ICU-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria in COVID-19 Patients: A Narrative Review
Abstract
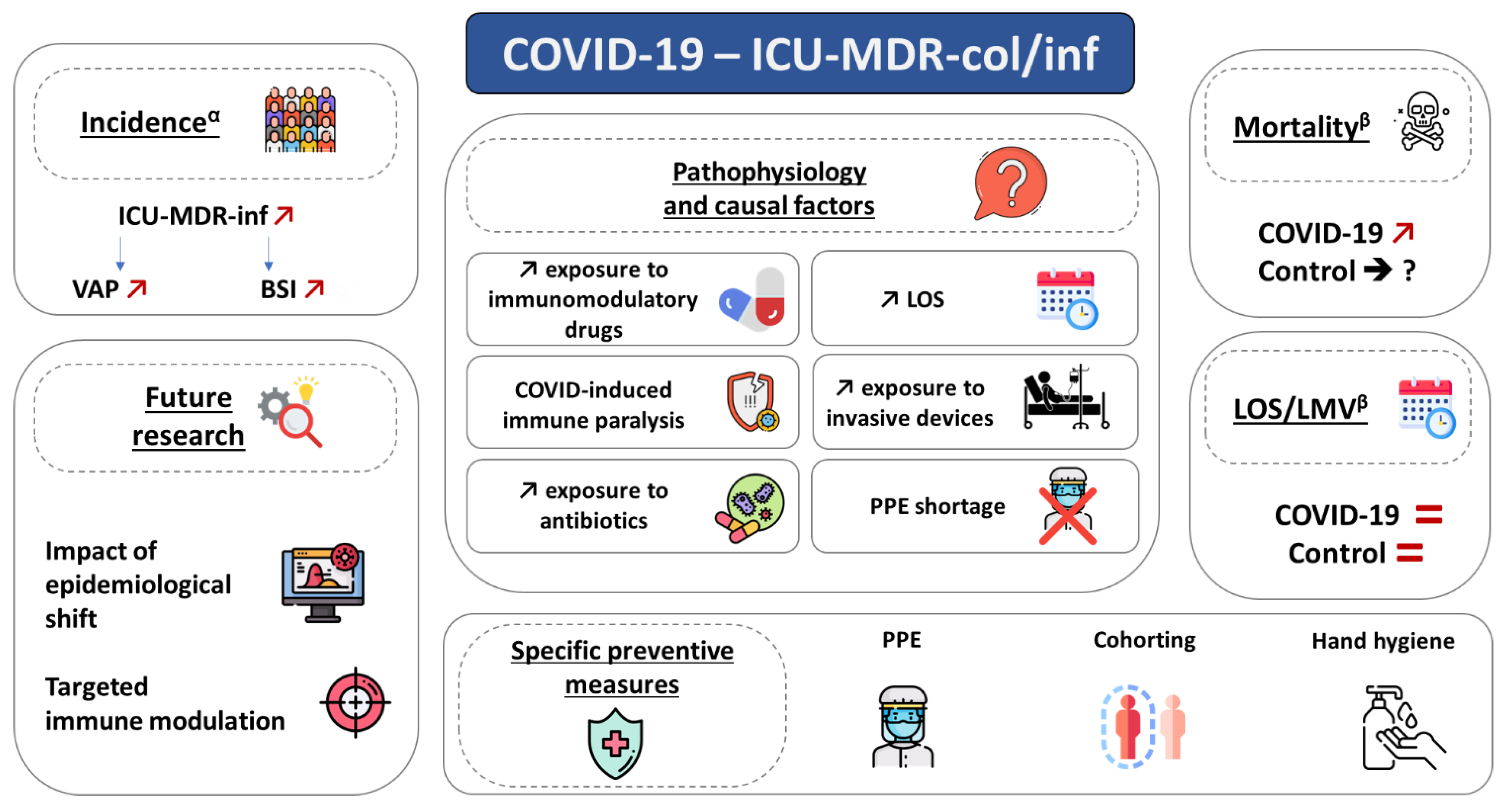
:1. Introduction
2. Methods
3. Epidemiology
4. Pathophysiology
5. Specific Preventive Measures
6. Future Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, M.; Hajage, D.; Demoule, A.; Pham, T.; Combes, A.; Dres, M.; Lebbah, S.; Kimmoun, A.; Mercat, A.; Beduneau, G.; et al. Clinical Characteristics and Day-90 Outcomes of 4244 Critically Ill Adults with COVID-19: A Prospective Cohort Study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Boulle Geronimi, C.; et al. Relationship between SARS-CoV-2 Infection and the Incidence of Ventilator-Associated Lower Respiratory Tract Infections: A European Multicenter Cohort Study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Buetti, N.; Ruckly, S.; de Montmollin, E.; Reignier, J.; Terzi, N.; Cohen, Y.; Siami, S.; Dupuis, C.; Timsit, J.-F. COVID-19 Increased the Risk of ICU-Acquired Bloodstream Infections: A Case-Cohort Study from the Multicentric OUTCOMEREA Network. Intensive Care Med. 2021, 47, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial Use, Drug-Resistant Infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Barbier, F.; Pommier, C.; Essaied, W.; Garrouste-Orgeas, M.; Schwebel, C.; Ruckly, S.; Dumenil, A.-S.; Lemiale, V.; Mourvillier, B.; Clec’h, C.; et al. Colonization and Infection with Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in ICU Patients: What Impact on Outcomes and Carbapenem Exposure? J. Antimicrob. Chemother. 2016, 71, 1088–1097. [Google Scholar] [CrossRef]
- Bickenbach, J.; Schöneis, D.; Marx, G.; Marx, N.; Lemmen, S.; Dreher, M. Impact of Multidrug-Resistant Bacteria on Outcome in Patients with Prolonged Weaning. BMC Pulm. Med. 2018, 18, 141. [Google Scholar] [CrossRef]
- Barbier, F.; Lisboa, T.; Nseir, S. Understanding Why Resistant Bacteria Are Associated with Higher Mortality in ICU Patients. Intensive Care Med. 2016, 42, 2066–2069. [Google Scholar] [CrossRef]
- Hu, S.; You, Y.; Zhang, S.; Tang, J.; Chen, C.; Wen, W.; Wang, C.; Cheng, Y.; Zhou, M.; Feng, Z.; et al. Multidrug-Resistant Infection in COVID-19 Patients: A Meta-Analysis. J. Infect. 2022, 86, P66–P117. [Google Scholar] [CrossRef]
- Langford, B.J.; Soucy, J.-P.R.; Leung, V.; So, M.; Kwan, A.T.H.; Portnoff, J.S.; Bertagnolio, S.; Raybardhan, S.; MacFadden, D.; Daneman, N. Antibiotic Resistance Associated with the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 29, P302–P309. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, R.M.; Julien, D.A.; Jelinski, D.C.; Larose, S.L.; Rennert-May, E.; Conly, J.M.; Dingle, T.C.; Chen, J.Z.; Tyrrell, G.J.; Ronksley, P.E.; et al. Antimicrobial Resistance (AMR) in COVID-19 Patients: A Systematic Review and Meta-Analysis (November 2019–June 2021). Antimicrob. Resist. Infect. Control 2022, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.; Nseir, S.; Rodriguez, A.; Martin-Loeches, I. Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19 Infection: A Narrative Review. ERJ Open Res. 2022, 8, 00046–02022. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Simeonova, M.; Leung, V.; Lo, J.; Kan, T.; Raybardhan, S.; Sapin, M.E.; Mponponsuo, K.; Farrell, A.; et al. Antimicrobial Resistance in Patients with COVID-19: A Systematic Review and Meta-Analysis. Lancet Microbe 2023, 4, e179–e191. [Google Scholar] [CrossRef] [PubMed]
- Micheli, G.; Sangiorgi, F.; Catania, F.; Chiuchiarelli, M.; Frondizi, F.; Taddei, E.; Murri, R. The Hidden Cost of COVID-19: Focus on Antimicrobial Resistance in Bloodstream Infections. Microorganisms 2023, 11, 1299. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Taccone, F.S.; Izzi, A.; Yin, N.; Garufi, A.; Hublet, S.; Njimi, H.; Ego, A.; Gorham, J.; Byl, B.; et al. The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms 2020, 8, 1821. [Google Scholar] [CrossRef]
- Razazi, K.; Arrestier, R.; Haudebourg, A.F.; Benelli, B.; Carteaux, G.; Decousser, J.-W.; Fourati, S.; Woerther, P.L.; Schlemmer, F.; Charles-Nelson, A.; et al. Risks of Ventilator-Associated Pneumonia and Invasive Pulmonary Aspergillosis in Patients with Viral Acute Respiratory Distress Syndrome Related or Not to Coronavirus 19 Disease. Crit. Care 2020, 24, 699. [Google Scholar] [CrossRef]
- Oliva, A.; Ceccarelli, G.; Borrazzo, C.; Ridolfi, M.; D’Ettorre, G.; Alessandri, F.; Ruberto, F.; Pugliese, F.; Raponi, G.M.; Russo, A.; et al. Comparison of Clinical Features and Outcomes in COVID-19 and Influenza Pneumonia Patients Requiring Intensive Care Unit Admission. Infection 2021, 49, 965–975. [Google Scholar] [CrossRef]
- Ong, C.C.H.; Farhanah, S.; Linn, K.Z.; Tang, Y.W.; Poon, C.Y.; Lim, A.Y.; Tan, H.R.; Binte Hamed, N.H.; Huan, X.; Puah, S.H.; et al. Nosocomial Infections among COVID-19 Patients: An Analysis of Intensive Care Unit Surveillance Data. Antimicrob. Resist. Infect. Control 2021, 10, 119. [Google Scholar] [CrossRef]
- Rouyer, M.; Strazzulla, A.; Youbong, T.; Tarteret, P.; Pitsch, A.; de Pontfarcy, A.; Cassard, B.; Vignier, N.; Pourcine, F.; Jochmans, S.; et al. Ventilator-Associated Pneumonia in COVID-19 Patients: A Retrospective Cohort Study. Antibiotics 2021, 10, 988. [Google Scholar] [CrossRef]
- Zuglian, G.; Ripamonti, D.; Tebaldi, A.; Cuntrò, M.; Riva, I.; Farina, C.; Rizzi, M. The Changing Pattern of Bacterial and Fungal Respiratory Isolates in Patients with and without COVID-19 Admitted to Intensive Care Unit. BMC Infect. Dis. 2022, 22, 185. [Google Scholar] [CrossRef] [PubMed]
- Bahçe, Y.G.; Acer, Ö.; Özüdoğru, O. Evaluation of Bacterial Agents Isolated from Endotracheal Aspirate Cultures of COVID-19 General Intensive Care Patients and Their Antibiotic Resistance Profiles Compared to Pre-Pandemic Conditions. Microb. Pathog. 2022, 164, 105409. [Google Scholar] [CrossRef] [PubMed]
- Sathyakamala, R.; Peace, A.R.; Shanmugam, P. A Comparative Study on Bacterial Co-Infections and Prevalence of Multidrug Resistant Organisms among Patients in COVID and Non-COVID Intensive Care Units. J. Prev. Med. Hyg. 2022, 63, E19–E26. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, C.-H.; Lepape, A.; Savey, A.; Machut, A.; Timsit, J.F.; Vanhems, P.; Le, Q.V.; Egbeola, J.; Martin, M.; Maxime, V.; et al. Increased Incidence of Ventilator-Acquired Pneumonia in Coronavirus Disease 2019 Patients: A Multicentric Cohort Study. Crit. Care Med. 2022, 50, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.-J.; Song, W.; Kim, H.-S.; Kim, H.S.; Kim, J.-S. Impact of COVID-19 on Antimicrobial Consumption and Spread of Multidrug-Resistance in Bacterial Infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef]
- Vacheron, C.-H.; Lepape, A.; Savey, A.; Machut, A.; Timsit, J.F.; Comparot, S.; Courno, G.; Vanhems, P.; Landel, V.; Lavigne, T.; et al. Attributable Mortality of Ventilator-Associated Pneumonia Among Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2022, 206, 161–169. [Google Scholar] [CrossRef]
- Cogliati Dezza, F.; Arcari, G.; Alessi, F.; Valeri, S.; Curtolo, A.; Sacco, F.; Ceccarelli, G.; Raponi, G.; Alessandri, F.; Mastroianni, C.M.; et al. Clinical Impact of COVID-19 on Multi-Drug-Resistant Gram-Negative Bacilli Bloodstream Infections in an Intensive Care Unit Setting: Two Pandemics Compared. Antibiotics 2022, 11, 926. [Google Scholar] [CrossRef]
- Metan, G.; Demir Çuha, M.; Hazirolan, G.; Telli Dizman, G.; Tanriverdi, E.S.; Otlu, B.; Tas, Z.; Zarakolu, P.; Arik, Z.; Topeli, A.; et al. The Impact of COVID-19 Pandemic on Nosocomial Multidrug-Resistant Bacterial Bloodstream Infections and Antibiotic Consumption in a Tertiary Care Hospital. GMS Hyg. Infect. Control 2022, 17, Doc15. [Google Scholar] [CrossRef]
- Buetti, N.; Tabah, A.; Loiodice, A.; Ruckly, S.; Aslan, A.T.; Montrucchio, G.; Cortegiani, A.; Saltoglu, N.; Kayaaslan, B.; Aksoy, F.; et al. Different Epidemiology of Bloodstream Infections in COVID-19 Compared to Non-COVID-19 Critically Ill Patients: A Descriptive Analysis of the Eurobact II Study. Crit. Care 2022, 26, 319. [Google Scholar] [CrossRef]
- Lepape, A.; Machut, A.; Bretonnière, C.; Friggeri, A.; Vacheron, C.-H.; Savey, A. REA-REZO network Effect of SARS-CoV-2 Infection and Pandemic Period on Healthcare-Associated Infections Acquired in Intensive Care Units. Clin. Microbiol. Infect. 2022, 29, 530–536. [Google Scholar] [CrossRef]
- Kinross, P.; Gagliotti, C.; Merk, H.; Plachouras, D.; Monnet, D.L.; Högberg, L.D.; Group, E.-N.S.; Participants, E.-N.S.G.; Strauss, R.; Mertens, K.; et al. Large Increase in Bloodstream Infections with Carbapenem-Resistant Acinetobacter Species during the First 2 Years of the COVID-19 Pandemic, EU/EEA, 2020 and 2021. Eurosurveillance 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Segala, F.V.; Pafundi, P.C.; Masciocchi, C.; Fiori, B.; Taddei, E.; Antenucci, L.; De Angelis, G.; Guerriero, S.; Pastorino, R.; Damiani, A.; et al. Incidence of Bloodstream Infections Due to Multidrug-Resistant Pathogens in Ordinary Wards and Intensive Care Units before and during the COVID-19 Pandemic: A Real-Life, Retrospective Observational Study. Infection 2023, 51, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Önal, U.; Tüzemen, Ü.; Kazak, E.; Gençol, N.; Souleiman, E.; İmer, H.; Heper, Y.; Yılmaz, E.; Özakın, C.; Ener, B.; et al. Effects of COVID-19 Pandemic on Healthcare-Associated Infections, Antibiotic Resistance and Consumption Rates in Intensive Care Units. Infez. Med. 2023, 31, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, V.; Panopoulou, M.; Rafailidis, P.; Lemonakis, N.; Lazaridis, G.; Terzi, I.; Papazoglou, D.; Panagopoulos, P. The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections. Pathogens 2023, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Kim, D.Y.; Kim, E.J.; Park, K.-H.; Lee, M.S. Impact of COVID-19 Pandemic on Healthcare-Associated Infections at Intensive Care Units in South Korea: Data from the Korean National Healthcare-Associated Infections Surveillance System (KONIS). J. Hosp. Infect. 2023, 138, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Chang, C.-H.; Tien, K.-L.; Tai, C.-H.; Lin, L.-M.; Lee, T.-F.; Ku, S.-C.; Fang, C.-T.; Chen, Y.-C.; Sheng, W.-H. Impact of Coronavirus Disease 2019 (COVID-19) on Antimicrobial Resistance among Major Pathogens Causing Healthcare-Associated Infection. J. Formos. Med. Assoc. 2023, S0929-6646(23)00254-1. [Google Scholar] [CrossRef]
- Kreitmann, L.; Jermoumi, S.; Vasseur, M.; Chabani, M.; Nourry, E.; Richard, J.-C.; Wallet, F.; Garçon, P.; Kachmar, S.; Zerbib, Y.; et al. Relationship between COVID-19 and ICU-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria: A Prospective Multicenter before-after Study. Intensive Care Med. 2023, 49, 796–807. [Google Scholar] [CrossRef]
- Piantoni, A.; Houard, M.; Piga, G.; Zebian, G.; Ruffier des Aimes, S.; Holik, B.; Wallet, F.; Rouzé, A.; Kreitmann, L.; Loiez, C.; et al. Relationship between COVID-19 and ICU-Acquired Bloodstream Infections Related to Multidrug-Resistant Bacteria. Antibiotics 2023, 12, 1105. [Google Scholar] [CrossRef]
- Nseir, S.; Martin-Loeches, I.; Povoa, P.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; Tamion, F.; Labruyere, M.; Makris, D.; Boulle Geronimi, C.; et al. Relationship between Ventilator-Associated Pneumonia and Mortality in COVID-19 Patients: A Planned Ancillary Analysis of the coVAPid Cohort. Crit. Care 2021, 25, 177. [Google Scholar] [CrossRef]
- Saura, O.; Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Kreitmann, L.; Torres, A.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; Tamion, F.; et al. Relationship between Corticosteroid Use and Incidence of Ventilator-Associated Pneumonia in COVID-19 Patients: A Retrospective Multicenter Study. Crit. Care 2022, 26, 292. [Google Scholar] [CrossRef]
- Lamouche-Wilquin, P.; Souchard, J.; Pere, M.; Raymond, M.; Asfar, P.; Darreau, C.; Reizine, F.; Hourmant, B.; Colin, G.; Rieul, G.; et al. Early Steroids and Ventilator-Associated Pneumonia in COVID-19-Related ARDS. Crit. Care 2022, 26, 233. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Ungaro, R.; Dirain, M.; Efron, P.A.; Mazer, M.B.; Remy, K.E.; Hotchkiss, R.S.; Zhong, L.; Bacher, R.; Starostik, P.; et al. Overlapping but Disparate Inflammatory and Immunosuppressive Responses to SARS-CoV-2 and Bacterial Sepsis: An Immunological Time Course Analysis. Front. Immunol. 2021, 12, 792448. [Google Scholar] [CrossRef] [PubMed]
- Garduno, A.; Martinez, G.S.; Ostadgavahi, A.T.; Kelvin, D.; Cusack, R.; Martin-Loeches, I. Parallel Dysregulated Immune Response in Severe Forms of COVID-19 and Bacterial Sepsis via Single-Cell Transcriptome Sequencing. Biomedicines 2023, 11, 778. [Google Scholar] [CrossRef]
- Bonnet, B.; Cosme, J.; Dupuis, C.; Coupez, E.; Adda, M.; Calvet, L.; Fabre, L.; Saint-Sardos, P.; Bereiziat, M.; Vidal, M.; et al. Severe COVID-19 Is Characterized by the Co-Occurrence of Moderate Cytokine Inflammation and Severe Monocyte Dysregulation. EBioMedicine 2021, 73, 103622. [Google Scholar] [CrossRef] [PubMed]
- Limmer, A.; Engler, A.; Kattner, S.; Gregorius, J.; Pattberg, K.T.; Schulz, R.; Schwab, J.; Roth, J.; Vogl, T.; Krawczyk, A.; et al. Patients with SARS-CoV-2-Induced Viral Sepsis Simultaneously Show Immune Activation, Impaired Immune Function and a Procoagulatory Disease State. Vaccines 2023, 11, 435. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Navarro, M.D.; Romero, L.; Muniain, M.A.; de Cueto, M.; Gálvez, J.; Perea, E.J.; Pascual, A. Risk-Factors for Emerging Bloodstream Infections Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli. Clin. Microbiol. Infect. 2008, 14, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalised with COVID-19 during the First Pandemic Wave from the ISARIC WHO CCP-UK Study: A Multicentre, Prospective Cohort Study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Calderon, M.; Gysin, G.; Gujjar, A.; McMaster, A.; King, L.; Comandé, D.; Hunter, E.; Payne, B. Bacterial Co-Infection and Antibiotic Stewardship in Patients with COVID-19: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2023, 23, 14. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020, 180, 1436–1447. [Google Scholar] [CrossRef]
- Kadri, S.S.; Sun, J.; Lawandi, A.; Strich, J.R.; Busch, L.M.; Keller, M.; Babiker, A.; Yek, C.; Malik, S.; Krack, J.; et al. Association Between Caseload Surge and COVID-19 Survival in 558 U.S. Hospitals, March to August 2020. Ann. Intern. Med. 2021, 174, 1240–1251. [Google Scholar] [CrossRef]
- Lasater, K.B.; Aiken, L.H.; Sloane, D.M.; French, R.; Martin, B.; Reneau, K.; Alexander, M.; McHugh, M.D. Chronic Hospital Nurse Understaffing Meets COVID-19: An Observational Study. BMJ Qual. Saf. 2021, 30, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the COVID-19 Pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P. Lung Microbiota and COVID-19 Severity. Nat. Microbiol. 2021, 6, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, R.F.J.; de Brabander, J.; Boers, L.S.; Biemond, J.J.; Nossent, E.J.; Heunks, L.M.A.; Vlaar, A.P.J.; Bonta, P.I.; van der Poll, T.; Duitman, J.; et al. Lung Microbiota of Critically Ill Patients with COVID-19 Are Associated with Nonresolving Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2022, 206, 846–856. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Y.; Zhong, J.; Li, X.; Xiao, Y.; Li, J.; Yang, J.; Fan, G.; Guo, L.; Shen, Z.; et al. Dynamics of the Upper Respiratory Tract Microbiota and Its Association with Mortality in COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Holter, J.C.; Holm, K.; Vestad, B.; Sazonova, T.; Granerud, B.K.; Dyrhol-Riise, A.M.; Holten, A.R.; Tonby, K.; Kildal, A.B.; et al. Gut Microbiota Composition during Hospitalization Is Associated with 60-Day Mortality after Severe COVID-19. Crit. Care 2023, 27, 69. [Google Scholar] [CrossRef]
- De Pascale, G.; De Maio, F.; Carelli, S.; De Angelis, G.; Cacaci, M.; Montini, L.; Bello, G.; Cutuli, S.L.; Pintaudi, G.; Tanzarella, E.S.; et al. Staphylococcus Aureus Ventilator-Associated Pneumonia in Patients with COVID-19: Clinical Features and Potential Inference with Lung Dysbiosis. Crit. Care 2021, 25, 197. [Google Scholar] [CrossRef]
- Tsitsiklis, A.; Zha, B.; Byrne, A.; DeVoe, C.; Levan, S.; Rackaityte, E.; Sunshine, S.; Mick, E.; Ghale, R.; Jauregui, A.; et al. Impaired Immune Signaling and Changes in the Lung Microbiome Precede Secondary Bacterial Pneumonia in COVID-19. Res. Sq. 2021, rs.3.rs-380803. [Google Scholar] [CrossRef]
- García-García, J.; Diez-Echave, P.; Yuste, M.E.; Chueca, N.; García, F.; Cabeza-Barrera, J.; Fernández-Varón, E.; Gálvez, J.; Colmenero, M.; Rodríguez-Cabezas, M.E.; et al. Gut Microbiota Composition Can Predict Colonization by Multidrug-Resistant Bacteria in SARS-CoV-2 Patients in Intensive Care Unit: A Pilot Study. Antibiotics 2023, 12, 498. [Google Scholar] [CrossRef]
- Singson, J.R.C.; Kirley, P.D.; Pham, H.; Rothrock, G.; Armistead, I.; Meek, J.; Anderson, E.J.; Reeg, L.; Lynfield, R.; Ropp, S.; et al. Factors Associated with Severe Outcomes Among Immunocompromised Adults Hospitalized for COVID-19—COVID-NET, 10 States, March 2020–February 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 878–884. [Google Scholar] [CrossRef]
- Kreitmann, L.; Vasseur, M.; Jermoumi, S.; Perche, J.; Richard, J.-C.; Wallet, F.; Chabani, M.; Nourry, E.; Garçon, P.; Zerbib, Y.; et al. Relationship between Immunosuppression and Intensive Care Unit-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria: A Prospective Multicenter Cohort Study. Intensive Care Med. 2023, 49, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.R.; Freeman, M.L.; Zidar, D.A. Immunohematologic Biomarkers in COVID-19: Insights into Pathogenesis, Prognosis, and Prevention. Pathog. Immun. 2023, 8, 17–50. [Google Scholar] [CrossRef] [PubMed]
- Price, J.R.; Mookerjee, S.; Dyakova, E.; Myall, A.; Leung, W.; Weiße, A.Y.; Shersing, Y.; Brannigan, E.T.; Galletly, T.; Muir, D. Development and Delivery of a Real-Time Hospital-Onset COVID-19 Surveillance System Using Network Analysis. Clin. Infect. Dis. 2021, 72, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Myall, A.; Price, J.R.; Peach, R.L.; Abbas, M.; Mookerjee, S.; Zhu, N.; Ahmad, I.; Ming, D.; Ramzan, F.; Teixeira, D.; et al. Prediction of Hospital-Onset COVID-19 Infections Using Dynamic Networks of Patient Contact: An International Retrospective Cohort Study. Lancet Digit. Health 2022, 4, e573–e583. [Google Scholar] [CrossRef] [PubMed]
- Fartoukh, M.; Nseir, S.; Mégarbane, B.; Cohen, Y.; Lafarge, A.; Contou, D.; Thille, A.W.; Galerneau, L.-M.; Reizine, F.; Cour, M.; et al. Respiratory Multiplex PCR and Procalcitonin to Reduce Antibiotic Exposure in Severe SARS-CoV-2 Pneumonia: A Multicentre Randomized Controlled Trial. Clin. Microbiol. Infect. 2023, 29, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Wolfensberger, A.; Clack, L.; von Felten, S.; Faes Hesse, M.; Saleschus, D.; Meier, M.-T.; Kusejko, K.; Kouyos, R.; Held, L.; Sax, H. Prevention of Non-Ventilator-Associated Hospital-Acquired Pneumonia in Switzerland: A Type 2 Hybrid Effectiveness-Implementation Trial. Lancet Infect. Dis. 2023, 23, 836–846. [Google Scholar] [CrossRef]
| Study | Year | Journal | Setting | Nb. of Centers | Design | Sample Size (Cases vs. Controls) |
|---|---|---|---|---|---|---|
| Bogossian et al. [16] | November 2020 | Microorganisms | Belgium | 1 | Retrospective | 72/72 |
| Razazi et al. [17] | December 2020 | Critical Care | France | 1 | Retrospective | 90/82 |
| Oliva et al. [18] | January 2021 | Infection | Italy | 1 | Retrospective | 55/19 |
| Rouzé et al. [2] | January 2021 | Intensive Care Medicine | Europe | 36 | Retrospective | 568/1008 |
| Ong et al. [19] | August 2021 | Antimicrobial Resistance and Infection Control | Singapore | 1 | Prospective | 71/487 |
| Rouyer et al. [20] | August 2021 | Antibiotics | France | 1 | Retrospective | 79/188 |
| Zuglian et al. [21] | February 2022 | BMC Infectious Diseases | Italy | 1 | Retrospective | 176/194 |
| Bahçe et al. [22] | March 2022 | Microbial Pathogenesis | Turkey | 1 | Retrospective | 602/971 |
| Sathyakamala et al. [23] | March 2022 | Journal of Preventive Medicine and Hygiene | India | 1 | Retrospective | 356/292 |
| Vacheron et al. [24] | March 2022 | Critical Care Medicine | France | 94 | Retrospective | 1879/1879 |
| Jeon et al. [25] | April 2022 | Antibiotics | Korea | 4 | Retrospective | 209,107 (total including ICU and non-ICU patients, pre- and per-COVID-19 periods) |
| Vacheron et al. [26] | July 2022 | American Journal of Respiratory and Critical Care Medicine | France | ? | Retrospective | 1687/72,258 |
| Cogliati Dezza et al. [27] | July 2022 | Antibiotics | Italy | 1 | Retrospective | 18/28 |
| Metan et al. [28] | August 2022 | GMS Hygiene and Infection Control | Turkey | 1 | Retrospective | ? |
| Buetti et al. [29] | October 2022 | Critical Care | World | 53 | Retrospective | 252/577 |
| Lepape et al. [30] | October 2022 | Clinical Microbiology and Infection | France | 110 | Retrospective | 4465/63,433 |
| Kinross et al. [31] | November 2022 | Eurosurveillance | Europe | ? | Retrospective | ? |
| Segala et al. [32] | March 2023 | Infection | Italy | 1 | Prospective | 14,884 (total including cases and controls) |
| Önal et al. [33] | April 2023 | Le Infezioni in Medicina | Turkey | 1 | Retrospective | 8157 (total including cases and controls) |
| Petrakis et al. [34] | May 2023 | Pathogens | Greece | 1 | Retrospective | 823/393 |
| Lee et al. [35] | June 2023 | Journal of Hospital Infection | Korea | 346 | Retrospective | ? |
| Chang et al. [36] | July 2023 | Journal of the Formosan Medical Association | Taiwan | 1 | Retrospective | 38,184 ICU patients (total) |
| Kreitmann et al. [37] | July 2023 | Intensive Care Medicine | France | 7 | Prospective | 367/680 |
| Piantoni et al. [38] | July 2023 | Antibiotics | France | 1 | Retrospective | 497/823 |
| Study | Data on ICU Patients | Control Group | Assessment of ICU-Acquired MDR Colonization | Systematic Screening of ICU-Acquired MDR Colonization | Assessment of ICU-Acquired MDR Infections | Reporting of ICU-Acquired Non-MDR Infections | Adjustment for Confounding Factors |
|---|---|---|---|---|---|---|---|
| Bogossian et al. [16] | Yes | Non-COVID-19 ICU patients before pandemic | Yes | Yes | No | No | Yes |
| Razazi et al. [17] | Yes | Non-COVID-19 ICU patients before and during pandemic | No | No | Yes (VAP) | Yes | Yes |
| Oliva et al. [18] | Yes | Flu patients (before pandemic) | Yes | Yes | Yes (all) | Yes | No |
| Rouzé et al. [2] | Yes | ICU patients with flu or no viral infection (before and during pandemic) | No | No | Yes (VA-LTRI, VAP and VAT) | Yes | Yes |
| Ong et al. [19] | Yes | Non-COVID-19 ICU patients during pandemic | No | No | Yes (all) | Yes | Yes |
| Rouyer et al. [20] | Yes | Non-COVID-19 ICU patients during pandemic | Yes | Yes | Yes (VAP) | Yes | No |
| Zuglian et al. [21] | Yes | Non-COVID-19 IUC patients before pandemic | No | No | Yes (respiratory samples) | Yes | No |
| Bahçe et al. [22] | Yes | Non-COVID-19 ICU patients before pandemic | No | No | Yes (respiratory samples) | Yes | No |
| Sathyakamala et al. [23] | Yes | Non-COVID-19 ICU patients during pandemic | No | No | Yes (all) | Yes | No |
| Vacheron et al. [24] | Yes | Non-COVID-19 ICU patients before pandemic | No | No | Yes (VAP) | Yes | Yes |
| Jeon et al. [25] | Yes | Non-COVID-19 ICU patients before pandemic | Yes | ? | Yes (all) | No | No |
| Vacheron et al. [26] | Yes | Non-COVID-19 ICU patients before and during pandemic | No | No | Yes (VAP) | Yes | No |
| Cogliati Dezza et al. [27] | Yes | Non-COVID-19 ICU patients before pandemic | Yes | Yes | Yes (MDR Gram-negative BSI) | No | No |
| Metan et al. [28] | Yes | Non-COVID-19 ICU patients during pandemic | No | No | Yes (BSI) | No | No |
| Buetti et al. [29] | Yes | Non-COVID-19 ICU patients before pandemic | No | No | Yes (BSI) | Yes | No |
| Lepape et al. [30] | Yes | Non-COVID-19 ICU patients during and before pandemic | Yes | ? | Yes (VAP, HAP, BSI) | Yes | Yes |
| Kinross et al. [31] | Yes | Non-COVID-19 ICU patients during and before pandemic | No | No | Yes (Acinetobacter spp. BSI) | Yes | No |
| Segala et al. [32] | Yes | Non-COVID-19 ICU patients before pandemic | Yes | ? | Yes (BSI) | Yes | No |
| Önal et al. [33] | Yes | Non-COVID-19 ICU patients before and during pandemic | No | No | Yes (all) | Yes | No |
| Petrakis et al. [34] | Yes | Non-COVID-19 ICU patients during pandemic | No | No | Yes (blood and respiratory samples) | Yes | No |
| Lee et al. [35] | Yes | Non-COVID-19 ICU patients before pandemic | No | No | Yes (all) | Yes | No |
| Chang et al. [36] | Yes | Non-COVID-19 ICU patients before pandemic | No | No | Yes (all) | No | No |
| Kreitmann et al. [37] | Yes | Non-COVID-19 ICU patients before pandemic | Yes | Yes | Yes (all) | No | Yes |
| Piantoni et al. [38] | Yes | Non-COVID-19 ICU patients during same period of follow-up | No | No | Yes (ICU-acquired MDR BSI) | No | Yes |
| Study | Incidence of ICU-Acquired MDR Colonization and Infection | Risk Factors for ICU-Acquired Colonization or Infection among COVID-19 Patients | Impact on Outcomes | Limitations |
|---|---|---|---|---|
| Bogossian et al. [16] | No significant association between COVID-19 status and the incidence of ICU-acquired MDR colonization (sHR 1.71 (CI 95% 0.93–3.21) | Risk factors for ICU-acquired MDR colonization: vasopressors, antimicrobial therapy | Longer duration of ICU and hospital LOS, but no impact on mortality in patients with ICU-acquired MDR colonization (among COVID-19 patients) | Small sample size, monocentric study |
| Razazi et al. [17] | COVID-19 patients had higher incidence of VAP (sHR 1.72, 95% CI 1.14–2.57), including MDR VAP (23% vs. 11%, p = 0.03), than non-COVID-19 controls | No data | No data | Small sample size, monocentric study |
| Oliva et al. [18] | No difference in the incidence of ICU-acquired MDR colonization and infection in COVID-19 vs. flu patients | No data | No data | Small sample size, monocentric study |
| Rouzé et al. [2] | Higher incidence of VA-LTRI and VAP in COVID-19 patients vs. patients with flu or no viral infection, with lower rate of MDR bacteria isolated in COVID-19 patients (23.3%) vs. patients with flu (38.4%) and no viral infection (33.8%) | No data | No data | |
| Ong et al. [19] | Similar incidence of ICU-acquired infections, including MDR infections in COVID-19 patients vs. controls | No data | No data | Monocentric study, small number of events |
| Rouyer et al. [20] | Similar rates of ICU-acquired MDR colonization in COVID-19 vs. controls | No data | No data | Incomplete data collection on ICU-acquired MDR infections |
| Zuglian et al. [21] | Similar frequency of MDR P. aeruginosa and Enterobacteriaceae among positive samples in COVID-19 patients and controls | No data | No data | |
| Bahçe et al. [22] | Rates of antibiotic resistance in K. pneumoniae, A. baumanii, and P. aeruginosa in ETA unchanged for most antibiotics. Increased rate of levofloxacin- and ceftazidime-resistant P. aeruginosa | No data | No data | Small number of positive ETA samples (lack of power?) |
| Sathyakamala et al. [23] | Similar rates of MDR Gram-negative bacteria among blood, urine, and respiratory samples in COVID-19 patients vs. controls | No data | No data | No formal statistical comparison, incomplete data collection |
| Vacheron et al. [24] | Higher incidence of VAP in COVID-19 patients vs. controls (adjusted sHR 1.68, 95% CI 1.45–1.96) with similar frequency of MDR pathogens (except for a lower frequency of MRSA) | No data | No data | |
| Jeon et al. [25] | ICU-acquired MDR colonization: decreased rates of MRSA, VRE, CRE, and CRAB in COVID-19 vs. non-COVID-19 patients. ICU-acquired MDR infection: decreased rates of MRSA, CRAB, and CRPA, and increased rates of VRE and CRE in COVID-19 vs. non-COVID-19 patients | No data | No data | Registry study with no patient-related data |
| Vacheron et al. [26] | Higher incidence of VAP in COVID-19 patients (36.9%) than in both control groups (13.4% and 10.6%), with similar proportions of resistant strains | No data | Higher mortality related to VAP in the COVID-19 group (but no data on the impact of COVID-19 status on the association between MDR VAP and outcomes) | |
| Cogliati Dezza et al. [27] | Lower incidence of ICU-acquired MDR colonization in COVID-19 (47.4%) vs. control patients (81.4%). No significant difference in incidence rate of ICU-acquired BSI with MDR Gram-negative bacteria in COVID-19 vs. control patients. | No data | Among patients with BSI related to MDR Gram-negative bacteria, 30-day mortality higher in COVID-19 patients than in controls (77.8% vs. 21.4%, p < 0.0001) | Small sample size, monocentric study |
| Metan et al. [28] | Similar rates of MDR bacteria in BSI in COVID-19 patients and controls | No data | No data | Small sample size, monocentric study, incomplete reporting |
| Buetti et al. [29] | Increased incidence of hospital-acquired BSI related to DTR Gram-negative bacteria in COVID-19 vs. controls (19.4% vs. 13%, p = 0.017) | No data | Among patients with Gram-negative DTR BSIs, 28-day mortality higher in COVID-19 patients than for controls (83.7% vs. 65.3%, p = 0.025) | No adjustment of statistical analysis on patient-related confounders |
| Lepape et al. [30] | ICU-acquired MDR colonization: higher incidence in COVID-19 patients vs. controls (6.8% vs. 3.7%, p < 0.001). ICU-acquired MDR infection: higher incidence of VAP and BSI related to MDR bacteria in COVID-19 patients (MRSA, CRE, ESBL, and ceftazidime-resistant P. aeruginosa) | No data | No data | No clear distinction between colonization and infection in some tables |
| Kinross et al. [31] | Increased incidence of blood cultures positive with Acinetobacter spp., including imipenem-resistant Acinetobacter spp., during COVID-19 periods vs. pre-pandemic period | No data | No data | Registry study, no patient-related data |
| Segala et al. [32] | Similar incidence of ICU-acquired infections in COVID-19 patients vs. controls | No data | No data | |
| Önal et al. [33] | COVID-19 patients had higher incidence of BSI (but similar rates of other ICU-acquired infections) than controls, with similar rates of MDR bacteria | No data | No data | Retrospective, monocentric study |
| Petrakis et al. [34] | Increased rate of resistance to common antibiotics among isolates of K. pneumonia, A. baumanii, and P. aeruginosa in COVID-19 patients vs. controls | No data | No data | Retrospective, monocentric study |
| Lee et al. [35] | Increased rate of imipenem-resistant K. pneumonia among ICU-acquired infections in COVID-19 vs. non-COVID-19 patients | No data | No data | Registry study with no patient-related data |
| Chang et al. [36] | No significant change in the incidence of ICU-acquired MDR infections before and after the COVID-19 pandemic | No data | No data | No patient data, no assessment of ICU-acquired non-MDR infection |
| Kreitmann et al. [37] | COVID-19 patients had higher incidence of ICU-acquired MDR infections (adjusted sHR 2.50, 95% CI 1.90–3.28), but similar incidence of ICU-acquired MDR colonization (adjusted sHR 1.27, 95% CI 0.85–1.88) vs. controls | No data | Occurrence of ICU-acquired MDR colonization and/or infection associated with decreased survival (adjusted cHR 2.61, 95% CI 1.59–4.27) in COVID-19 patients, but not in controls. No impact of the occurrence of ICU-acquired MDR colonization and/or infection on ICU LOS and on duration of IMV in the overall cohort, in COVID-19 patients, and in controls | No assessment of ICU-acquired non-MDR infection |
| Piantoni et al. [38] | Increased incidence of ICU-acquired BSI related to MDR bacteria, mostly during the period starting after day 15 post-ICU admission (adjusted cHR 4.35, 95% CI 1.58–11.90) | No data | ICU-acquired MDR BSI associated with increased mortality in overall cohort (adjusted HR 1.73, 95% CI 1.0–3.0, with no effect of COVID-19 status). No association between occurrence of ICU-acquired MDR BSI and ICU LOS and IMV duration in overall cohort, in COVID-19 patients, and in controls | No assessment of ICU-acquired non-MDR BSI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudet, A.; Kreitmann, L.; Nseir, S. ICU-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria in COVID-19 Patients: A Narrative Review. Antibiotics 2023, 12, 1464. https://doi.org/10.3390/antibiotics12091464
Gaudet A, Kreitmann L, Nseir S. ICU-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria in COVID-19 Patients: A Narrative Review. Antibiotics. 2023; 12(9):1464. https://doi.org/10.3390/antibiotics12091464
Chicago/Turabian StyleGaudet, Alexandre, Louis Kreitmann, and Saad Nseir. 2023. "ICU-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria in COVID-19 Patients: A Narrative Review" Antibiotics 12, no. 9: 1464. https://doi.org/10.3390/antibiotics12091464
APA StyleGaudet, A., Kreitmann, L., & Nseir, S. (2023). ICU-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria in COVID-19 Patients: A Narrative Review. Antibiotics, 12(9), 1464. https://doi.org/10.3390/antibiotics12091464






