Exploring Roles of the Polysaccharide Capsule in Pathogenesis of Hypervirulent Acinetobacter baumannii Clinical Isolate Lac-4
Abstract
:1. Introduction
2. Results
2.1. Generation of an A. baumannii Lac-4 Capsule-Deficient Mutant
2.2. Capsule Contribution to A. baumannii Lac-4 Antibiotic Susceptibility
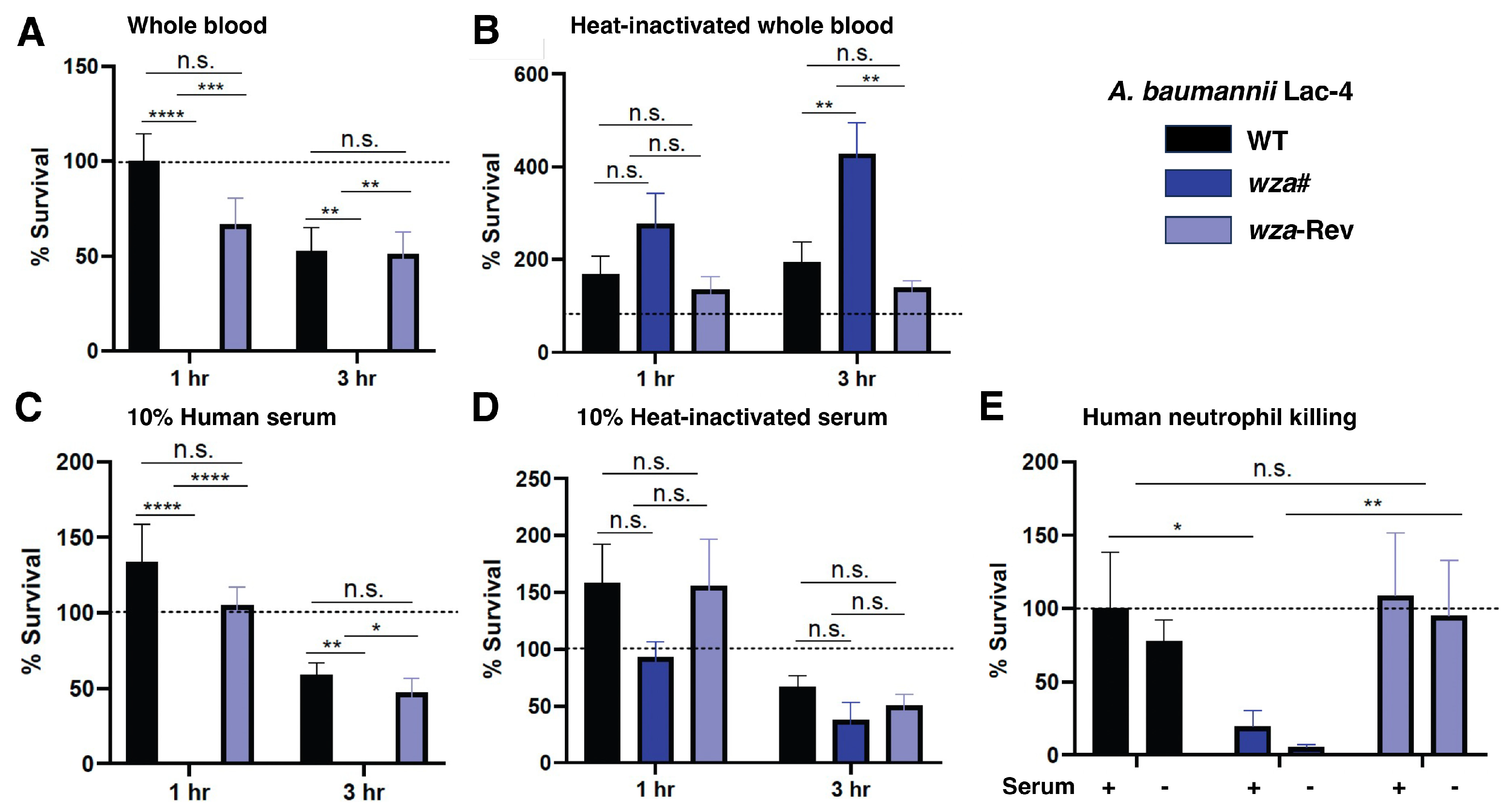
2.3. A. baumannii Lac-4 Capsule Promotes Resistance to Human Innate Immune Factors
2.4. Capsule Contributes to A. baumannii Lac-4 Hypervirulence in Murine Infection Models
2.5. Capsule Expression Reduces A. baumannii Lac-4 Lung Epithelial Cell Adherence
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture
4.2. Creation of A. baumannii Mutant wza#
4.3. Minimum Inhibitory Concentration (MIC) Testing
4.4. Whole Blood and Serum Killing Assays
4.5. Opsonophagocytic Killing Assays
4.6. Adhesion Assays
4.7. Murine Studies
4.8. Murine Sepsis and Pneumonia
4.9. Statistical Analysis and Programs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the critically ill: Complex infections get complicated. Front. Microbiol. 2023, 14, 1196774. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Zhou, Y.; Kanakamedala, H.; Cai, B. Burden of illness in carbapenem-resistant Acinetobacter baumannii infections in US hospitals between 2014 and 2019. BMC Infect. Dis. 2022, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Cincinnati, OH, USA, 2019.
- Russo, T.A.; Luke, N.R.; Beanan, J.M.; Olson, R.; Sauberan, S.L.; MacDonald, U.; Schultz, L.W.; Umland, T.C.; Campagnari, A.A. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 2010, 78, 3993–4000. [Google Scholar] [CrossRef]
- Singh, J.K.; Adams, F.G.; Brown, M.H. Diversity and function of capsular polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2018, 9, 3301. [Google Scholar] [CrossRef]
- Yang, J.-L.; Yang, C.-J.; Chuang, Y.-C.; Sheng, W.-H.; Chen, Y.-C.; Chang, S.-C. Association of capsular polysaccharide locus 2 with prognosis of Acinetobacter baumannii bacteraemia. Emerg. Microbes Infect. 2022, 11, 83–90. [Google Scholar] [CrossRef]
- Akoolo, L.; Pires, S.; Kim, J.; Parker, D. The capsule of Acinetobacter baumannii protects against the innate immune response. J. Innate Immun. 2022, 14, 543–554. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Wan, F.; Liu, P.; Du, F.; Shao, Y.; Zhang, Q.; Cheng, Z.; Liu, Y. Virulence characteristics and drug resistance of the prevalent capsule types in Acinetobacter baumannii. Microb. Drug Resist. 2023, 29, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, E.; Isberg, R.R. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 2015, 11, e1004691. [Google Scholar] [CrossRef] [PubMed]
- Luke, N.R.; Sauberan, S.L.; Russo, T.A.; Beanan, J.M.; Olson, R.; Loehfelm, T.W.; Cox, A.D.; St Michael, F.; Vinogradov, E.V.; Campagnari, A.A. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect. Immun. 2010, 78, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Talyansky, Y.; Nielsen, T.B.; Yan, J.; Carlino-Macdonald, U.; Di Venanzio, G.; Chakravorty, S.; Ulhaq, A.; Feldman, M.F.; Russo, T.A.; Vinogradov, E.; et al. Capsule carbohydrate structure determines virulence in Acinetobacter baumannii. PLoS Pathog. 2021, 17, e1009291. [Google Scholar] [CrossRef] [PubMed]
- Rakovitsky, N.; Lellouche, J.; Ben David, D.; Frenk, S.; Elmalih, P.; Weber, G.; Kon, H.; Schwartz, D.; Wolfhart, L.; Temkin, E.; et al. Increased capsule thickness and hypermotility are traits of carbapenem-resistant Acinetobacter baumannii ST3 strains causing fulminant infection. Open Forum Infect. Dis. 2021, 8, ofab386. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Kuo Lee, R.; Xu, H.H.; Chen, W. Acute intraperitoneal infection with a hypervirulent Acinetobacter baumannii isolate in mice. Sci. Rep. 2019, 9, 6538. [Google Scholar] [CrossRef]
- Harris, G.; Kuo Lee, R.; Lam, C.K.; Kanzaki, G.; Patel, G.B.; Xu, H.H.; Chen, W. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob. Agents Chemother. 2013, 57, 3601–3613. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-Y.; Kuang, S.N.; He, X.; Molgora, B.M.; Ewing, P.J.; Deng, Z.; Osby, M.; Chen, W.; Xu, H.H. Complete genome sequence of hypervirulent and outbreak-associated Acinetobacter baumannii strain LAC-4: Epidemiology, resistance, genetic determinants, and potential virulence factors. Sci. Rep. 2015, 5, 8643. [Google Scholar] [CrossRef]
- Niu, T.; Guo, L.; Luo, Q.; Zhou, K.; Yu, W.; Chen, Y.; Huang, C.; Xiao, Y. Wza Gene knockout decreases Acinetobacter baumannii virulence and affects Wzy-dependent capsular polysaccharide synthesis. Virulence 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Amin, I.M.; Richmond, G.E.; Sen, P.; Koh, T.H.; Piddock, L.J.V.; Chua, K.L. A method for generating marker-less gene deletions in multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2013, 13, 158. [Google Scholar] [CrossRef]
- van Faassen, H.; KuoLee, R.; Harris, G.; Zhao, X.; Conlan, J.W.; Chen, W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect. Immun. 2007, 75, 5597–5608. [Google Scholar] [CrossRef] [PubMed]
- Joly-Guillou, M.L.; Wolff, M.; Pocidalo, J.J.; Walker, F.; Carbon, C. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 1997, 41, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sahly, H.; Podschun, R.; Oelschlaeger, T.A.; Greiwe, M.; Parolis, H.; Hasty, D.; Kekow, J.; Ullmann, U.; Ofek, I.; Sela, S. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect. Immun. 2000, 68, 6744–6749. [Google Scholar] [CrossRef] [PubMed]
- Schembri, M.A.; Dalsgaard, D.; Klemm, P. Capsule shields the function of short bacterial adhesins. J. Bacteriol. 2004, 186, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Alrahmany, D.; Omar, A.F.; Alreesi, A.; Harb, G.; Ghazi, I.M. Acinetobacter baumannii infection-related mortality in hospitalized patients: Risk factors and potential targets for clinical and antimicrobial stewardship interventions. Antibiotics 2022, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xu, X.; Yao, J.; Deng, K.; Chen, S.; Shen, Z.; Yang, L.; Feng, G. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: A systematic review and meta-analysis. Am. J. Infect. Control 2019, 47, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fraga, L.; Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Vallejo, J.A.; Bou, G.; Beceiro, A.; Poza, M. Pneumonia infection in mice reveals the involvement of the feoA gene in the pathogenesis of Acinetobacter baumannii. Virulence 2018, 9, 496–509. [Google Scholar] [CrossRef]
- Qiu, H.; KuoLee, R.; Harris, G.; Chen, W. High susceptibility to respiratory Acinetobacter baumannii infection in A/J mice is associated with a delay in early pulmonary recruitment of meutrophils. Microbes Infect. 2009, 11, 946–955. [Google Scholar] [CrossRef]
- Joly-Guillou, M.L.; Wolff, M.; Farinotti, R.; Bryskier, A.; Carbon, C. In vivo activity of levofloxacin alone or in combination with imipenem or amikacin in a mouse model of Acinetobacter baumannii pneumonia. J. Antimicrob. Chemother. 2000, 46, 827–830. [Google Scholar] [CrossRef]
- de Léséleuc, L.; Harris, G.; KuoLee, R.; Xu, H.H.; Chen, W. Serum resistance, gallium nitrate tolerance, and extrapulmonary dissemination are linked to heme consumption in a bacteremic strain of Acinetobacter baumannii. Int. J. Med. Microbiol. 2014, 304, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, E.; Maclean, L.; Xu, H.H.; Chen, W. The Structure of the polysaccharide isolated from Acinetobacter baumannii strain LAC-4. Carbohydr. Res. 2014, 390, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Lundgren, B.R.; Chaumun, M.; Whitfield, D.M.; Clark, B.; Schoenhofen, I.C.; Boddy, C.N. Total biosynthesis of legionaminic acid, a bacterial sialic acid analogue. Angew. Chem. Int. Ed. Engl. 2016, 55, 12018–12021. [Google Scholar] [CrossRef]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A.G. Masquerading microbial pathogens: Capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.-E.; Vallenet, D.; Barbe, V.; Audic, S.; Ogata, H.; Poirel, L.; Richet, H.; Robert, C.; Mangenot, S.; Abergel, C.; et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006, 2, e7. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.E.; Kinsella, R.L.; Edwards, A.V.G.; Larsen, M.R.; Dutta, S.; Saba, J.; Foster, L.J.; Feldman, M.F. Diversity within the O-linked protein glycosylation systems of Acinetobacter species. Mol. Cell. Proteom. 2014, 13, 2354–2370. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Nickel, G.C.; Bajaksouzian, S.; Lavender, H.; Murthy, A.R.; Jacobs, M.R.; Bonomo, R.A. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 2009, 53, 3628–3634. [Google Scholar] [CrossRef]
- Boll, J.M.; Crofts, A.A.; Peters, K.; Cattoir, V.; Vollmer, W.; Davies, B.W.; Trent, M.S. A Penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc. Natl. Acad. Sci. USA 2016, 113, E6228–E6237. [Google Scholar] [CrossRef]
- Bjanes, E.; Zhou, J.; Qayum, T.; Krishnan, N.; Zurich, R.H.; Menon, N.D.; Hoffman, A.; Fang, R.H.; Zhang, L.; Nizet, V. Outer membrane vesicle-coated nanoparticle vaccine protects against Acinetobacter baumannii pneumonia and sepsis. Adv. Nanobiomed Res. 2023, 3, 2200130. [Google Scholar] [CrossRef]


| wza# | wza-Rev | |||||
|---|---|---|---|---|---|---|
| Mean | SEM | p | Mean | SEM | p | |
| Doxycycline | 1.75 | 0.75 | n.s | 1.37 | 0.22 | n.s |
| Cefapime | 1.15 | 0.30 | n.s | 1.65 | 0.24 | n.s |
| Colistin | 1.00 | 0.00 | n.s | 1.00 | 0.00 | n.s |
| Polymyxin B | 1.00 | 0.00 | n.s | 1.00 | 0.00 | n.s |
| Ampicillin | 0.86 | 0.14 | n.s | 0.71 | 0.29 | n.s |
| Meropenem | 0.88 | 0.15 | n.s | 0.97 | 0.11 | n.s |
| Penicillin | 0.89 | 0.06 | n.s | 0.78 | 0.15 | n.s |
| Imipenem | 0.67 | 0.17 | n.s | 3.00 | 1.53 | n.s. |
| Streptomycin | 0.56 | 0.06 | n.s | 1.00 | 0.00 | n.s |
| Vancomycin | 0.33 | 0.04 | n.s | 0.78 | 0.31 | n.s |
| Piperacillin/Tazobactam | 0.43 | 0.10 | n.s | 0.86 | 0.21 | n.s |
| Levofloxacin | 0.25 | 0.00 | n.s | 2.00 | 0.00 | n.s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjånes, E.; Koh, T.; Qayum, T.; Zurich, R.; McCabe, S.; Hampel, K.; Cartwright, L.; Nizet, V. Exploring Roles of the Polysaccharide Capsule in Pathogenesis of Hypervirulent Acinetobacter baumannii Clinical Isolate Lac-4. Antibiotics 2024, 13, 10. https://doi.org/10.3390/antibiotics13010010
Bjånes E, Koh T, Qayum T, Zurich R, McCabe S, Hampel K, Cartwright L, Nizet V. Exploring Roles of the Polysaccharide Capsule in Pathogenesis of Hypervirulent Acinetobacter baumannii Clinical Isolate Lac-4. Antibiotics. 2024; 13(1):10. https://doi.org/10.3390/antibiotics13010010
Chicago/Turabian StyleBjånes, Elisabet, Truman Koh, Tariq Qayum, Raymond Zurich, Sinead McCabe, Kegan Hampel, Lisa Cartwright, and Victor Nizet. 2024. "Exploring Roles of the Polysaccharide Capsule in Pathogenesis of Hypervirulent Acinetobacter baumannii Clinical Isolate Lac-4" Antibiotics 13, no. 1: 10. https://doi.org/10.3390/antibiotics13010010
APA StyleBjånes, E., Koh, T., Qayum, T., Zurich, R., McCabe, S., Hampel, K., Cartwright, L., & Nizet, V. (2024). Exploring Roles of the Polysaccharide Capsule in Pathogenesis of Hypervirulent Acinetobacter baumannii Clinical Isolate Lac-4. Antibiotics, 13(1), 10. https://doi.org/10.3390/antibiotics13010010






