Linezolid Resistance Genes and Mutations among Linezolid-Susceptible Enterococcus spp.—A Loose Cannon?
Abstract
1. Introduction
2. Results
2.1. Distribution of LIN Resistance Genes/Mutations among Phenotypically Susceptible Enterococci
2.2. Comparison of BMD with Additional AST Methods
2.2.1. Performance of AST Methods on the Set of Isolates with a LIN MIC of 4 mg/L
2.2.2. Predictive Ability of Different AST Methods for Genotype-Phenotype Correlation
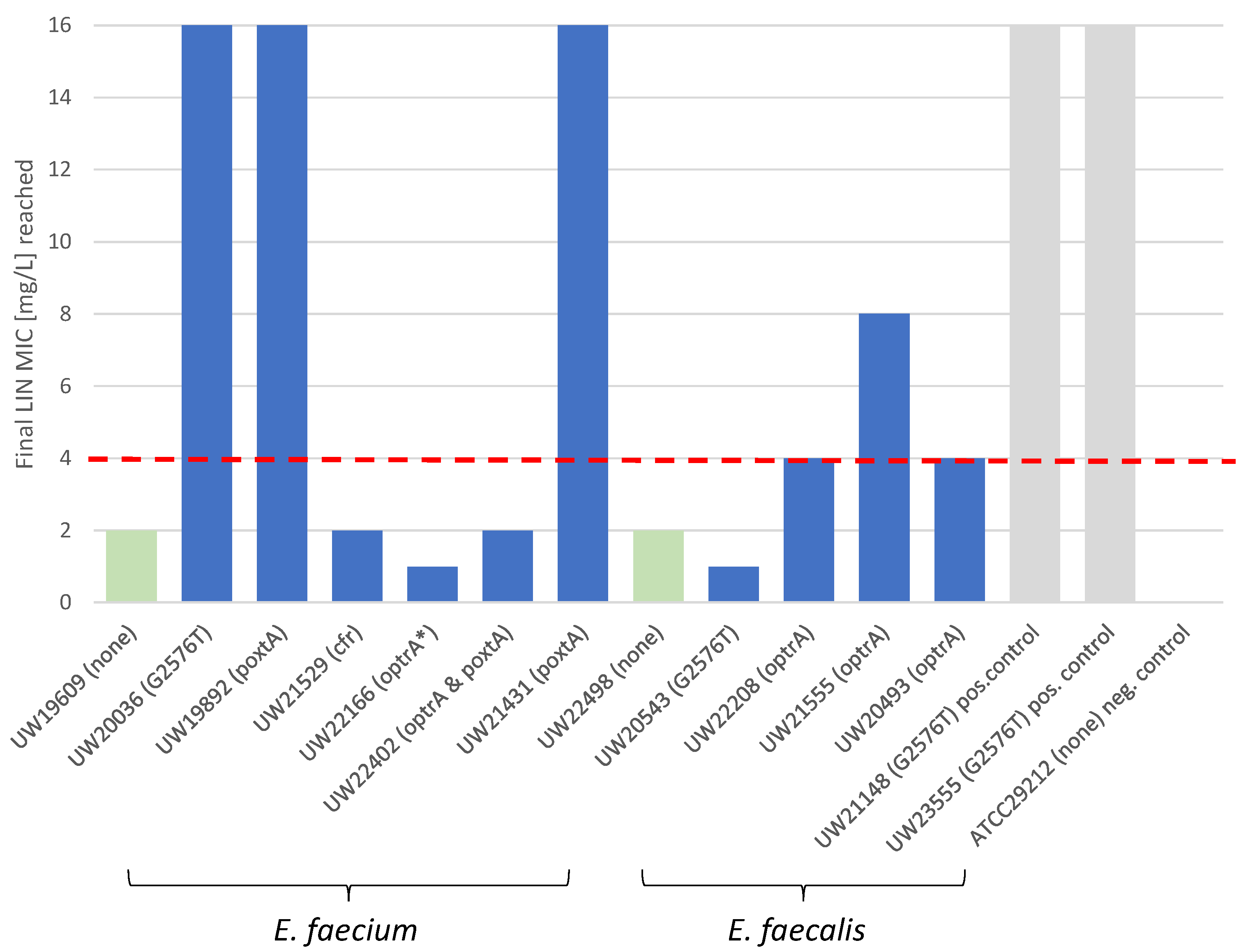
2.3. LIN Selection Experiments
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Ji, J.; Ying, C.; Liu, Z.; Yang, Q.; Kong, H.; Xiao, Y.; Blood Bacterial Resistant Investigation Collaborative System (BRICS) Study Group. Blood bacterial resistant investigation collaborative system (BRICS) report: A national surveillance in China from 2014 to 2019. Antimicrob. Resist. Infect. Control 2022, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Heininger, A.; Zimmermann, S.; Bootsveld, C.; Boutin, S.; Nurjadi, D. Low prevalence of combined linezolid- and vancomycin-resistant Enterococcus faecium from hospital admission screening in an endemic region in Germany. J. Glob. Antimicrob. Resist. 2020, 22, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Markwart, R.; Willrich, N.; Eckmanns, T.; Werner, G.; Ayobami, O. Low Proportion of Linezolid and Daptomycin Resistance Among Bloodborne Vancomycin-Resistant Enterococcus faecium and Methicillin-Resistant Staphylococcus aureus Infections in Europe. Front. Microbiol. 2021, 12, 664199. [Google Scholar] [CrossRef]
- Coombs, G.W.; Daley, D.A.; Mowlaboccus, S.; Lee, Y.T.; Pang, S.; Australian Group on Antimicrobial Resistance. Australian Group on Antimicrobial Resistance (AGAR) Australian Enterococcal Sepsis Outcome Programme (AESOP) Annual Report 2018. Commun. Dis. Intell. (2018) 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Bender, J.K.; Kriebel, N.; Weber, R.E.; Wohlfarth, E.; Maechler, F.; Noll, I.; Abu Sin, M.; Werner, G. Eigenschaften, Häufigkeit und Verbreitung von Vancomycin-resistenten Enterokokken in Deutschland—Update. Epidemiol. Bull. 2023, 28, 14. [Google Scholar] [CrossRef]
- Bender, J.K.; Cattoir, V.; Hegstad, K.; Sadowy, E.; Coque, T.M.; Westh, H.; Hammerum, A.M.; Schaffer, K.; Burns, K.; Murchan, S.; et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: Towards a common nomenclature. Drug Resist. Updates 2018, 40, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Zhang, W.; Du, X.D.; Kruger, H.; Fessler, A.T.; Ma, S.; Zhu, Y.; Wu, C.; Shen, J.; Wang, Y. Mobile Oxazolidinone Resistance Genes in Gram-Positive and Gram-Negative Bacteria. Clin. Microbiol. Rev. 2021, 34, e0018820. [Google Scholar] [CrossRef]
- Bender, J.K.; Fleige, C.; Klare, I.; Fiedler, S.; Mischnik, A.; Mutters, N.T.; Dingle, K.E.; Werner, G. Detection of a cfr(B) Variant in German Enterococcus faecium Clinical Isolates and the Impact on Linezolid Resistance in Enterococcus spp. PLoS ONE 2016, 11, e0167042. [Google Scholar] [CrossRef]
- Lobritz, M.; Hutton-Thomas, R.; Marshall, S.; Rice, L.B. Recombination proficiency influences frequency and locus of mutational resistance to linezolid in Enterococcus faecalis. Antimicrob. Agents Chemother. 2003, 47, 3318–3320. [Google Scholar] [CrossRef]
- Raad, I.I.; Hanna, H.A.; Hachem, R.Y.; Dvorak, T.; Arbuckle, R.B.; Chaiban, G.; Rice, L.B. Clinical-use-associated decrease in susceptibility of vancomycin-resistant Enterococcus faecium to linezolid: A comparison with quinupristin-dalfopristin. Antimicrob. Agents Chemother. 2004, 48, 3583–3585. [Google Scholar] [CrossRef]
- Rodriguez-Lucas, C.; Fernandez, J.; Vazquez, X.; de Toro, M.; Ladero, V.; Fuster, C.; Rodicio, R.; Rodicio, M.R. Detection of the optrA Gene Among Polyclonal Linezolid-Susceptible Isolates of Enterococcus faecalis Recovered from Community Patients. Microb. Drug Resist. 2022, 28, 773–779. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Reading Guide for Broth Microdilution. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Reading_guide_BMD_v_4.0_2022.pdf (accessed on 26 November 2023).
- Dejoies, L.; Boukthir, S.; Pean de Ponfilly, G.; Le Guen, R.; Zouari, A.; Potrel, S.; Collet, A.; Auger, G.; Jacquier, H.; Fihman, V.; et al. Performance of commercial methods for linezolid susceptibility testing of Enterococcus faecium and Enterococcus faecalis. J. Antimicrob. Chemother. 2020, 75, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Moet, G.J.; Woosley, L.N.; Sader, H.S.; Fritsche, T.R. Critical Evaluation of Linezolid Susceptibility Testing using two Automated Systems (Vitek, Vitek 2). In Proceedings of the 47th ICAAC Abstracts: 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL USA, 17–20 September 2007; American Society for Microbiology: Washington, DC, USA, 2007. [Google Scholar]
- Nguyen, C.T.; Bethel, C.; Pettit, N.N.; Charnot-Katsikas, A. From Etest to Vitek 2: Impact of Enterococcal Linezolid Susceptibility Testing Methodology on Time to Active Therapy. Antimicrob. Agents Chemother. 2020, 64, e00302-20. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zheng, X.; Obias, A.; Scheetz, M.H.; Malczynski, M.; Warren, J.R. Comparison of testing methods for detection of decreased linezolid susceptibility due to G2576T mutation of the 23S rRNA gene in Enterococcus faecium and Enterococcus faecalis. J. Clin. Microbiol. 2006, 44, 1098–1100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tenover, F.C.; Williams, P.P.; Stocker, S.; Thompson, A.; Clark, L.A.; Limbago, B.; Carey, R.B.; Poppe, S.M.; Shinabarger, D.; McGowan, J.E., Jr. Accuracy of six antimicrobial susceptibility methods for testing linezolid against staphylococci and enterococci. J. Clin. Microbiol. 2007, 45, 2917–2922. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.H.; Donskey, C.J.; Hutton-Thomas, R.; Salata, R.A.; Rice, L.B. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 2002, 46, 3334–3336. [Google Scholar] [CrossRef]
- Chen, H.; Wu, W.; Ni, M.; Liu, Y.; Zhang, J.; Xia, F.; He, W.; Wang, Q.; Wang, Z.; Cao, B.; et al. Linezolid-resistant clinical isolates of enterococci and Staphylococcus cohnii from a multicentre study in China: Molecular epidemiology and resistance mechanisms. Int. J. Antimicrob. Agents 2013, 42, 317–321. [Google Scholar] [CrossRef]
- Lee, S.M.; Huh, H.J.; Song, D.J.; Shim, H.J.; Park, K.S.; Kang, C.I.; Ki, C.S.; Lee, N.Y. Resistance mechanisms of linezolid-nonsusceptible enterococci in Korea: Low rate of 23S rRNA mutations in Enterococcus faecium. J. Med. Microbiol. 2017, 66, 1730–1735. [Google Scholar] [CrossRef]
- Long, K.S.; Vester, B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob. Agents Chemother. 2012, 56, 603–612. [Google Scholar] [CrossRef]
- Mendes, R.E.; Deshpande, L.M.; Jones, R.N. Linezolid update: Stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist. Updates 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Schwarz, S.; Wang, S.; Chen, L.; Wu, C.; Shen, J. Investigation of a multiresistance gene cfr that fails to mediate resistance to phenicols and oxazolidinones in Enterococcus faecalis. J. Antimicrob. Chemother. 2014, 69, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Nuesch-Inderbinen, M.; Heyvaert, L.; Treier, A.; Zurfluh, K.; Cernela, N.; Biggel, M.; Stephan, R. High occurrence of Enterococcus faecalis, Enterococcus faecium, and Vagococcus lutrae harbouring oxazolidinone resistance genes in raw meat-based diets for companion animals—A public health issue, Switzerland, September 2018 to May 2020. Eurosurveillance 2023, 28, 2200496. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Y.; Ding, L.; Xu, X.; Lin, D. Molecular Investigations of Linezolid Resistance in Enterococci OptrA Variants from a Hospital in Shanghai. Infect. Drug Resist. 2020, 13, 2711–2716. [Google Scholar] [CrossRef]
- Ha, H.T.A.; Nguyen, P.T.L.; Hung, T.T.M.; Tuan, L.A.; Thuy, B.T.; Lien, T.H.M.; Thai, P.D.; Thanh, N.H.; Bich, V.T.N.; Anh, T.H.; et al. Prevalence and Associated Factors of optrA-Positive-Enterococcus faecalis in Different Reservoirs around Farms in Vietnam. Antibiotics 2023, 12, 954. [Google Scholar] [CrossRef]
- Pai, M.P.; Rodvold, K.A.; Schreckenberger, P.C.; Gonzales, R.D.; Petrolatti, J.M.; Quinn, J.P. Risk factors associated with the development of infection with linezolid- and vancomycin-resistant Enterococcus faecium. Clin. Infect. Dis. 2002, 35, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Olearo, F.; Both, A.; Belmar Campos, C.; Hilgarth, H.; Klupp, E.M.; Hansen, J.L.; Maurer, F.P.; Christner, M.; Aepfelbacher, M.; Rohde, H. Emergence of linezolid-resistance in vancomycin-resistant Enterococcus faecium ST117 associated with increased linezolid-consumption. Int. J. Med. Microbiol. 2021, 311, 151477. [Google Scholar] [CrossRef] [PubMed]
- Deekshit, V.K.; Srikumar, S. ‘To be, or not to be’-The dilemma of ‘silent’ antimicrobial resistance genes in bacteria. J. Appl. Microbiol. 2022, 133, 2902–2914. [Google Scholar] [CrossRef]
- Dimitriu, T.; Matthews, A.C.; Buckling, A. Increased copy number couples the evolution of plasmid horizontal transmission and plasmid-encoded antibiotic resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107818118. [Google Scholar] [CrossRef]
- Cook, L.C.; Dunny, G.M. Effects of biofilm growth on plasmid copy number and expression of antibiotic resistance genes in Enterococcus faecalis. Antimicrob. Agents Chemother. 2013, 57, 1850–1856. [Google Scholar] [CrossRef]
- Stasiak, M.; Mackiw, E.; Kowalska, J.; Kucharek, K.; Postupolski, J. Silent Genes: Antimicrobial Resistance and Antibiotic Production. Pol. J. Microbiol. 2021, 70, 421–429. [Google Scholar] [CrossRef]
- Carvalho, K.R.; Carvalho-Assef, A.P.; Santos, L.G.; Pereira, M.J.; Asensi, M.D. Occurrence of blaOXA-23 gene in imipenem-susceptible Acinetobacter baumannii. Mem. Inst. Oswaldo Cruz 2011, 106, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; White, D.G.; Ge, B.; Ayers, S.; Friedman, S.; English, L.; Wagner, D.; Gaines, S.; Meng, J. Identification and characterization of integron-mediated antibiotic resistance among Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 2001, 67, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.K.; Fleige, C.; Klare, I.; Werner, G. Development of a multiplex-PCR to simultaneously detect acquired linezolid resistance genes cfr, optrA and poxtA in enterococci of clinical origin. J. Microbiol. Methods 2019, 160, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.; Strommenger, B.; Klare, I.; Witte, W. Molecular detection of linezolid resistance in Enterococcus faecium and Enterococcus faecalis by use of 5′ nuclease real-time PCR compared to a modified classical approach. J. Clin. Microbiol. 2004, 42, 5327–5331. [Google Scholar] [CrossRef]
| E. faecium (N = 174) | cfr | optrA | poxtA | 23S rDNA G2576T | n | % |
|---|---|---|---|---|---|---|
| - | - | - | - | 113 | 64.9 | |
| - | - | - | + | 49 | 28.2 | |
| - | - | + | - | 7 | 4.0 | |
| - | + | - | - | 1 | 0.6 | |
| - | + | - | + | 1 | 0.6 | |
| - | + | + | - | 1 | 0.6 | |
| + | - | - | - | 1 | 0.6 | |
| + | - | - | + | 1 | 0.6 | |
| total | 174 | 100 | ||||
| E. faecalis (N = 21) | cfr | optrA | poxtA | 23S rDNA G2576T | n | % |
| - | + | - | - | 16 | 76.0 | |
| - | - | - | - | 4 | 19.0 | |
| - | - | - | + | 1 | 5.0 | |
| total | 21 | 100 |
| LIN MIC | BMD | ETEST® | VITEK2 | CHROMagarTM LIN-R 24 h 1 | CHROMagarTM LIN-R 48 h 1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| 0.75 | - | 13 | 6.7% | - | ||||||
| 1 | - | 44 | 23% | 4 | 2.1% | |||||
| 1.5 | - | 35 | 18% | - | ||||||
| 2 | - | 33 | 17% | 121 | 62% | |||||
| 3 | - | 31 | 16% | - | ||||||
| 4 | 195 | 100% | 26 | 13% | 13 | 6.7% | 120 | 62% | 113 | 58% |
| >4 | - | 13 | 6.7% | 57 | 29% | 75 | 38% | 82 | 42% | |
| BMD | ETEST® | VITEK2 | CHROMagarTM LIN-R 24 h 1 | CHROMagarTM LIN-R 48 h 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| ME 1 | n.a. | 0 | 0% | 4 | 2.1% | 6 | 3.1% | 8 | 4.1% | |
| VME 2 | 78 | 40% | 65 | 33% | 25 | 13% | 9 | 4.6% | 4 | 2.1% |
| AST Method | AST Result | cfr | optrA | poxtA | G2576T | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | PPV% | n | PPV% | n | PPV% | n | PPV% | ||
| ETEST® | Susceptible | 1 | 0% | 15 | 11.8% | 7 | 0% | 39 | 22% |
| Resistant | 0 | 2 | 0 | 11 | |||||
| VITEK2 | Susceptible | 0 | 100% | 7 | 58.8% | 4 | 42.9% | 36 | 72% |
| Resistant | 1 | 10 | 3 | 14 | |||||
| CHROMagarTM LIN-R 24 h | Susceptible | 1 | 0% | 1 | 94.1% | 0 | 100% | 7 | 86% |
| Resistant | 0 | 16 | 7 | 43 | |||||
| CHROMagarTM LIN-R 48 h | Susceptible | 0 | 100% | 1 | 94.1% | 0 | 100% | 3 | 94% |
| Resistant | 1 | 16 | 7 | 47 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, J.K.; Fleige, C.; Funk, F.; Moretó-Castellsagué, C.; Fischer, M.A.; Werner, G. Linezolid Resistance Genes and Mutations among Linezolid-Susceptible Enterococcus spp.—A Loose Cannon? Antibiotics 2024, 13, 101. https://doi.org/10.3390/antibiotics13010101
Bender JK, Fleige C, Funk F, Moretó-Castellsagué C, Fischer MA, Werner G. Linezolid Resistance Genes and Mutations among Linezolid-Susceptible Enterococcus spp.—A Loose Cannon? Antibiotics. 2024; 13(1):101. https://doi.org/10.3390/antibiotics13010101
Chicago/Turabian StyleBender, Jennifer K., Carola Fleige, Finn Funk, Clara Moretó-Castellsagué, Martin A. Fischer, and Guido Werner. 2024. "Linezolid Resistance Genes and Mutations among Linezolid-Susceptible Enterococcus spp.—A Loose Cannon?" Antibiotics 13, no. 1: 101. https://doi.org/10.3390/antibiotics13010101
APA StyleBender, J. K., Fleige, C., Funk, F., Moretó-Castellsagué, C., Fischer, M. A., & Werner, G. (2024). Linezolid Resistance Genes and Mutations among Linezolid-Susceptible Enterococcus spp.—A Loose Cannon? Antibiotics, 13(1), 101. https://doi.org/10.3390/antibiotics13010101






