Abstract
Antibiotic resistance (AR) associated with chronic limb-threatening ischemia (CLTI) poses additional challenges for the management of ischemic leg ulcers, increasing the likelihood of severe outcomes. This study assessed AR prevalence in bacteria isolated from CLTI-associated leg ulcers before (1 January 2017–10 March 2020; n = 69) and during (11 March 2020–31 December 2022; n = 59) the COVID-19 pandemic from patients admitted with positive wound cultures to a regional hospital in Chiang Mai (Thailand). There was a marked reduction in AR rates from 78% pre-pandemic to 42% during the pandemic (p < 0.0001), with rates of polymicrobial infections 22 percentage points lower (from 61% to 39%, respectively; p = 0.014). There were reduced AR rates to amoxicillin/clavulanate (from 42% to 4%; p < 0.0001) and ampicillin (from 16% to 2%; p = 0.017), as well as multidrug resistance (19% to 8%; p = 0.026). Factors associated with increased AR odds were polymicrobial infections (adjusted odds ratio (aOR) 5.6 (95% CI 2.1, 15.0); p = 0.001), gram-negative bacteria (aOR 7.0 (95% CI 2.4, 20.5); p < 0.001), and prior use of antibiotics (aOR 11.9 (95% CI 1.1, 128.2); p = 0.041). Improvements in infection control measures and hygiene practices in the community during the pandemic were likely key factors contributing to lower AR rates. Thus, strategic public health interventions, including community education on hygiene and the informed use of antibiotics, may be crucial in mitigating the challenges posed by AR in CLTI. Further, advocating for more judicious use of empirical antibiotics in clinical settings can balance effective treatment against AR development, thereby improving patient outcomes.
1. Introduction
Chronic limb-threatening ischemia (CLTI) is the advanced stage of atherosclerosis-induced chronic occlusive peripheral arterial disease (PAD) [1]. PAD results from severe blockage of lower-limb arteries, resulting in reduced blood perfusion; this may lead to tissue damage, unhealed ulcers, necrosis, and an increased susceptibility to infections [1,2,3]. The co-occurrence of infection and ischemia poses a substantial risk to the patient, potentially leading to major limb amputations or even fatality [4]. Severe limb infections extend to tendons, ligaments, bones, and even the proximal limb, requiring aggressive debridement and potentially leading to limb loss. As a result, CLTI has a 1-year mortality rate of 25%, and approximately 30% of CLTI patients require lower limb amputation [5].
Concurrent infections present at ulcer sites and the presence of necrotic tissue markedly affect treatment outcomes, particularly following revascularization surgery [6,7]. When superimposed infections occur in ischemic leg ulcers, treatment typically consists of multiple courses of high-potency, empirical, broad-spectrum antibiotics over an extended period before referral to a tertiary care center. However, the compromised blood flow in ischemic tissue hinders adequate antibiotic delivery, facilitating polymicrobial infections and the proliferation of highly virulent organisms [8]. Consequently, superimposed infections in ischemic leg ulcers increase the risk of multidrug-resistant (MDR) pathogens occurring [9,10], potentially leading to life-threatening untreatable infections [8]. Additionally, MDR pathogens can evolve into extensively drug-resistant strains, requiring aggressive treatment regimens involving combinations of different drugs [11,12,13].
A systematic review and meta-analysis included 148 studies and approximately 363,000 patients covering 2019–2021 [14]. It reported a 5% prevalence of bacterial co-infections and an 18% prevalence of secondary bacterial infections among hospitalized COVID-19 patients [14]. However, only 42 of the included studies reported comprehensive data on antimicrobial susceptibility, with 61% of recorded bacterial infections involving resistant pathogens [14]. Another meta-analysis of 23 studies yielded inconclusive results [15], but there have been reports of increased antibiotic resistance during the COVID-19 pandemic [16,17]. In contrast, several studies have reported reductions in antibiotic-resistance prevalence during the pandemic [18,19,20]. The uncertainties regarding COVID-19 diagnosis and concerns over bacterial co-infections or secondary infections likely underpinned substantial but inappropriate prescription of antibiotics [15]. While the latter might have led to increased antibiotic resistance, the evidence remains conflicting and, thus, inconclusive.
For the physician, ischemic leg ulcers are a concern as they frequently lead to severe pain, systemic inflammation, and a high risk of treatment failure, potentially leading to hospitalization and the need for both antibiotic therapy and surgical interventions [3]. In 2020–2022, during the COVID-19 pandemic, inevitable delays occurred in accessing minor clinical procedures, such as special dressing or debridement, due to the strict screening protocols that restricted hospital entry and exit and limited non-urgent admissions [21]. The consequences of delayed treatment were exacerbated by the demands on medical resources for COVID-19 patients, which put substantial pressure on healthcare systems [22,23]. In this context, physicians frequently resorted to empiric broad-spectrum antibiotics to mitigate the risks of deep infection and potential limb loss. However, this practice led to adverse public health consequences, such as variations in antibiotic-resistant genes, as well as changes in the spectrum of pathogens and the severity of associated foot infections. Conversely, it was suggested that the COVID-19 pandemic would not substantially increase the prevalence of antimicrobial resistance in most countries, owing to enhanced infection prevention and control efforts in community and healthcare settings [24].
Although several studies have explored changes in antibiotic resistance associated with diabetes-related foot ulcers during the COVID-19 pandemic [16,21], to our knowledge, no studies have specifically reported the prevalence and spectrum of bacteria isolated from ischemic leg ulcers. Therefore, we examined the infection rates of antibiotic-resistant bacteria in ischemic leg ulcers among PAD patients admitted to a tertiary medical center before and during the COVID-19 pandemic. In addition, we aimed to characterize the recorded bacterial taxa, including the antibiotics they were resistant to. Lastly, we explored the associated risk factors and characteristics of the infected wounds.
2. Results
2.1. Study Population
During the six years covered by this study, 197 patients were admitted with CLTI of Rutherford class ≥5, including 106 patients pre-COVID-19 and 91 during the pandemic, but 37 and 32 patients, respectively, were subsequently excluded (Figure 1). Thus, 69 and 59 patients, respectively, met the main inclusion criteria of confirmed chronic ischemic leg ulcers with signs of infection and positive wound cultures (Figure 1).
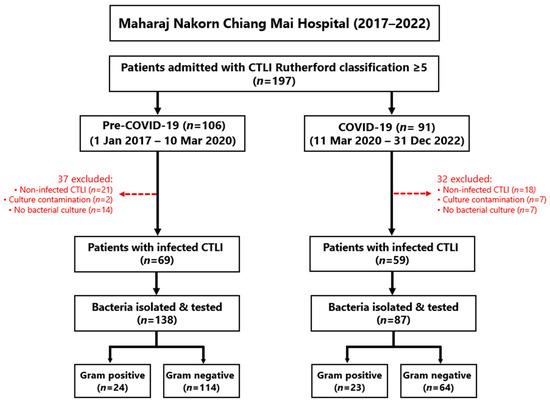
Figure 1.
Flow diagram showing the recruitment of patients into this study, including the reasons for exclusion.
The demographic characteristics of the study population are shown in Table 1. Most patients were male (62%) and elderly, with a median age of 69 years (range 35–99 years) (Table 1). Pre-pandemic and pandemic patients had similar demographic and clinical characteristics, except for higher rates of revascularization procedures during the COVID-19 pandemic (51 vs. 32%; p = 0.029). There was also indication that the median duration of ulceration before hospitalization was longer in the pre-pandemic compared to the pandemic period (30 vs. 20 days, respectively; p = 0.052) (Table 1). Importantly, rates of systemic inflammatory response syndrome (SIRS) and severity of wound infections were similar in the two periods (Table 1).

Table 1.
Demographic and clinical characteristics of patients admitted to hospital with CLTI-associated ischemic leg ulcers before (1 January 2017–10 March 2020) and during (11 March 2020–31 December 2022) the COVID-19 pandemic.
2.2. Infection Types and Prescribed Antibiotics
Microbiological analyses showed a markedly higher rate of polymicrobial infections pre-pandemic compared to the pandemic period (61% vs. 39%, respectively, or +22% (95% CI 5, 39%); p = 0.014) (Table 2). All but one patient was prescribed an antibiotic at admission (Table 2). Over two-thirds of patients (68%) across the six years had exclusive gram-negative infections, with similar rates in the two periods (69% vs. 67%, respectively; Table 2). However, rates of mixed infections were more than two-fold higher pre-pandemic (23% vs. 10% or +13.0% (95% CI 0.4, 26%); p = 0.043).

Table 2.
Bacterial infection types and prescribed antibiotics in patients admitted to hospital with CLTI-associated ischemic leg ulcers before (1 January 2017–10 March 2020) and during (11 March 2020–31 December 2022) the COVID-19 pandemic.
Overall, 95% of patients received empirical antibiotic treatment before wound culture, with little change between periods (Table 2). Among the empirical treatment regimens, clindamycin and ciprofloxacin were the most prescribed antibiotics (75% and 69%, respectively), without evidence of rate differences between periods (Table 2). Similarly, combined treatment rates with ciprofloxacin and clindamycin did not change (62% pre-pandemic and 64% during the pandemic).
2.3. Differences in AR Rates
The six-year rate of patients with at least one bacterial taxon with AR cultured from a leg ulcer was 61.7% (79/128). However, there were marked differences between study periods (Figure 2). Pre-pandemic, the three-year AR rate was 78% (54/69) compared to 42% (25/59) during the COVID-19 pandemic (+36% (95% CI 20, 52%); p < 0.0001) (Figure 2). Notably, while there was relatively limited variation in yearly AR rates pre-pandemic, rates progressively increased throughout the COVID-19 pandemic (29% in 2020, 43% in 2021, and 50% in 2022; Figure 2). From the 128 patients, 225 bacterial samples were isolated, of which 79.1% were gram-negative and 20.9% were gram-positive.
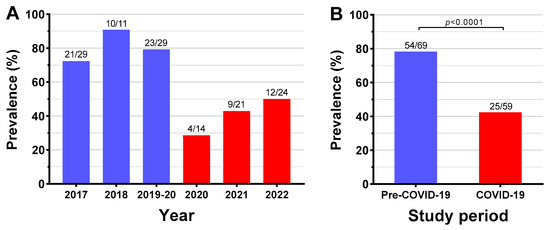
Figure 2.
Yearly (A) and overall (B) prevalence of antibiotic resistance in 128 patients with CLTI-associated leg ulcers before (1 January 2017–10 March 2020) and during (11 March 2020–31 December 2022) the COVID-19 pandemic.
When data on the 225 bacterial samples tested were compared between the two periods, pre-pandemic AR rates for amoxicillin/clavulanate were 10-fold higher (42% vs. 4%; +38% (95% CI 27, 51%); p < 0.0001) and for ampicillin 8-fold higher (16% vs. 2%; +14% (95% CI 5, 24%); p = 0.017) (Table 3). Despite the proportionally lower numbers of antibiotic-resistant isolates during the pandemic for seven of the 12 antibiotics tested, no other statistically significant differences were observed between the two periods (Table 3).

Table 3.
Prevalence of antibiotic resistance in bacteria isolated from CLTI-associated leg ulcers before (1 January 2017–10 March 2020) and during (11 March 2020–31 December 2022) the COVID-19 pandemic.
There were 33 bacterial isolates (14.7%) considered of greater clinical and public health relevance (Table 4). Consistent with the above-described findings, the pre-pandemic 3-year rate of MDR bacteria was markedly higher compared to the pandemic rate (18.8% vs. 8.0%; +10.8% (95% CI 2.1, 19.5%); p = 0.026) (Table 4). Nonetheless, most of these bacteria were relatively rare in our study population, ranging from 0.4% to 1.3%, except for the higher prevalence of ESBL Escherichia coli (4.9%; 11/225), ESBL Proteus spp. (3.6%; 8/225), and ESBL Klebsiella spp. (3.1%; 7/225) (Table 4).

Table 4.
Clinically important antibiotic-resistant bacteria isolated from CLTI-associated leg ulcers before (1 January 2017–10 March 2020) and during (11 March 2020–31 December 2022) the COVID-19 pandemic.
2.4. Gram-Positive Bacteria
The analysis of the 47 samples of gram-positive bacteria showed that overall AR rates in the pre-pandemic and pandemic periods were 29% and 22%, respectively (Table 5). Although the proportion of antibiotic-resistant isolates seemed to be lower for four of the ten drugs tested during the pandemic, no statistically significant differences were detected between the two periods (Table 5).

Table 5.
Antibiotic resistance in gram-positive bacteria isolated from CLTI-associated leg ulcers before (1 January 2017–10 March 2020) and during (11 March 2020–31 December 2022) the COVID-19 pandemic.
The overall AR rate among gram-positive bacteria was 26% (12/47), with at least one isolated taxon displaying AR against one out of 10 antibiotics (Table 5 and Supplementary Table S2). Staphylococcus aureus was the predominant gram-positive bacterium isolated (40%; 19/47), with 16% (3/19) of cultures found to be antibiotic-resistant (Supplementary Table S2 and Table 5). Enterococcus spp. was the second most common gram-positive taxon (n = 17; 36%) with a 35% AR rate (6/17; Supplementary Table S2 and Table 5). These two taxa comprised 77% (36/47) of gram-positive isolates (Supplementary Table S2 and Table 5). The antibiotics with the greatest AR rates among gram-positive bacteria were clindamycin and erythromycin (both 17% (5/30); Supplementary Table S2 and Table 5). Among the two Staphylococcus epidermidis isolates, AR was observed against six of the seven antibiotics tested (Supplementary Table S2 and Table 5).
2.5. Gram-Negative Bacteria
In Table 6, the 178 gram-negative bacterial samples tested were compared between the two periods. The overall pre-pandemic AR rate was higher than the pandemic rate (61% vs. 39%, respectively; +22% (95% CI 7, 37%); p = 0.007). This observation aligns with the markedly higher pre-pandemic AR rates for specific antibiotics: ampicillin was 19-fold higher (19% vs. 0%; +19% (95% CI 9, 29%); p = 0.006) and amoxicillin/clavulanate was 10-fold higher (42% vs. 4%; +38% (95% CI 27, 51%); p < 0.0001) (Table 6). Among individual gram-negative bacteria, there was a detectable AR rate reduction for Morganella morganii despite very few pandemic isolates (n = 3), as all ten pre-pandemic isolates tested were antibiotic-resistant (33% vs. 100%; +67% (95% CI 13, 100%); p = 0.014) (Table 6).

Table 6.
AR in gram-negative bacteria from CLTI-associated leg ulcers before (1 January 2017–10 March 2020) and during (11 March 2020–31 December 2022) the COVID-19 pandemic.
Over half of the individual gram-negative bacterial cultures (53%; 94/178) were resistant to at least one of the eight antibiotics tested (Table 6 and Supplementary Table S3). This AR rate was markedly higher compared to the observed 26% rate for gram-positive bacteria (+27% (95% CI 13, 42%); p < 0.001). Further, 12 out of 13 gram-negative bacterial taxa in the study were resistant to at least two antibiotics (Supplementary Table S3 and Table 6). Escherichia coli and Pseudomonas aeruginosa were the most common gram-negative bacteria isolated, each making up 15% of the overall records for this group (27/178 and 26/178, respectively; Supplementary Table S3 and Table 6). Notably, the AR rate against at least one antibiotic for both Escherichia coli and Morganella morganii was 85% (23/27 and 11/13, respectively), with 77% of Morganella morganii found to be resistant against amoxicillin/clavulanate (10/13) (Supplementary Table S3 and Table 6). The highest AR rates were recorded against fluoroquinolones (30%; 53/178) and amoxicillin/clavulanate (29%; 39/134) (Supplementary Table S3 and Table 6). Importantly, among Escherichia coli, AR was recorded against all eight antibiotics tested and against seven antibiotics for Citrobacter freundii and Klebsiella pneumoniae (Supplementary Table S3 and Table 6).
2.6. AR Risk Factors
In univariable analyses, the odds ratio of AR during the COVID-19 pandemic was nearly five-fold lower compared to the pre-pandemic period (OR 0.21; p < 0.001) (Table 7). Other risk factors associated with increased odds of AR were having a polymicrobial infection (OR 3.37 vs. monomicrobial; p = 0.002) or a wound infection with gram-negative bacteria alone (OR 2.57; p = 0.015) and hospitalization within 6 months before admission (OR 2.10; p = 0.038) (Table 7).

Table 7.
Risk factors for antibiotic resistance among the study population.
The multivariable logistic regression yielded adjusted odds ratios (aOR) identical to the univariable analysis, also showing odds of AR approximately five-fold lower during the pandemic period (0.21 (95% CI 0.08, 0.51); p = 0.001) (Table 7). Risk factors that remained associated with increased odds of AR were a polymicrobial infection (aOR 5.58; p = 0.001) or a gram-negative only infection (aOR 6.98; p < 0.001) (Table 7). In addition, there were markedly higher odds of AR in association with previous empirical antibiotic treatment (aOR 11.9; p = 0.041) and increased respiratory rate at admission (aOR 6.17; p = 0.047) (Table 7).
3. Discussion
The COVID-19 pandemic has profoundly affected healthcare services worldwide, particularly regarding resource distribution and, thus, patient care. However, this study shows that the pandemic was associated with markedly lower AR rates among patients admitted to our tertiary hospital in Chiang Mai with infected ischemic leg ulcers due to CLTI. These were accompanied by considerably lower AR rates for certain antibiotics (e.g., amoxicillin/clavulanate and ampicillin) and lower rates of polymicrobial and mixed infections, while the rate of MDR infections more than halved. The latter is particularly notable as MDR bacteria are associated with more severe infections and worse patient outcomes [6].
Our observations are corroborated by previous studies consistently showing decreases in AR rates among inpatients during the pandemic, such as in Italy [18,20], Turkey [19], and Singapore [25]. These were largely attributed to improved hospital infection prevention and control measures [18,19,20]. Practices that reportedly mitigate the risk of nosocomial infections with AR pathogens include screening potential COVID cases, segregating patients with respiratory symptoms, enforcing physical distancing between beds, mandating masks, and intensifying disinfection protocols [25]. Further evidence that lower AR rates in Chiang Mai were likely due to COVID-19 measures was provided by the steady increase in yearly AR rates throughout the 3-year study period following the pandemic declaration in March 2020.
Importantly, apart from the pandemic-associated reduction in rates of nosocomial infections (for pathogens other than SARS-CoV-2), our study also indicated reductions in home- and community-acquired AR infection rates. Our samples were collected directly from wound sites at the time of patient admission, which indicates frequent bacterial colonization of wounds acquired in the community. Thus, it is likely that many CLTI infections with secondary complications originate from community settings. Several factors may contribute to community-acquired AR infections, such as inadequate wound care, inadequate use and/or overuse of antibiotics [26], exposure to infected sources, and poor hygiene practices [27]. In addition, AR bacteria can also increase in community settings via contaminated food and water [28].
Additional strong evidence of marked reductions in community transmission of common pathogens during the pandemic was reported in New Zealand [29]. During the winter of 2020, stringent government-imposed lockdowns led to the near complete elimination of influenza virus transmission, when surveillance data showed a 99.9% reduction in influenza cases compared to previous years [29]. Our study’s apparent reduction in community-acquired infections with antibiotic-resistant bacteria may be partially attributed to government-imposed measures and heightened public awareness following the COVID-19 pandemic declaration in March 2020 [30]. In Thailand, the government imposed a range of measures, including the inception of the Centre for COVID-19 Situation Administration (CCSA), the imposition of lockdowns, border controls, quarantine protocols, and public hygiene campaigns [30]. The success of the collaborative effort between government initiatives and public compliance halted community transmission of SARS-CoV-2 during the first wave, achieving 220 consecutive days with no confirmed cases of community transmission [31]. While the CCSA was officially closed in October 2020 and lockdowns were lifted, several key preventative measures remained in place to some extent throughout the three-year duration of the pandemic. These included physical distancing, mask-wearing, regular handwashing, frequent antigen self-testing, and continuous risk communication to the public [31].
Clinical practice guidelines for ischemic leg infections are mostly based on studies of diabetic foot infections [32]. Here, we examined bacterial pathogens associated with CLTI, which are less explored. Our results suggest different infection patterns in CLTI-associated leg ulcers compared to diabetic foot infections. Regarding Gram stain characteristics, diabetic foot infections appear to be colonized mostly by gram-positive bacteria (≈75%) [10,32] compared to 21% among our patients and ≈42% in a Romanian study [33]. Notably, early-stage wounds are mainly infected by gram-positive bacteria, such as Staphylococcus spp. and Streptococcus spp., which come from normal skin flora [34]. In contrast, chronic wounds, if deprived of oxygen due to arterial insufficiency, can lead to prolonged infections, often colonized by gram-negative bacterial genera such as Pseudomonas, Acinetobacter, and Stenotrophomonas [35]. In our study, Staphylococcus aureus and Enterococcus spp. were the main gram-positive bacteria recorded. Still, infections were primarily associated with gram-negative bacteria, particularly Escherichia coli and Pseudomonas aeruginosa.
The choice of antibiotic treatment in CLTI often relies on expert opinion rather than evidence-based recommendations [6,33]. While there seems to be no consensus on the optimal antibiotic regimen for CLTI, empirical antibiotic therapy is the most common approach for such infections, targeting the most likely pathogens before the exact bacterium species is identified. However, these infections can lead to complications such as failed revascularization, resulting in the incomplete healing of ischemic lesions and subsequently increasing the risk of major limb amputations [36,37]. A previous study showed that infected CLTI patients who received empirical antibiotics before revascularization had amputation and death rates similar to those without infection [36]. Therefore, it is concerning that we observed relatively high AR rates for commonly used empirical antibiotics for CLTI, especially ciprofloxacin, clindamycin, and amoxicillin/clavulanate. Antibiotic resistance to fluoroquinolone and amoxicillin/clavulanate was approximately 30% among gram-negative bacteria. While robust conclusions cannot be drawn from routinely collected retrospective data, 95% of our patients hospitalized with CLTI reported a history of previous antibiotic treatment, mostly prescribed by general practitioners. Therefore, our findings suggest that excessive use of empirical antibiotics was likely an important contributor to antibiotic resistance in CLTI, especially when empirical therapy involves ineffective antibiotics against the causative organism(s) [10]. As a result, future studies should compare previous empirical antibiotic treatments with the laboratory results of antibiotic resistance in patients with CLTI.
A limitation of our study was its retrospective design involving a single center with a relatively limited number of cases. While our hospital served as a tertiary center during the pandemic, we faced a manageable number of COVID-19 inpatients compared to secondary or provincial hospitals. Therefore, caution is required when generalizing our results. In addition, our cross-sectional study examined data collected only at the patient’s hospital admission, which might have biased our observations regarding antibiotic-resistance rates in the absence of longitudinal data. Further, we did not evaluate how different wound dressing and care approaches might have affected secondary infections. Lastly, several bacterial taxa (e.g., Citrobacter freundii and Klebsiella pneumoniae) exhibit natural resistance to specific antibiotics [38,39], which would likely affect susceptibility assessments, as reported, for example, for Proteus vulgaris and Enterobacter cloacae to aminoglycosides and Acinetobacter baumannii to fluoroquinolones [38,40,41]. However, distinguishing between natural and acquired resistance is challenging. While underlying natural resistance is most unlikely to have yielded Type I errors (i.e., concluding that AR rates changed when they did not), such bacterial taxa might have masked certain changes in AR rates, leading to Type II errors. Despite these considerations, our research is among the initial efforts to compare AR in ischemic limbs pre- and post-COVID-19. Additionally, comprehensive descriptions of antibiotic resistance and the related bacterial taxa in CLTI-associated leg ulcer infections within Thailand had not been previously explored.
4. Conclusions
We observed markedly lower rates of antibiotic resistance among patients admitted to our regional hospital with infected ischemic leg ulcers due to CLTI. We speculate that improvements in infection control measures and hygiene practices in the community during the pandemic were likely key factors contributing to lower AR rates. Thus, strategic public health interventions, including community education on hygiene and the informed use of antibiotics, may be crucial in mitigating the challenges posed by antibiotic resistance in CLTI. Further, advocating for more judicious use of empirical antibiotics in clinical settings can balance effective treatment against AR development, thereby improving patient outcomes.
5. Material and Methods
5.1. Ethics Approval
This study was approved by the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University (SUR-2565-09322). Informed consent from individual patients was not necessary, as this was a retrospective audit of data from routine clinical care based on de-identified patient data.
5.2. Study Design
This study was conducted at the Maharaj Nakorn Chiang Mai Hospital, a teaching hospital affiliated with the Faculty of Medicine at Chiang Mai University (Chiang Mai, Thailand). It was the first university hospital established outside Bangkok, and it is an important regional medical center providing tertiary care in Northern Thailand. This hospital is well-equipped to manage complex medical cases, including CLTI and AR. Patient data for this study were sourced from the Department of Vascular Surgery, spanning six years from 1 January 2017 to 31 December 2022.
We extracted data from electronic medical records, including admission and discharge summaries, laboratory data, and microbiological diagnostic information for infected ulcers. Eligible participants were all patients with PAD assessed for CLTI and diagnosed with chronic ischemic leg ulcers using Rutherford categories before admission [3]. PAD was diagnosed based on abnormal pedal pulse palpation due to chronic atherosclerosis and confirmed by poor arterial perfusion using at least one of the following methods [1,42,43]:
- ▪
- ankle-brachial index (ABI)
- ▪
- toe pressure measurements
- ▪
- transcutaneous oxygen measurement (TCOM)
- ▪
- color Doppler ultrasound
- ▪
- computed tomographic angiography (CTA)
The 6-stage Rutherford classification was used to categorize PAD severity [1]:
- ▪
- 0—asymptomatic
- ▪
- 1—mild claudication
- ▪
- 2—moderate claudication
- ▪
- 3—severe claudication
- ▪
- 4—ischemic rest pain
- ▪
- 5—minor tissue loss
- ▪
- 6—major tissue loss
CLTI, indicative of advanced PAD stage, was defined as persistent foot pain or tissue necrosis for at least two weeks. In this study, ischemic leg ulcers were specifically defined as CLTI cases classified under the Rutherford Classification stage ≥ 5. Hemodynamic criteria for CLTI included [3,44]:
- ▪
- resting ankle systolic pressure < 50–70 mmHg
- ▪
- toe pressure < 40 mmHg in non-diabetic or <50 mmHg in diabetic patients
- ▪
- TCOM < 30 mmHg
Using the guidelines from the Infectious Diseases Society of America/International Working Group on Diabetic Foot (IDSA/IWGDF) [32], we classified the severity of infected ischemic wounds based on the presence of at least two of the following:
- ▪
- local swelling or induration
- ▪
- erythema (redness) >0.5 cm around the wound
- ▪
- local tenderness or pain
- ▪
- local hyperthermia
- ▪
- purulent discharge not attributable to other conditions (e.g., trauma, gout, or venous stasis
The depth of infection into bone structures was evaluated using the probe-to-bone test, with positive results leading to further investigation by X-ray for osteomyelitis diagnosis. The Society of Vascular Surgery’s Wound, Ischemia, and Foot Infection (SVS-WIfI) classification system [2] was used to evaluate the overall severity of ulcers and infections. Information regarding patient demographics, medical history, clinical evaluation, and diagnosis were extracted at the first admission date. Revascularization refers to open surgical bypass, endarterectomy, and endovascular procedure. Minor limb amputations were defined as the transverse removal of any part of the lower limb at the level of the ankle joint or below; major amputations were classified as any amputation above the ankle joint up to the thigh [32,45].
For patients diagnosed with CLTI multiple times throughout the study period, only the data from their first admission were included in this study. Other exclusion criteria were the absence of systemic or local symptoms, lack of signs of infection alongside positive wound cultures, and instances where specimens were contaminated during handling and/or processing.
5.3. Specimens and Microbiology
Bacterial pathogens were primarily identified through microbiological tests on pus samples or wound biopsies collected within the first few days of admission and before the administration of antibiotics at our hospital. It should be noted, however, that most patients had likely already received empirical antibiotics from primary healthcare providers before admission to our facility. We included only the first isolates per patient to prevent the overestimation of microbiological isolations resulting from multiple samples within the same patients. Inconsistencies were resolved using wound biopsy results. Bacterial identification was performed using MALDI-TOF [46], and antibiotic susceptibility was tested using the Vitek2 system (bioMerieux, Marcy L’Etoile, France). The results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [47].
The levels of antibiotic resistance of each identified pathogen were categorized into susceptible, intermediate, and resistant phenotypes, with intermediate results treated as susceptible. We assessed AR risks across various antibiotic groups and identified numerous bacterial pathogens resistant to antibiotics commonly used in routine clinical practice. These pathogens pose a considerable threat to public health, leading to prolonged illness and, in some cases, death, as well as increased healthcare costs. To assess the risk of AR across antibiotic groups (penicillins, clindamycin, macrolides, sulfonamides, cephalosporins, quinolones, and aminoglycosides), we considered a loss of susceptibility affecting the bactericidal or bacteriostatic properties of an antibiotic agent, as indicated by laboratory results [8,12,16]. Subsequently, we analyzed the most frequently isolated agents over the study period, including Methicillin-resistant Staphylococcus aureus (MRSA), Methicillin-resistant Staphylococcus epidermidis (MRSE), extended-spectrum β-lactamase (ESBL)-producing bacteria (including those resistant to third-generation cephalosporin), Vancomycin-resistant Enterococcus (VRE), Carbapenem-resistant Enterobacterales (CRE, including Escherichia coli, Klebsiella spp., and Proteus spp.), Carbapenem-resistant Pseudomonas spp., and Carbapenem-resistant Acinetobacter spp. Multidrug resistance (MDR) was defined as acquired non-susceptibility to at least one agent in a minimum of three antibiotic categories, following the criteria established by the Clinical Laboratory Standards Institute [13].
5.4. Outcomes
The rates of antibiotic resistance in infected CLTI patients were compared between the COVID-19 pandemic and pre-pandemic years, determined based on the WHO’s declaration on 11 March 2020 [48]. The primary outcome was the rate of AR, defined as the number of patients with at least one antibiotic-resistant pathogen in their infected leg wound, stratified by the period before and during the COVID-19 pandemic. We selected the potential risk factors likely to influence AR outcomes independently, as previously reported [9,49]. These factors were assessed at hospital admission and coded as binary variables to simplify the comparison of descriptors and AR results: gender (male vs. female); age (≥60 vs. <60 years); diabetes mellitus (with vs. without); renal replacement therapy (undergoing vs. not undergoing); fever (≤38 vs. >38 °C) [32]; respiratory rate (≤20 vs. >20 breaths/min) [32], leukocytosis (≤12,000 vs. >12,000 cells/mm3) [32]; recurrent ulcer on the same limb (yes vs. no); duration of ulcer (<3 vs. ≥3 months); limb infection grade(<3 vs. >3) [32]; wound grade (<3 vs. 3) [32]; ischemic grade (<3 vs. 3) [32]; osteomyelitis (with vs. without) [32]; hospitalization within six months before admission (yes vs. no); referral case (yes vs. no); infection type (polymicrobial vs. monomicrobial); Gram stain (negative or positive vs mixed infection); any empirical antibiotic treatment in the last 30 days (yes vs. no).
5.5. Statistical Analysis
Differences in baseline clinical data between the two periods were evaluated using two-sample t-tests or the non-parametric Mann–Whitney U test for continuous data and chi-squared tests or Fisher’s exact tests for categorical data, as appropriate. The AR rates before and during the COVID-19 pandemic were compared using Fisher’s exact tests. The 95% confidence intervals (CI) for the differences between the two rates were derived using the normal approximation test for two proportions. Continuous data approximating a normal distribution were reported as the mean ± standard deviation (SD). Skewed data were reported as the median [quartile 1, quartile 3]. A multivariable logistic regression was used to explore the risk factors for infected ischemic leg ulcers in CLTI patients with antibiotic-resistant bacteria. A backward stepwise selection procedure was used to identify the predictors associated with AR likelihood. Variables with a p-value ≥ 0.1 were removed from the model at each step, and variables with a p-value < 0.05 were retained. Multicollinearity among risk factors was checked by the variance inflation factor. All tests were 2-sided with statistical significance set at p < 0.05. Statistical analyses were performed using STATA (16.1, Stata Corp LLC, College Station, TX, USA) and Minitab v21.4 (Pennsylvania State University, State College, PA, USA).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics13010035/s1; Table S1: List of antibiotics and their respective classes against which gram-positive and gram-negative bacteria were tested for resistance in our study; Table S2: Antibiotic resistance and susceptibility of gram-positive bacteria isolated from leg ulcers in patients admitted to hospital with CLTI over the six-year study period (2017–2022); Table S3: Antibiotic resistance and susceptibility of gram-negative bacteria isolated from leg ulcers in patients admitted to hospital with CLTI over the six-year study period (2017–2022).
Author Contributions
Conceptualization, A.R., B.S., J.G.B.D., K.R. and S.O. (Saritphat Orrapin); Methodology, A.R., B.S., J.G.B.D., K.R. and S.O. (Saritphat Orrapin); Data collection, all authors; Data validation, all authors; Formal analysis, A.R., J.G.B.D., K.R. and P.T.; Writing—original draft preparation, A.R., J.G.B.D., K.R. and P.T.; Writing—review and editing, all authors; Supervision, J.G.B.D. and K.R.; Funding acquisition, A.R. and K.R. All authors have read and approved the published version of the manuscript.
Funding
José G. B. Derraik was partially supported by Chiang Mai University.
Institutional Review Board Statement
Ethics approval for this study was provided by the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University (SUR-2565-09322).
Informed Consent Statement
Patient consent was not necessary due to the study’s retrospective nature and the use of de-identified data.
Data Availability Statement
The data supporting this study are available within the article. Raw data supporting this study’s findings are available from the corresponding author upon reasonable request, conditional on the necessary ethics approval of the statistical analysis plan.
Acknowledgments
This research was partially supported by Chiang Mai University. Amaraporn Rerkasem and Pak Thaichana contributed equally to this study as the first authors. ChatGPT-4 (OpenAI, San Francisco, CA, USA) was used to assist with the translation and initial revision of text from Thai to English. In addition, ChatGPT-4 and Claude 2 (Anthropic, San Francisco, CA, USA) were used for additional editorial revision before manuscript submission.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart. J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef]
- Sidawy, A.N.; Perler, B.A. Rutherford’s Vascular Surgery and Endovascular Therapy; Elsevier Health Sciences: Philadephia, PA, USA, 2022. [Google Scholar]
- Azuma, N. The diagnostic classification of critical limb ischemia. Ann. Vasc. Dis. 2018, 11, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ventoruzzo, G.; Mazzitelli, G.; Ruzzi, U.; Liistro, F.; Scatena, A.; Martelli, E. Limb salvage and survival in chronic limb-threatening ischemia: The need for a fast-track team-based approach. J. Clin. Med. 2023, 12, 6081. [Google Scholar] [CrossRef]
- Izumi, Y. Countermeasures against infection in critical limb ischemia treatments. Ann. Vasc. Dis. 2018, 11, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, J.; Lobmann, R.; Koenig, B.; Halloul, Z.; Lippert, H.; Buerger, T. The influence of polymorbidity, revascularization, and wound therapy on the healing of arterial ulceration. Vasc. Health Risk Manag. 2008, 4, 683–689. [Google Scholar] [CrossRef]
- Matta-Gutiérrez, G.; García-Morales, E.; García-Álvarez, Y.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. The influence of multidrug-resistant bacteria on clinical outcomes of diabetic foot ulcers: A systematic review. J. Clin. Med. 2021, 10, 1948. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; He, W.; Luo, T.; Tang, N. Risk factors for multidrug-resistant bacterial infections in patients with diabetic foot ulcers: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 12618–12630. [Google Scholar] [CrossRef]
- Salm, J.; Bohme, T.; Noory, E.; Beschorner, U.; Kramer, T.S.; Westermann, D.; Zeller, T. Arterial leg ulcers—Bacterial patterns, antimicrobial resistance and clinical characteristics, a retrospective single-centre cohort, 2012–2021. PLoS ONE 2023, 18, e0290103. [Google Scholar] [CrossRef]
- Bansal, E.; Garg, A.; Bhatia, S.; Attri, A.K.; Chander, J. Spectrum of microbial flora in diabetic foot ulcers. Indian J. Pathol. Microbiol. 2008, 51, 204–208. [Google Scholar] [CrossRef]
- Boyanova, L.; Mitov, I. Antibiotic resistance rates in causative agents of infections in diabetic patients: Rising concerns. Expert Rev. Anti-Infect. Ther. 2013, 11, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Simeonova, M.; Leung, V.; Lo, J.; Kan, T.; Raybardhan, S.; Sapin, M.E.; Mponponsuo, K.; Farrell, A.; et al. Antimicrobial resistance in patients with COVID-19: A systematic review and meta-analysis. Lancet Microbe 2023, 4, e179–e191. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; Soucy, J.R.; Leung, V.; So, M.; Kwan, A.T.H.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.R.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; Maiorino, M.I.; Macera, M.; Signoriello, G.; Castellano, L.; Scappaticcio, L.; Longo, M.; Gicchino, M.; Campitiello, F.; Bellastella, G.; et al. Antibiotic resistance in diabetic foot infection: How it changed with COVID-19 pandemic in a tertiary care center. Diabetes Res. Clin. Pract. 2021, 175, 108797. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, V.; Panopoulou, M.; Rafailidis, P.; Lemonakis, N.; Lazaridis, G.; Terzi, I.; Papazoglou, D.; Panagopoulos, P. The impact of the COVID-19 pandemic on antimicrobial resistance and management of bloodstream infections. Pathogens 2023, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Micozzi, A.; Assanto, G.M.; Cesini, L.; Minotti, C.; Cartoni, C.; Capria, S.; Ciotti, G.; Alunni Fegatelli, D.; Donzelli, L.; Martelli, M.; et al. Reduced transmission of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-KP) in patients with haematological malignancies hospitalized in an Italian hospital during the COVID-19 pandemic. JAC Antimicrob. Resist. 2021, 3, dlab167. [Google Scholar] [CrossRef]
- Guven, D.C.; Eroglu, I.; Ismayilov, R.; Ulusoydan, E.; Aktepe, O.H.; Telli Dizman, G.; Arik, Z.; Dizdar, O.; Unal, S.; Metan, G.; et al. Lesson learned from the pandemic: Isolation and hygiene measures for COVID-19 could reduce the nosocomial infection rates in oncology wards. J. Oncol. Pharm. Pract. 2022, 28, 1807–1811. [Google Scholar] [CrossRef]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of multidrug-resistant (MDR) bacterial infections during the COVID-19 pandemic: A retrospective study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef]
- Tao, F.; Tang, X.; Tao, H.; Luo, Y.; Cao, H.; Xiang, W.; Zhao, Y.; Jin, L. Surgical treatment of diabetic foot ulcers during the COVID-19 pandemic in China. J. Diabetes Complicat. 2020, 34, 107622. [Google Scholar] [CrossRef]
- Saleem, Z.; Haseeb, A.; Godman, B.; Batool, N.; Altaf, U.; Ahsan, U.; Khan, F.U.; Mustafa, Z.U.; Nadeem, M.U.; Farrukh, M.J.; et al. Point prevalence survey of antimicrobial use during the COVID-19 pandemic among different hospitals in Pakistan: Findings and implications. Antibiotics 2023, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Vaughan Sarrazin, M.; Wang, X.; Nordby, P.; Yu, M.; DeLonay, A.J.; Jaffery, J. Risk from delayed or missed care and non-COVID-19 outcomes for older patients with chronic conditions during the pandemic. J. Am. Geriatr. Soc. 2022, 70, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Beggs, J.J. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC Antimicrob. Resist. 2020, 2, dlaa051. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.I.; Conceicao, E.P.; Tan, J.Y.; Magesparan, K.D.; Amin, I.B.M.; Ismail, B.B.S.; Toh, H.X.; Jin, P.; Zhang, J.; Wee, E.G.L.; et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Luu, L.; Muhsin, A. A retrospective study of the overuse of extended-spectrum antibiotics in patients with community-acquired pneumonia with risk for methicillin-resistant Staphylococcus aureus and/or Pseudomonas aeruginosa. Cureus 2022, 14, e31126. [Google Scholar] [CrossRef]
- Goossens, H.; Sprenger, M.J. Community acquired infections and bacterial resistance. BMJ 1998, 317, 654–657. [Google Scholar] [CrossRef][Green Version]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Huang, Q.S.; Wood, T.; Jelley, L.; Jennings, T.; Jefferies, S.; Daniells, K.; Nesdale, A.; Dowell, T.; Turner, N.; Campbell-Stokes, P.; et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat. Commun. 2021, 12, 1001. [Google Scholar] [CrossRef]
- Plipat, T. Lessons from Thailand’s response to the COVID-19 pandemic. Thai J Public Health 2020, 50, 268–277. [Google Scholar]
- Wilasang, C.; Jitsuk, N.C.; Sararat, C.; Modchang, C. Reconstruction of the transmission dynamics of the first COVID-19 epidemic wave in Thailand. Sci. Rep. 2022, 12, 2002. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3280. [Google Scholar] [CrossRef] [PubMed]
- IluȚ, P.A. Antibiotic susceptibility and resistance of bacterial pathogens in chronic leg ulcers: A retrospective cohort study. Farmacia 2023, 71, 38–43. [Google Scholar] [CrossRef]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Nahid, M.A.; Griffin, J.M.; Lustik, M.B.; Hayes, J.J.; Fong, K.S.K.; Horseman, T.S.; Menguito, M.; Snesrud, E.C.; Barnhill, J.C.; Washington, M.A. A longitudinal evaluation of the bacterial pathogens colonizing chronic non-healing wound sites at a United States military treatment facility in the Pacific Region. Infect. Drug. Resist. 2021, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, Y.; Walther, S.; Anderson, S.; Tauber, M.; Kummer, O.; Baumgartner, R.; Diehm, N.; Dörffler-Melly, J.; Baumgartner, I. Influence of secondary infection on amputation in chronic critical limb ischemia. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 605–609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kummer, O.; Widmer, M.K.; Pluss, S.; Willenberg, T.; Vogele, J.; Mahler, F.; Baumgartner, I. Does infection affect amputation rate in chronic critical leg ischemia? Vasa 2003, 32, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pagès, J.-M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.J.; Harding, K. 2015 International Working Group on the Diabetic Foot Guidance on the prevention and management of foot problems in diabetes. Int. Wound J. 2015, 12, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.B.; Bhattacharyya, S.; Kehl, S.C.; Matukas, L.M.; Pentella, M.A.; Salfinger, M.; Schuetz, A.N. Practical guidance for clinical microbiology laboratories: Implementing a quality management system in the medical microbiology laboratory. Clin. Microbiol. Rev. 2018, 31, e00062-17. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Orsted, H.; Schultz, G.S.; Coutts, P.; Keast, D. Preparing the wound bed 2003: Focus on infection and inflammation. Ostomy Wound Manag. 2003, 49, 24–51. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).