes and to identify the enrichment of Kyoto Encyclopedia of Genes and Genomes (KEGG) terms and Gene Ontology (GO) terms based on the identified SR genes.

Table 2.
Mapping and read counts of Tn5 insertions to K-12 MG1655 reference genome.
2.4. Construction of E. coli MG1655/pTF2 Mutant Strains
Mutant strains were made in MG1655/pTF2 for the genes acrZ, cls (synonymous with clsA), mdfA, arcA, hfq, and nlpI using the Lambda Red recombination system essentially as described [17,18]. Deletion mutants were confirmed via PCR. The primers used for creating and confirming mutants are listed in Table S3.
2.5. Construction of Plasmid pACYC184_Backbone Lacking TETR
The pACYC184-CHL harboring a CHL resistance gene (cat, which encodes a CHL acetyltransferase) was generated from pACYC184 as template (GenBank X06403) using HiFi cloning (New England BioLabs, Ipswich, MA, USA) and PCR. The PCR fragment for cloning was amplified with primers listed in Table S3 using Q5 DNA polymerase (NEB). The purified HiFi cloning product (pACYC184-CHL) was used to transform electrocompetent E. coli DH5α. The recombinant plasmid was extracted using GeneJET Plasmid Miniprep (Thermo Fisher Scientific, Roskilde, Denmark) and confirmed by Sanger sequencing (Macrogen Europe). Then, MG1655/pTF2 and mutants were transformed with the recombinant plasmid using electroporation.
2.6. Growth Experiments
To validate the predictions revealed by the TraDIS analysis, the WT strain (MG1655/pTF2) and its mutant derivatives (ΔacrZ, Δcls, ΔmdfA, ΔarcA, Δhfq, and ΔnlpI) were compared in terms of their ability to grow without antibiotics and in the presence of CHL using Bioscreen C (Thermo Labsystems, Helsinki, Finland) as previously described with slight modifications [19]. Briefly, a 10−2 dilution of 0.5 MacFarland (1–2 × 108 CFU/mL) was prepared in MHB-II for each strain, resulting in a final cell density of approximately 106 CFU/mL. Then, 250 μL of the bacterial suspension was inoculated in each well. Next, the cultures were grown without antibiotics and in the presence of 2 mg/L CHL. The OD600 was measured every 30 min with continuous shaking for 24 h at 37 °C. This experiment was performed with two biological replicates using two technical replicates, and the growth curves were generated using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA).
2.7. Homology to Human Proteins
To determine whether the potential targets, which displayed increased susceptibility to CHL upon deletion, have similarities to the human proteome, the protein sequences of ArcA, Hfq, AcrZ, CLS, MdfA, and NlpI were retrieved from Artemis. These sequences were then subjected to a sequence homology search with human proteins using the NCBI Homo sapiens Protein BLAST (BLASTp) tool, accessible at http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 February 2023). Proteins that did not yield any matches with an E-value cutoff of 10−10 were identified as non-homologous proteins [23].
3. Results
3.1. MIC Testing of MG1655/pTF2
In our previous study, we reported that MG1655/pTF2 displayed resistance to CTX, with an MIC value of 256 mg/L [19]. This resistance is attributed to the presence of blaCTX-M-1 in the IncI1 plasmid. In the current study, we assessed the MIC of CHL for the same strain. The results showed that MG1655/pTF2 exhibited an MIC value of 4 mg/L for CHL. Based on the CLSI and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [14,24], MG1655/pTF2 is considered to be susceptible to CHL.
3.2. Uncovering the SR Genes to CHL in MG1655/pTF2
To identify the SR genes during the exposure to CHL, transposon insertions were compared between the control output libraries (input libraries grown in the absence of antibiotics), which had been previously sequenced and analyzed [19], and the input libraries grown in the presence of a ½ MIC of CHL. The bioinformatic analysis reveals that 67 genes were identified as SR genes to CHL (Table S2).
The top 20 significantly affected/disrupted genes during growth of the bacteria in the presence of CHL, encompassing the genes selected for validation studies, are listed in Table 3. The gene nlpI, encoding the lipoprotein NlpI, which co-ordinates peptidoglycan synthesis and hydrolysis [25], was predicted to yield the highest fitness defect (Log2FC = −6.45), followed by acrZ, encoding an assessor protein in a multidrug efflux pump [26] (Log2FC = −5.41).

Table 3.
Top twenty affected genes in the SR to CHL in MG1655/pTF2.
A visual representation of the interactions between the genes that were significantly affected during growth in the presence of a ½ MIC of CHL is shown in Figure S1. Our analysis revealed the enrichment of only one KEGG pathway (cation antimicrobial peptide (CAMP) resistance). Five GO terms were enriched in the biological processes category, including lipid transport, membrane organization, intermembrane lipid transfer, Gram-negative-bacterium-type cell outer membrane assembly, and biological regulation. No terms were enriched in the molecular functions category, while the terms protein containing complex, efflux pump complex, and cell envelope were enriched in the cellular components category (Table S4).
The genes highlighted in Table 3 were chosen for further validation through site-specific deletions, followed by an assessment of their growth performance in the absence and presence of CHL and an MIC analysis.
3.3. Validation of SR Genes to CHL
Based on the analyses performed above, acrZ, cls, mdfA, arcA, hfq, and nlpI were knocked out in MG1655/pFT2 by site-specific deletion to investigate their importance for growth in the absence and presence of CHL and to examine their effect on the MIC. These genes are associated with multidrug efflux pumps (acrZ, mdfA) [26,27], cardiolipin biosynthesis (cls) [28], transcriptional and post-transcriptional regulation (arcA, hfq) [29,30], and cell division (nlpI) [31].
In the absence of CHL, our analysis revealed that the mutations in acrZ, cls, and mdfA did not affect the growth compared to the WT strain. However, mutations in arcA, hfq, and nlpI yielded a slight growth defect (Figure 1), which should be considered in the interpretation of the results below.
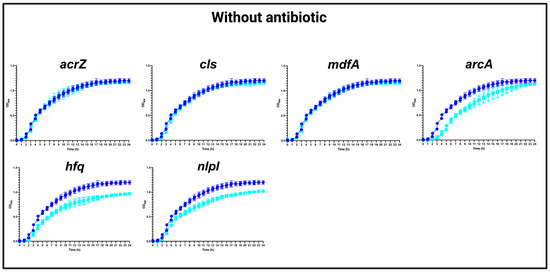
Figure 1.
Growth curves of MG1655/pTF2 (WT) and acrZ, cls, mdfA, arcA, hfq, and nlpI mutants in the absence of antibiotics. Growth curves of WT (blue line) against mutants (cyan lines) in MHB-II without CHL. The data shown are means ± standard deviations of two biological replicates with two technical replicates.
In the presence of a ½ MIC (2 mg/L) of CHL, the most obvious growth defects were found in mutants with a specific deletion of arcA, hfq, acrZ, and nlpI, respectively, while a slighter growth defect was observed for ΔmdfA, and almost no difference in growth was detected for Δcls compared to the WT strain (Figure 2).
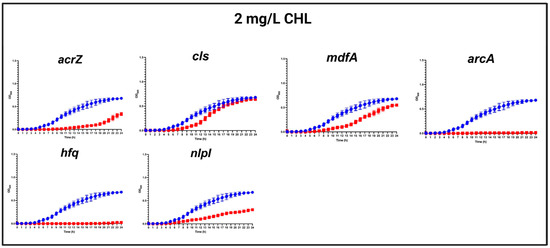
Figure 2.
Growth curves of MG1655/pTF2 (WT) and acrZ, cls, mdfA, arcA, hfq, and nlpI mutants in the presence of 2 mg/L CHL. Growth curves of WT (blue line) against mutants (red lines) in MHB-II in the presence of CHL. The data shown are means ± standard deviations of two biological replicates with two technical replicates.
3.4. Improved Efficacy to CHL
To further explain the growth experiments mentioned above, we analyzed the MICs of CHL for the selected mutant strains. The mutations in arcA and hfq led to a four-fold reduction in the MIC of CHL, whereas the mutations in acrZ, cls, mdfA, and nlpI led to a two-fold reduction in the MIC of CHL (Table 4).

Table 4.
MICs of CHL against MG1655 mutants.
Since MG1655/pTF2 is sensitive to CHL with an MIC of 4 mg/L, we extended our analysis to investigate if these findings are applicable to CHL-resistant strains. Consequently, the plasmid pACYC184-CHL harboring the cat gene that was constructed in the present study was transformed into the WT and mutant strains. Based on our susceptibility testing, a similar decreasing trend was documented in the MIC of CHL in the CHL-resistant mutant strains compared to the CHL-sensitive mutant strains (Table 4).
3.5. Homology of Proteins Encoded by the Selected Genes to Human Proteins
To assess the potential suitability of the identified targets for the development of antibiotic adjuvants, the protein sequences of ArcA, Hfq, AcrZ, CLS, MdfA, and NlpI were compared to the human proteome using NCBI-BLAST tools. This analysis aimed to minimize any undesired side effects on the host. The in silico analysis did not reveal any similarity between the ArcA, Hfq, AcrZ, CLS, MdfA, and NlpI proteins from MG1655/pTF2 and human proteins.
4. Discussion
AMR has become a pressing issue in the healthcare sector, affecting individuals worldwide. As a result, AMR has been recognized by the World Health Organization (WHO) as a major threat to global health security [32]. The growing prevalence of infections caused by multidrug-resistant Gram-negative bacilli (MDR-GNB) poses a serious challenge in healthcare facilities due to the lack of effective treatment options [33]. Additionally, no new antibacterial agents with activity exclusively against Gram-negative bacteria or resistant bacteria that withstand currently available antibiotics are currently being developed [34]. Due to this, there is a renewed interest in reconsidering older and forgotten antibiotics that have been largely abandoned due to their toxic side effects. One such antibiotic is CHL, which was extensively used in 1950s [35]. CHL’s popularity waned in the 1960s due to its toxicity, which caused two types of bone marrow effects: dose-dependent reversible anemia and unpredictable, irreversible, frequently fatal aplastic anemia. The occurrence rate of aplastic anemia was estimated to be 1 case in 24,000 to 40,000 therapy courses [36]. The rationale for reconsidering CHL is strengthened by its limited recent usage, which reduces the opportunity for bacteria to develop resistance against it [33]. Also, CHL is a broad-spectrum antibiotic that displays a powerful activity against diverse types of microorganisms, including Gram-positive and Gram-negative bacteria [37]. In addition to its ability to penetrate different tissues, CHL can be administered by several different routes, including oral, parenteral, or topical routes. Moreover, CHL is considered an inexpensive antibiotic and has been recommended by the WHO in circumstances in which modern alternatives are scarce, which is a frequently encountered scenario in low-income countries [37,38,39]. Hence, this study employed a high-throughput TraDIS screening method to identify the genes, other than the resistance genes, necessary for the growth of E. coli when exposed to CHL. Our analysis showed that a total of 67 genes were significantly affected by transposon insertions upon CHL exposure, representing the SR to CHL in MG1655/pTF2 E. coli. Therefore, their encoded products might be considered as target(s) for antibiotic adjuvants which, when applied in combination with CHL, may enhance the efficacy of antibiotics.
The SR to CHL has not previously been characterized. The pathway analysis in the CHL-sensitive strain under study via KEGG showed enrichment of only one pathway: resistance to antimicrobial peptides. The biological reason for this was not investigated further, but the genes involved included acrB, acrA, phoQ, phoP, marA, and tolC, which are related to two-component systems involved in stress response and to efflux pumps [40,41,42,43]. This suggests that efflux may play an important role in tolerating CHL in this strain. Our analysis of GO terms in different categories, however, suggested that a broader set of biological processes and cellular components (lipid transport, membrane organization, intermembrane lipid transfer, outer membrane assembly, protein-containing complex, and cell envelope) were important for growth in the presence of CHL. It remains to be investigated whether this is also the case in CHL-resistant E. coli.
To validate our results, we knocked out six genes that are linked to multidrug efflux pumps (acrZ, mdfA) [26,27], cardiolipin biosynthesis (cls) [28], transcriptional and post-transcriptional regulation (arcA, hfq) [29,30], and cell division (nlpI) [31]. The deletion of these genes in MG1655/pTF2 was found to increase sensitivity to CHL both in the original CHL-sensitive strain and in the derived CHL-resistant mutants, suggesting that the SR genes to CHL might be shared between sensitive and resistant strains. Moreover, our results agreed with those of previous studies showing that acrZ and mdfA are important for the efflux of CHL [26,44], which suggests the accuracy and reliability of the TraDIS approach used in this study. The other genes identified here (cls, hfq, nlpI, and arcA) have not previously been shown to play a role in resistance to CHL.
The cls gene encodes a cardiolipin synthase categorized as a non-essential protein for growth in many culture conditions [28,45], and in agreement with this, the mutant was not affected in terms of growth in the absence of antimicrobials. The deletion of cls was previously found to increase susceptibility to novobiocin [46], which, together with the increased susceptibility to CHL detected in our study, suggests that it may represent a target for an antibiotic adjuvant for several antimicrobials.
Our TraDIS analysis showed that hfq and nlpI were also important for growth of MG1655/pTF2 in the presence of CHL. Additionally, these genes have been previously proven to be relevant for growth when exposed to CTX [19]. Thus, as mentioned for cls, hfq and nlpI might be investigated as potential targets for different antimicrobials. Based on the log2FC, however, both genes might be more important for growth in the presence of CHL than in the presence of CTX. The deletion of nlpI was previously found to be associated with the production of high levels of MepS during growth of the bacteria [25] and the increased production of outer membrane vesicles (OMVs) [47,48]. This hypervesiculating phenotype, which is associated with improved E. coli survival in the presence of β-lactam antibiotics [49], was suggested to be the result of the high levels of peptidoglycan synthesis and the low-level production of lipoprotein outer membranes. Since the deletion of mepS was previously observed to suppress the high production of OMVs associated with the deletion of nlpI [50], we hypothesize that the nlpI mutant may exhibit a decreased susceptibility to CTX due to its association with the hypervesiculating phenotype; however, further studies are needed to confirm this hypothesis.
Among the genes we validated, the deletion of arcA led to a four-fold reduction in the MIC of CHL in MG1655/pTF2. arcA encodes the response regulator of the two-component system ArcA/B, which is a global regulator of gene expression under anaerobic and microaerobic conditions [51], and under anaerobic conditions, this system is known to repress the protein expression involved in aerobic respiration [52]. The deletion of arcA has been previously reported to be associated with activating aerobic respiration in anaerobic and microaerobic conditions [53], resulting in the enhancement of the reactive oxygen species (ROS) production in E. coli BW25113 [54]. Furthermore, it was demonstrated that the deletion of arcA significantly suppresses the evolution of resistance to ciprofloxacin, cefixime, and CHL [54], and the expression of arcA was found to be increased in E. coli strains resistant to quinolones, β-lactams, and CHL [54,55]. Since ArcA is not an essential protein [56], and several studies have proposed that ROS production contributes to antibiotic-mediated cell killing [57,58], we suggest that arcA could be a potential target to enhance the efficacy of CHL to treat infections caused by E. coli.
Unlike the development of conventional antibiotics, developing antibiotic adjuvants has the advantage of not requiring the identification of a target that is essential for bacterial survival, but a target, that, when inactivated, improves the potency of conventional antibiotics [59]. Nonetheless, there are several essential safety considerations that must be taken into account to consider a target as a promising candidate for antibiotic adjuvant development. For instance, the target should have minimal or no antibacterial activity when blocked, and it should not have similarities to host proteins [13]. These characteristics are crucial in reducing the likelihood of resistance development against antibiotic adjuvants and minimizing any undesirable effects on the host. In this context, our analysis of the growth experiments revealed no significant impacts on growth when acrZ, cls, and mdfA were inactivated compared to the WT strain in the absence of CHL. On the other hand, deletions of arcA, hfq, and nlpI resulted in a slight growth defect under similar conditions. This suggests that blocking these genes has limited or no antibacterial effects against the bacteria. Additionally, no significant similarities between AcrZ, CLS, MdfA, ArcA, Hfq, NlpI, and human proteins were detected. Taken together, these genes could serve as potential targets for antibiotic adjuvants to enhance the effectiveness of CHL, enabling treatment with lower doses and minimizing its toxic side effects. However, caution is needed when extrapolating the findings of this study, because they were based on a single E. coli strain, MG1655, which is non-pathogenic. Therefore, the possibility of using the identified genes as antibiotic adjuvant targets needs to be further validated by evaluating the effect of their deletion in pathogenic E. coli strains. This would also allow for the testing of the effect of the deletion of genes on the treatment efficacy of CHL against resistant strains in animal models by a comparison of infection outcomes following the challenge and treatment of wild-type and gene knockout mutants.
The small RNA molecule, arcA, is involved in the control of chemotaxis and motility in E. coli, and it has been shown to influence virulence in avian pathogenic E. coli [60]. Additionally, cardiolipin, whose synthesis requires cls, is important for virulence in Shigella flexnerii, a close relative of E. coli [61]. Thus, blocking these genes may have additional effects, reducing resistance to CHL, and further studies into the effects of blocking all the genes we have selected on virulence are warranted. Further, as already discussed for some of the genes, there may be effects on the susceptibility to other antibiotics than CHL. Thus, the deletion of hfq has pleiotropic effects because it encodes a chaperone, which assists several small RNA molecules when they exert their effects on gene expression [62]. A study has shown that the deletion of this gene affects the susceptibility to antibiotics of several classes [63]. Similarly, MFS efflux pumps, to which MdfA belongs, can export several drug classes and are present in many bacteria [64]. Thus, further studies on the possible effects of blocking these genes in bacteria other than E. coli are highly relevant.
In conclusion, this study provided the first list of genes that are fitness genes for growth in the presence of CHL in an E. coli-sensitive strain. Moreover, the deletion of the six selected genes increased the sensitivity to CHL, not only in the WT sensitive strain but also in the derived mutants resistant to CHL. Such genes could potentially serve as targets for antibiotic adjuvants, enabling more effective treatments of MDR bacteria with lower CHL concentrations compared to using CHL alone and therefore yielding a lower level of toxicity. Besides CHL, some of these might be helper drugs for other antibiotics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics13010073/s1. Table S1: DNA quality and quantity of CHL-treated libraries; Table S2: The full output from R scripts; Table S3: Primers used in mutant strains and plasmid pACYC184 backbone construction; Table S4: KEGG pathway and GO terms enrichment analysis for genes identified as SR to CHL; Figure S1: Representation of the interactions between the genes identified as SR to CHL.
Author Contributions
Conceptualization, M.S.A.A., A.H.-F. and J.E.O.; methodology, M.S.A.A., L.E.T., A.H.-F. and S.M.W.; software, M.S.A.A. and V.G; validation, M.S.A.A.; formal analysis, M.S.A.A.; investigation, M.S.A.A. and V.G.; resources, A.H.-F., L.E.T. and S.M.W.; data curation, M.S.A.A.; writing—original draft preparation, M.S.A.A.; writing—review and editing, M.S.A.A., A.H.-F. and J.E.O.; visualization, M.S.A.A., A.H.-F., V.G.; supervision, J.E.O.; project administration, M.S.A.A. and J.E.O.; funding acquisition, M.S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a PhD scholarship from King Saud bin Abdulaziz University for Health Sciences (KSAU-HS) (grant number SO/3289/2012) via the Saudi Arabian Cultural Office.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
The authors would like to thank the staff at the GeoGenetics Sequencing Core at the Globe Institute, University of Copenhagen for their authorization to use Covaris M220 to shear the DNA. V.G. acknowledges the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia for her post-doctoral grants (Grant Numbers ED481B-2018-018 and ED481D-2022-012). A.H.-F. is supported by a grant from the “Ministerio de Universidades”, Spain (BG22/00150—Beatriz Galindo program).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CHL | Chloramphenicol |
| SR | Secondary resistome |
| MIC | Minimum inhibitory concentration |
| AMR | Antimicrobial resistance |
| ExPEC | Extra-intestinal pathogenic E. coli |
| ESBL | Extended-spectrum β-Lactamase |
| UTIs | Urinary tract infections |
| CTX-M | Cefotaximase from Munich |
| WT | Wild-type |
| LB | Lysogeny broth |
| CTX | Cefotaxime |
| KAN | Kanamycin |
| GEN | Gentamicin |
| AMP | Ampicillin |
| TET | Tetracycline |
| MHB-II | Mueller–Hinton Broth-II |
| TraDIS | Transposon-directed insertion site sequencing |
| PCR/qPCR | Polymerase Chain Reaction/Quantitative Polymerase Chain Reaction |
| UIS | Unique insertion sites |
| ENA | European Nucleotide Archive |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| CAT | Chloramphenicol acetyltransferase |
| CFU | Colony forming unit |
| NCBI | National Center for Biotechnology Information |
| BLAST | Basic Local Alignment Search Tool |
| CLSI | Clinical and Laboratory Standards Institute |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| CAMP | Cation Antimicrobial Peptide |
| WHO | World Health Organization |
| MDR | Multidrug resistance |
| GNB | Gram-negative bacilli |
| OMV | Outer membrane vesicles |
| ROS | Reactive oxygen species |
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.; Bartz, Q.R.; Smith, R.M.; Joslyn, D.A.; Burkholder, P.R. Chloromycetin, a New Antibiotic from a Soil Actinomycete. Science 1947, 106, 417. [Google Scholar] [CrossRef] [PubMed]
- Schlünzen, F.; Zarivach, R.; Harms, J.; Bashan, A.; Tocilj, A.; Albrecht, R.; Yonath, A.; Franceschi, F. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 2001, 413, 814–821. [Google Scholar] [CrossRef]
- Settepani, J.A. The hazard of using chloramphenicol in food animals. J. Am. Vet. Med. Assoc. 1984, 184, 930–931. [Google Scholar]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Martelo, O.J.; Manyan, D.R.; Smith, U.S.; Yunis, A.A. Chloramphenicol and bone marrow mitochondria. J. Lab. Clin. Med. 1969, 74, 927–940. [Google Scholar]
- Shaw, W.V. Chloramphenicol acetyltransferase: Enzymology and molecular biology. CRC Crit. Rev. Biochem. 1983, 14, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Mascaretti, O.A. Bacteria versus Antibacterial Agents: An Integrated Approach; American Society for Microbiology (ASM): Washington, DC, USA, 2003. [Google Scholar]
- Falagas, M.E.; Kopterides, P. Old antibiotics for infections in critically ill patients. Curr. Opin. Crit. Care 2007, 13, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.T.; Wacker, M. Extraintestinal Pathogenic Escherichia coli, a Common Human Pathogen: Challenges for Vaccine Development and Progress in the Field. J. Infect. Dis. 2016, 213, 6–13. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef]
- Perdigão Neto, L.V.; Machado, A.S.; da Silva, R.G.; de Souza, R.B.C.; Coutinho, S.M.; Comello, F.; Porto, A.P.M.; Lima, D.S.; di Gioia, T.S.R.; Castro Lima, V.A.C.; et al. Case Report: Successful Treatment of Recurrent Urinary Tract Infection Due to Extensively Drug-Resistant Klebsiella Pneumoniae in a Kidney Transplant Recipient Using Chloramphenicol. Transplant. Proc. 2023, 55, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Cain, A.K.; Doerrler, W.T.; Boinett, C.J.; Fookes, M.C.; Parkhill, J.; Guardabassi, L. The secondary resistome of multidrug-resistant Klebsiella pneumoniae. Sci. Rep. 2017, 7, 42483. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B.; Cockerill, F.; Bradford, P.A. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2015. [Google Scholar]
- Kjeldsen, T.S.; Overgaard, M.; Nielsen, S.S.; Bortolaia, V.; Jelsbak, L.; Sommer, M.; Guardabassi, L.; Olsen, J.E. CTX-M-1 β-lactamase expression in Escherichia coli is dependent on cefotaxime concentration, growth phase and gene location. J. Antimicrob. Chemother. 2015, 70, 62–70. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Doublet, B.; Douard, G.; Targant, H.; Meunier, D.; Madec, J.Y.; Cloeckaert, A. Antibiotic marker modifications of lambda Red and FLP helper plasmids, pKD46 and pCP20, for inactivation of chromosomal genes using PCR products in multidrug-resistant strains. J. Microbiol. Methods 2008, 75, 359–361. [Google Scholar] [CrossRef]
- Alobaidallah, M.S.A.; García, V.; De Mets, R.; Wellner, S.M.; Thomsen, L.E.; Herrero-Fresno, A.; Olsen, J.E. Uncovering the Important Genetic Factors for Growth during Cefotaxime-Gentamicin Combination Treatment in bla(CTX-M-1) Encoding Escherichia coli. Antibiotics 2023, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Grønnemose, R.B.; Torres-Puig, S.; Kudirkiene, E.; Piantelli, M.; Ahmed, S.; Andersen, T.E.; Møller-Jensen, J.; Olsen, J.E.; Herrero-Fresno, A. Genome-wide analysis of fitness-factors in uropathogenic Escherichia coli during growth in laboratory media and during urinary tract infections. Microb. Genom. 2021, 7, 000719. [Google Scholar] [CrossRef]
- Barquist, L.; Mayho, M.; Cummins, C.; Cain, A.K.; Boinett, C.J.; Page, A.J.; Langridge, G.C.; Quail, M.A.; Keane, J.A.; Parkhill, J. The TraDIS toolkit: Sequencing and analysis for dense transposon mutant libraries. Bioinformatics 2016, 32, 1109–1111. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Mondal, S.I.; Ferdous, S.; Jewel, N.A.; Akter, A.; Mahmud, Z.; Islam, M.M.; Afrin, T.; Karim, N. Identification of potential drug targets by subtractive genome analysis of Escherichia coli O157:H7: An in silico approach. Adv. Appl. Bioinform. Chem. 2015, 8, 49–63. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.1. 2023. Available online: http://www.eucast.org (accessed on 29 October 2023).
- Singh, S.K.; Parveen, S.; SaiSree, L.; Reddy, M. Regulated proteolysis of a cross-link-specific peptidoglycan hydrolase contributes to bacterial morphogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 10956–10961. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Yin, X.; Paul, B.J.; Astarita, J.L.; Storz, G. Conserved small protein associates with the multidrug efflux pump AcrB and differentially affects antibiotic resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 16696–16701. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Brown, M.H.; Skurray, R.A. Proton-dependent multidrug efflux systems. Microbiol. Rev. 1996, 60, 575–608. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, S.; Asami, Y.; Uetake, N.; Yamagoe, S.; Ohta, A.; Shibuya, I. Disruption of the Escherichia coli cls gene responsible for cardiolipin synthesis. J. Bacteriol. 1988, 170, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Lin, E.C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc. Natl. Acad. Sci. USA 1988, 85, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Møller, T.; Franch, T.; Højrup, P.; Keene, D.R.; Bächinger, H.P.; Brennan, R.G.; Valentin-Hansen, P. Hfq: A bacterial Sm-like protein that mediates RNA-RNA interaction. Mol. Cell 2002, 9, 23–30. [Google Scholar] [CrossRef]
- Ohara, M.; Wu, H.C.; Sankaran, K.; Rick, P.D. Identification and characterization of a new lipoprotein, NlpI, in Escherichia coli K-12. J. Bacteriol. 1999, 181, 4318–4325. [Google Scholar] [CrossRef]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef]
- Sood, S. Chloramphenicol—A Potent Armament Against Multi-Drug Resistant (MDR) Gram Negative Bacilli? J. Clin. Diagn. Res. 2016, 10, Dc01–Dc03. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Flavin, M.T.; Flavin, J. Combating multidrug-resistant Gram-negative bacterial infections. Expert. Opin. Investig. Drugs 2014, 23, 163–182. [Google Scholar] [CrossRef]
- Best, W.R. Chloramphenicol-associated blood dyscrasias: A review of cases submitted to the American Medical Association Registry. JAMA 1967, 201, 181–188. [Google Scholar] [CrossRef]
- Wiest, D.B.; Cochran, J.B.; Tecklenburg, F.W. Chloramphenicol toxicity revisited: A 12-year-old patient with a brain abscess. J. Pediatr. Pharmacol. Ther. 2012, 17, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Cardoso, C.; Monteiro, R.; Vidal, S. Readdressing Chloramphenicol: Data from a Pediatric Tertiary Care Center. Lus. Sci. J. 2022, 3. [Google Scholar] [CrossRef]
- WHO. Recommendations for Management of Common Childhood Conditions: Evidence for Technical Update of Pocket Book Recommendations: Newborn Conditions, Dysentery, Pneumonia, Oxygen Use and Delivery, Common Causes of Fever, Severe Acute Malnutrition and Supportive Care. 2012. Available online: https://iris.who.int/bitstream/handle/10665/44774/9789241502825_eng.pdf?sequence=1 (accessed on 1 November 2023).
- Fuller, D.G.; Duke, T.; Shann, F.; Curtis, N. Antibiotic treatment for bacterial meningitis in children in developing countries. Ann. Trop. Paediatr. 2003, 23, 233–253. [Google Scholar] [CrossRef]
- Ma, D.; Cook, D.N.; Alberti, M.; Pon, N.G.; Nikaido, H.; Hearst, J.E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 1995, 16, 45–55. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Carey, J.N.; Leibman, R.S.; Chen, A.I.; Stern, A.M.; Roggiani, M.; Lippa, A.M.; Goulian, M. Antimicrobial peptides trigger a division block in Escherichia coli through stimulation of a signalling system. Nat. Commun. 2016, 7, 12340. [Google Scholar] [CrossRef]
- Ruiz, C.; Levy, S.B. Many chromosomal genes modulate MarA-mediated multidrug resistance in Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 2125–2134. [Google Scholar] [CrossRef]
- Fralick, J.A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 1996, 178, 5803–5805. [Google Scholar] [CrossRef]
- Edgar, R.; Bibi, E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J. Bacteriol. 1997, 179, 2274–2280. [Google Scholar] [CrossRef]
- Ivanisevic, R.; Milić, M.; Ajdić, D.; Rakonjac, J.; Savić, D.J. Nucleotide sequence, mutational analysis, transcriptional start site, and product analysis of nov, the gene which affects Escherichia coli K-12 resistance to the gyrase inhibitor novobiocin. J. Bacteriol. 1995, 177, 1766–1771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tropp, B.E.; Ragolia, L.; Xia, W.; Dowhan, W.; Milkman, R.; Rudd, K.E.; Ivanisević, R.; Savić, D.J. Identity of the Escherichia coli cls and nov genes. J. Bacteriol. 1995, 177, 5155–5157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McBroom, A.J.; Johnson, A.P.; Vemulapalli, S.; Kuehn, M.J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 2006, 188, 5385–5392. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.B.; Im, S.P.; Lee, J.S.; Jung, J.W.; Gong, T.W.; Lazarte, J.M.S.; Kim, J.; Seo, J.S.; Kim, J.H.; et al. Outer membrane vesicles from β-lactam-resistant Escherichia coli enable the survival of β-lactam-susceptible E. coli in the presence of β-lactam antibiotics. Sci. Rep. 2018, 8, 5402. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Rodriguez, D.L.; Kuehn, M.J. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. Microbiologyopen 2015, 4, 375–389. [Google Scholar] [CrossRef]
- Salmon, K.A.; Hung, S.P.; Steffen, N.R.; Krupp, R.; Baldi, P.; Hatfield, G.W.; Gunsalus, R.P. Global gene expression profiling in Escherichia coli K12: Effects of oxygen availability and ArcA. J. Biol. Chem. 2005, 280, 15084–15096. [Google Scholar] [CrossRef] [PubMed]
- Partridge, J.D.; Sanguinetti, G.; Dibden, D.P.; Roberts, R.E.; Poole, R.K.; Green, J. Transition of Escherichia coli from aerobic to micro-aerobic conditions involves fast and slow reacting regulatory components. J. Biol. Chem. 2007, 282, 11230–11237. [Google Scholar] [CrossRef]
- Nizam, S.A.; Zhu, J.; Ho, P.Y.; Shimizu, K. Effects of arcA and arcB genes knockout on the metabolism in Escherichia coli under aerobic condition. Biochem. Eng. J. 2009, 44, 240–250. [Google Scholar] [CrossRef]
- Horinouchi, T.; Maeda, T.; Kotani, H.; Furusawa, C. Suppression of antibiotic resistance evolution by single-gene deletion. Sci. Rep. 2020, 10, 4178. [Google Scholar] [CrossRef]
- Suzuki, S.; Horinouchi, T.; Furusawa, C. Prediction of antibiotic resistance by gene expression profiles. Nat. Commun. 2014, 5, 5792. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K. Epistatic Effect of Regulators to the Adaptive Growth of Escherichia coli. Sci. Rep. 2020, 10, 3661. [Google Scholar] [CrossRef] [PubMed]
- Lobritz, M.A.; Belenky, P.; Porter, C.B.; Gutierrez, A.; Yang, J.H.; Schwarz, E.G.; Dwyer, D.J.; Khalil, A.S.; Collins, J.J. Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 8173–8180. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Collins, J.J.; Walker, G.C. Unraveling the physiological complexities of antibiotic lethality. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 313–332. [Google Scholar] [CrossRef]
- Gill, E.E.; Franco, O.L.; Hancock, R.E. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem. Biol. Drug Des. 2015, 85, 56–78. [Google Scholar] [CrossRef]
- Jiang, F.; An, C.; Bao, Y.; Zhao, X.; Jernigan, R.L.; Lithio, A.; Nettleton, D.; Li, L.; Wurtele, E.S.; Nolan, L.K.; et al. ArcA Controls Metabolism, Chemotaxis, and Motility Contributing to the Pathogenicity of Avian Pathogenic Escherichia coli. Infect. Immun. 2015, 83, 3545–3554. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.M.; Yum, L.; Agaisse, H.; Payne, S.M. Cardiolipin Synthesis and Outer Membrane Localization Are Required for Shigella flexneri Virulence. mBio 2017, 8, e-01199-17. [Google Scholar] [CrossRef]
- Hoekzema, M.; Romilly, C.; Holmqvist, E.; Wagner, E.G.H. Hfq-dependent mRNA unfolding promotes sRNA-based inhibition of translation. Embo J. 2019, 38, e101199. [Google Scholar] [CrossRef]
- Gaffke, L.; Kubiak, K.; Cyske, Z.; Węgrzyn, G. Differential Chromosome- and Plasmid-Borne Resistance of Escherichia coli hfq Mutants to High Concentrations of Various Antibiotics. Int. J. Mol. Sci. 2021, 22, 8886. [Google Scholar] [CrossRef]
- De Gaetano, G.V.; Lentini, G.; Famà, A.; Coppolino, F.; Beninati, C. Antimicrobial Resistance: Two-Component Regulatory Systems and Multidrug Efflux Pumps. Antibiotics 2023, 12, 965. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).