Assessing the Activity under Different Physico-Chemical Conditions, Digestibility, and Innocuity of a GAPDH-Related Fish Antimicrobial Peptide and Analogs Thereof
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity
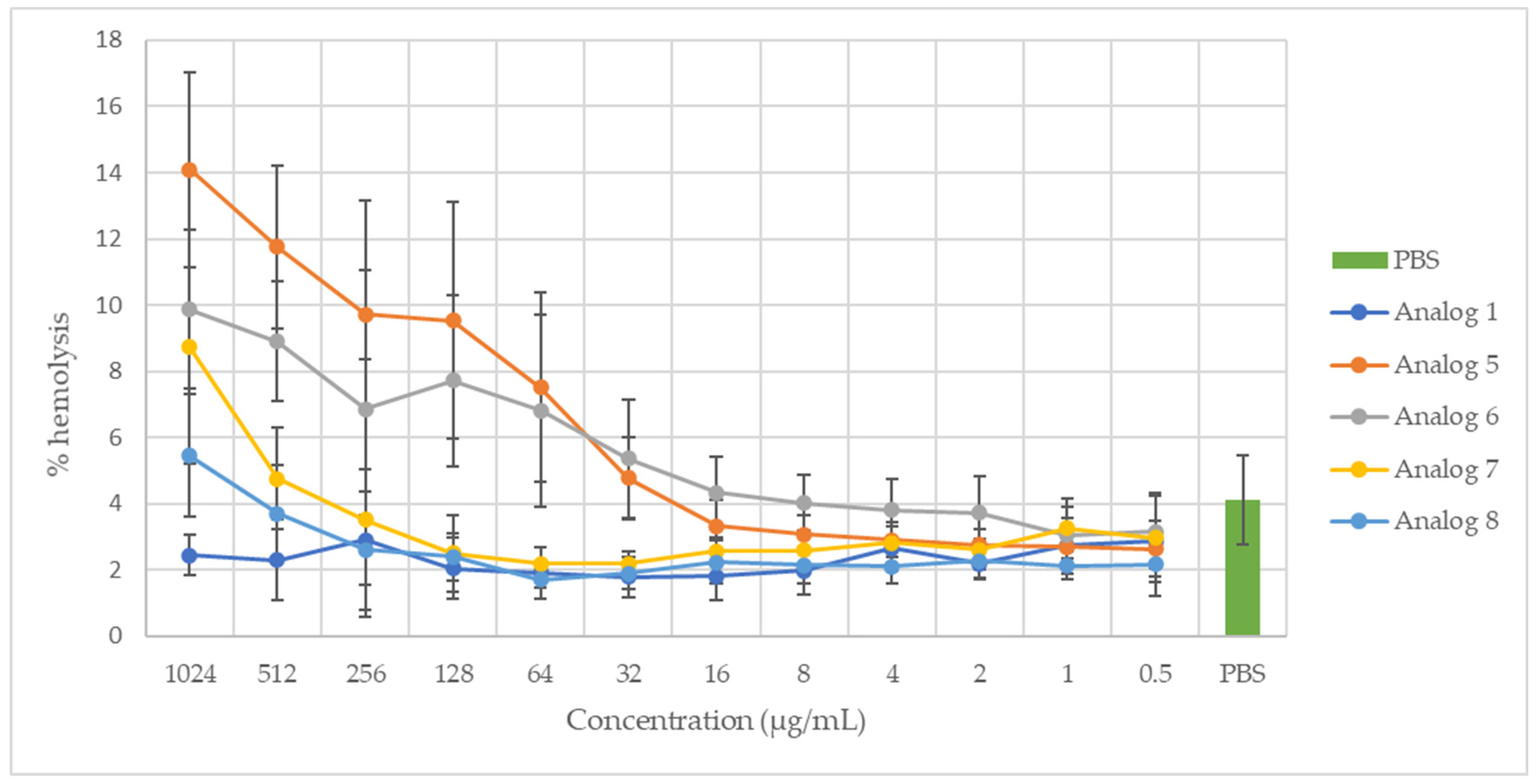
2.2. Hemolytic Activity
2.3. Digestibility of Peptide Analogs 6 and 8 in a Dynamic Gastro-Intestinal Model
3. Discussion
3.1. Impact of pH, Salts, and Heat Treatment
3.2. Hemolytic Activity
3.3. Stability in Gastro-Intestinal Conditions
4. Materials and Methods
4.1. Peptide Design and Synthesis
4.2. Antimicrobial Activity Assays
4.2.1. Bacterial Strain and Culture Conditions
4.2.2. Minimal Inhibitory and Bactericidal Concentrations (MICs and MBCs)
4.2.3. Impact of Salts, pH, and Heating on Antimicrobial Activity
4.3. Hemolytic Activity Assays
4.4. In Vitro Digestibility Assays in a Dynamic Gastro-Intestinal Tract Model
4.5. Analysis of GI TIM-1 Fractions
4.5.1. Fast Protein Liquid Chromatography (FPLC)
4.5.2. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Chetia, M.; Chatterjee, S. Antimicrobial Peptides and Proteins: From Nature’s Reservoir to the Laboratory and Beyond. Front. Chem. 2021, 9, 691532. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Evangelista, A.G.; Nazareth, T.d.M.; Luciano, F.B. Fundamentals on the molecular mechanism of action of antimicrobial peptides. Materialia 2019, 8, 100494. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet. Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Said, L.B.; Fliss, I.; Offret, C.; Beaulieu, L. Antimicrobial Peptides: The New Generation of Food Additives. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 576–582. [Google Scholar] [CrossRef]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Antimicrobial Peptides with Enhanced Activities and Specificity/Therapeutic Index. J. Biol. Chem. 2005, 13, 12316–12329. [Google Scholar] [CrossRef]
- Shabir, U.; Ali, S.; Magray, A.R.; Ganai, B.A.; Firdous, P.; Hassan, T.; Nazir, R. Fish antimicrobial peptides (AMP’s) as essential and promising molecular therapeutic agents: A review. Microb. Pathog. 2018, 114, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rajanbabu, V.; Chen, J.Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, D.G. Influence of the hydrophobic amino acids in the N- and C-terminal regions of pleurocidin on antifungal activity. J. Microbiol. Biotechnol. 2010, 20, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Mannion, M.; Pike, D.; Lewis, K.; Flynn, A.; Brannan, A.M.; Browne, M.J.; Jackman, D.; Madera, L.; Power Coombs, M.R.; et al. Structure-function relationships in histidine-rich antimicrobial peptides from Atlantic cod. Biochim. Biophys. Acta 2015, 1848, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Lauth, X.; Shike, H.; Burns, J.C.; Westerman, M.E.; Ostland, V.E.; Carlberg, J.M.; Van Olst, J.C.; Nizet, V.; Taylor, S.W.; Shimizu, C.; et al. Discovery and Characterization of Two Isoforms of Moronecidin, a Novel Antimicrobial Peptide from Hybrid Striped Bass. J. Biol. Chem. 2002, 277, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Ross, N.W.; MacKinnon, S.L. Myxinidin, a novel antimicrobial peptide from the epidermal mucus of hagfish, Myxine glutinosa L. Mar. Biotechnol. 2009, 11, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Lee, M.J.; Go, H.J.; Kim, Y.J.; Park, N.G. Antimicrobial function of the GAPDH-related antimicrobial peptide in the skin of skipjack tuna, Katsuwonus pelamis. Fish Shellfish. Immunol. 2014, 36, 571–581. [Google Scholar] [CrossRef]
- Cashman-Kadri, S.; Lagüe, P.; Fliss, I.; Beaulieu, L. Determination of the Relationships between the Chemical Structure and Antimicrobial Activity of a GAPDH-Related Fish Antimicrobial Peptide and Analogs Thereof. Antibiotics 2022, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; Schneider, J.J.; Baxmann, S.; Kalbacher, H.; Borelli, C.; Nuding, S.; Kuchler, R.; Wehkamp, J.; Kaeser, M.D.; Mailander-Sanchez, D.; et al. A peptide derived from the highly conserved protein GAPDH is involved in tissue protection by different antifungal strategies and epithelial immunomodulation. J. Invest. Dermatol. 2013, 133, 144–153. [Google Scholar] [CrossRef]
- Branco, P.; Francisco, D.; Chambon, C.; Hebraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef]
- Branco, P.; Kemsawasd, V.; Santos, L.; Diniz, M.; Caldeira, J.; Almeida, M.G.; Arneborg, N.; Albergaria, H. Saccharomyces cerevisiae accumulates GAPDH-derived peptides on its cell surface that induce death of non-Saccharomyces yeasts by cell-to-cell contact. FEMS Microbiol. Ecol. 2017, 93, fix055. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Albergaria, H.; Arneborg, N.; Prista, C. Effect of GAPDH-derived antimicrobial peptides on sensitive yeasts cells: Membrane permeability, intracellular pH and H+-influx/-efflux rates. FEMS Yeast Res. 2018, 18, foy030. [Google Scholar] [CrossRef]
- Offret, C.; Fliss, I.; Bazinet, L.; Marette, A.; Beaulieu, L. Identification of A Novel Antibacterial Peptide from Atlantic Mackerel belonging to the GAPDH-Related Antimicrobial Family and Its In Vitro Digestibility. Mar. Drugs 2019, 17, 413. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Liu, Y.; Zhao, X.; Lian, X.; Zhang, J.; Zhao, D.; Wang, Y.; Zhong, J.; Wang, J.; et al. A Peptide from Budding Yeast GAPDH Serves as a Promising Antifungal against Cryptococcus neoformans. Microbiol. Spectr. 2022, 10, e00826-21. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Walkenhorst, W.F.; Klein, J.W.; Vo, P.; Wimley, W.C. pH Dependence of microbe sterilization by cationic antimicrobial peptides. Antimicrob. Agents Chemother. 2013, 57, 3312–3320. [Google Scholar] [CrossRef]
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. pH Dependent Antimicrobial Peptides and Proteins, Their Mechanisms of Action and Potential as Therapeutic Agents. Pharmaceuticals 2016, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Naimi, S.; Zirah, S.; Hammami, R.; Fernandez, B.; Rebuffat, S.; Fliss, I. Fate and Biological Activity of the Antimicrobial Lasso Peptide Microcin J25 Under Gastrointestinal Tract Conditions. Front. Microbiol. 2018, 9, 1764. [Google Scholar] [CrossRef]
- Shwaiki, L.N.; Lynch, K.M.; Arendt, E.K. Future of antimicrobial peptides derived from plants in food application-A focus on synthetic peptides. Trends Food Sci. Technol. 2021, 112, 312–324. [Google Scholar] [CrossRef]
- Ma, Q.-Q.; Dong, N.; Shan, A.-S.; Lv, Y.-F.; Li, Y.-Z.; Chen, Z.-H.; Cheng, B.-J.; Li, Z.-Y. Biochemical property and membrane-peptide interactions of de novo antimicrobial peptides designed by helix-forming units. Amino Acids Forum Amino Acid Pept. Protein Res. 2012, 43, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- León Madrazo, A.; Segura Campos, M.R. Review of antimicrobial peptides as promoters of food safety: Limitations and possibilities within the food industry. J. Food Saf. 2020, 40, e12854. [Google Scholar] [CrossRef]
- Wu, G.; Ding, J.; Li, H.; Li, L.; Zhao, R.; Shen, Z.; Fan, X.; Xi, T. Effects of cations and pH on antimicrobial activity of thanatin and s-thanatin against Escherichia coli ATCC25922 and B. subtilis ATCC 21332. Curr. Microbiol. 2008, 57, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Chou, S.; Li, J.; Xue, C.; Li, X.; Cheng, B.; Shan, A.; Xu, L. Short Symmetric-End Antimicrobial Peptides Centered on β-Turn Amino Acids Unit Improve Selectivity and Stability. Front. Microbiol. 2018, 9, 2832. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Di Luca, M.; Esin, S.; Florio, W.; Brancatisano, F.L.; Bottai, D.; Campa, M.; Batoni, G. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides 2008, 29, 1–6. [Google Scholar] [CrossRef]
- Kerenga, B.K.; McKenna, J.A.; Harvey, P.J.; Quimbar, P.; Garcia-Ceron, D.; Lay, F.T.; Phan, T.K.; Veneer, P.K.; Vasa, S.; Parisi, K.; et al. Salt-Tolerant Antifungal and Antibacterial Activities of the Corn Defensin ZmD32. Front. Microbiol. 2019, 10, 795. [Google Scholar] [CrossRef]
- Natthaporn, K.; Poom, A.; Warunee, H.; Sangdao, S.; Ratchaneewan, A. A novel, rationally designed, hybrid antimicrobial peptide, inspired by cathelicidin and aurein, exhibits membrane-active mechanisms against Pseudomonas aeruginosa. In Scientific Reports; Nature Portfolio: Berlin, Germany, 2020; Volume 10, pp. 1–17. [Google Scholar]
- Murzyn, K.; Róg, T.; Pasenkiewicz-Gierula, M. Phosphatidylethanolamine-Phosphatidylglycerol Bilayer as a Model of the Inner Bacterial Membrane. Biophys. J. 2005, 88, 1091–1103. [Google Scholar] [CrossRef]
- Chanh Thi Minh, L.; Aamd, H.; Nimalka, B.; Brian, J.S.; Adam, M. Interaction of Small Ionic Species With Phospholipid Membranes: The Role of Metal Coordination. Front. Mater. 2019, 5, 80. [Google Scholar]
- Elmore, D.E. Molecular dynamics simulation of a phosphatidylglycerol membrane. FEBS Lett. 2006, 580, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Saha, B.; Kumar, G.S.; Karmakar, S. Binding of monovalent alkali metal ions with negatively charged phospholipid membranes. BBA-Biomembr. 2016, 1858, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.K.; Larson, R.G. Effect of salt on the interactions of antimicrobial peptides with zwitterionic lipid bilayers. BBA-Biomembr. 2006, 1758, 1274–1284. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Vattulainen, I. Effect of NaCl and KCl on phosphatidylcholine and phosphatidylethanolamine lipid membranes: Insight from atomic-scale simulations for understanding salt-induced effects in the plasma membrane. J. Phys. Chem. B 2008, 112, 1953–1962. [Google Scholar] [CrossRef]
- Mao, Y.; Du, Y.; Cang, X.; Wang, J.; Chen, Z.; Yang, H.; Jiang, H. Binding competition to the POPG lipid bilayer of Ca2+, Mg2+, Na+, and K+ in different ion mixtures and biological implication. J. Phys. Chem. B 2013, 117, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Y.; Dong, M.; Hang, B.; Sun, Y.; Wang, L.; Wang, Y.; Hu, J.; Zhang, W. HJH-1, a Broad-Spectrum Antimicrobial Activity and Low Cytotoxicity Antimicrobial Peptide. Molecules 2018, 23, 2026. [Google Scholar] [CrossRef]
- Jeucken, A.; Helms, J.B.; Brouwers, J.F. Cardiolipin synthases of Escherichia coli have phospholipid class specific phospholipase D activity dependent on endogenous and foreign phospholipids. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1345–1353. [Google Scholar] [CrossRef]
- Sathappa, M.; Alder, N.N. The ionization properties of cardiolipin and its variants in model bilayers. BBA-Biomembr. 2016, 1858, 1362–1372. [Google Scholar] [CrossRef]
- Hitchner, M.A.; Santiago-Ortiz, L.E.; Necelis, M.R.; Shirley, D.J.; Palmer, T.J.; Tarnawsky, K.E.; Vaden, T.D.; Caputo, G.A. Activity and characterization of a pH-sensitive antimicrobial peptide. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2019, 1861, 182984. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 1997, 65, 2898–2903. [Google Scholar] [CrossRef]
- Vaezi, Z.; Bortolotti, A.; Luca, V.; Perilli, G.; Mangoni, M.L.; Khosravi-Far, R.; Bobone, S.; Stella, L. Aggregation determines the selectivity of membrane-active anticancer and antimicrobial peptides: The case of killerFLIP. BBA-Biomembr. 2020, 1862, 183107. [Google Scholar] [CrossRef]
- Haney, E.F.; Wu, B.; Lee, K.; Hilchie, A.L.; Hancock, R.E.W. Aggregation and Its Influence on the Immunomodulatory Activity of Synthetic Innate Defense Regulator Peptides. Cell Chem. Biol. 2017, 24, 969–980. [Google Scholar] [CrossRef]
- Marquette, A.; Mason, A.J.; Bechinger, B. Aggregation and membrane permeabilizing properties of designed histidine-containing cationic linear peptide antibiotics. J. Pept. Sci. 2008, 14, 488–495. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.v.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Rodríguez, A.; Villegas, E.; Montoya-Rosales, A.; Rivas-Santiago, B.; Corzo, G. Characterization of antibacterial and hemolytic activity of synthetic pandinin 2 variants and their inhibition against Mycobacterium tuberculosis. PLoS ONE 2014, 9, e101742. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Aarestrup, F.M.; Hansen, E.B.; Butko, P. Dissection of the antimicrobial and hemolytic activity of Cap18: Generation of Cap18 derivatives with enhanced specificity. PLoS ONE 2018, 13, e0197742. [Google Scholar] [CrossRef] [PubMed]
- Ugurlu, T.; Turkoglu, M.; Gurer, U.S.; Akarsu, B.G. Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur. J. Pharm. Biopharm. 2007, 67, 202–210. [Google Scholar] [CrossRef]
- Soltani, S.; Zirah, S.; Rebuffat, S.; Couture, F.; Boutin, Y.; Biron, E.; Subirade, M.; Fliss, I. Gastrointestinal Stability and Cytotoxicity of Bacteriocins From Gram-Positive and Gram-Negative Bacteria: A Comparative in vitro Study. Front. Microbiol. 2021, 12, 780355. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kovics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Lowe, M.E. Human pancreatic digestive enzymes. Dig. Dis. Sci. 2007, 52, 1–17. [Google Scholar] [CrossRef]
- Heavner, G.A.; Kroon, D.J.; Audhya, T.; Goldstein, G. Biologically active analogs of thymopentin with enhanced enzymatic stability. Peptides 1986, 7, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Brinckerhoff, L.H.; Kalashnikov, V.V.; Thompson, L.W.; Yamshchikov, G.V.; Pierce, R.A.; Galavotti, H.S.; Engelhard, V.H.; Slingluff, C.L., Jr. Terminal modifications inhibit proteolytic degradation of an immunogenic mart-127–35 peptide: Implications for peptide vaccines. Int. J. Cancer 1999, 83, 326–334. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Bo, J.; Yang, Y.; Zheng, R.; Fang, C.; Jiang, Y.; Liu, J.; Chen, M.; Hong, F.; Bailey, C.; Segner, H.; et al. Antimicrobial activity and mechanisms of multiple antimicrobial peptides isolated from rockfish Sebastiscus marmoratus. Fish Shellfish. Immunol. 2019, 93, 1007–1017. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Gomaa, A.; Bedard, F.; Biron, E.; Subirade, M.; Beaulieu, L.; Fliss, I. Collagencin, an antibacterial peptide from fish collagen: Activity, structure and interaction dynamics with membrane. Biochem. Biophys. Res. Commun. 2016, 473, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Nielsen, C.G.; Aarestrup, F.M.; Hansen, E.B. Comparative Evaluation of the Antimicrobial Activity of Different Antimicrobial Peptides against a Range of Pathogenic Bacteria. PLoS ONE 2015, 10, e0144611. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R.; Veld, J.H.J.H.i.t. A Multicompartmental Dynamic Computer-controlled Model Simulating the Stomach and Small Intestine. Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar] [CrossRef]


| Antimicrobial Compounds | Antibacterial Activity (MIC; MBC) (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| CaCl2 | MgCl2 | MHB (Ctrl −) | |||||
| 1 mM | 5 mM | 10 mM | 1 mM | 5 mM | 10 mM | ||
| Analog 1 | n.a. 2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Analog 5 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 16; 32 |
| Analog 6 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 16; 32 |
| Analog 7 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Analog 8 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 32; 64 |
| Chloramphenicol 1 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Antimicrobial Compounds | Antibacterial Activity (MIC; MBC) (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| NaCl | KCl | MHB (Ctrl −) | |||||
| 50 mM | 150 mM | 300 mM | 50 mM | 150 mM | 300 mM | ||
| Analog 1 | n.a. 2 | 128; n.b.a. 3 | 128; n.b.a. | n.a. | n.a. | n.a. | n.a. |
| Analog 5 | 32; 32 | n.a. | n.a. | 32; 32 | n.a. | n.a. | 16; 32 |
| Analog 6 | 64; 64 | n.a. | n.a. | 32; 64 | n.a. | n.a. | 16; 32 |
| Analog 7 | 64 *; n.b.a. | 128 *; n.b.a. | n.a. | 64 *; n.b.a. | 128 *; n.b.a. | n.a. | n.a. |
| Analog 8 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 32; 64 |
| Chloramphenicol 1 | 8 | 4 | 4 | 8 | 4 | 2 | 8 |
| Antimicrobial Compounds | Antibacterial Activity (MIC; MBC) (μg/mL) | |||||
|---|---|---|---|---|---|---|
| pH 5 | pH 6 | pH 7 | pH 8 | 100 °C, 15 min | MHB (Ctrl −) | |
| Analog 1 | 4; n.b.a. | 16; n.b.a. | 64; 128 | 128; 128 | n.a. 2 | n.a. |
| Analog 5 | 2; 8 | 8; 16 | 16; 128 | 32; 128 | n.a. | 16; 32 |
| Analog 6 | 8; n.b.a. | 16; 128 | 16; 32 | 16; 16 | 32; 64 | 16; 32 |
| Analog 7 | 8 *; 8 | 8 *; 32 | n.a. | n.a. | n.a. | n.a. |
| Analog 8 | 2; n.b.a. 3 | 16; 32 | 64; 64 | 128; n.b.a. | 64; 128 | 32; 64 |
| Chloramphenicol 1 | 0.5 | 2 | 4 | 4 | 8 | 8 |
| Analog Identification | Sequence | Net Charge | pI | Molar Weight (g/mol) | GRAVY Index |
|---|---|---|---|---|---|
| (1) SJGAP | VKVGINGFGRIGRLVTRAAFHGKKVEIVAIND | +4 | 11.4 | 3436.07 | 0.272 |
| (5) | VKVGINGFGRIGRLVTRAAFHGKKVAIVAINA | +6 | 12.4 | 3334.02 | 0.603 |
| (6) | VKVGINGFGRIGRLVTRAAFHGKKVKIVAINK | +8 | 12.5 | 3448.21 | 0.247 |
| (7) | VKVGINGFGRIGRLVTRLLFHGKKVEIVLIND | +4 | 11.4 | 3562.21 | 0.459 |
| (8) | VKVGINGFGRIGRLVTRAAFHGKKVEIVAIND-NH2 | +5 | 11.9 | 3435.08 | 0.272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cashman-Kadri, S.; Lagüe, P.; Fliss, I.; Beaulieu, L. Assessing the Activity under Different Physico-Chemical Conditions, Digestibility, and Innocuity of a GAPDH-Related Fish Antimicrobial Peptide and Analogs Thereof. Antibiotics 2023, 12, 1410. https://doi.org/10.3390/antibiotics12091410
Cashman-Kadri S, Lagüe P, Fliss I, Beaulieu L. Assessing the Activity under Different Physico-Chemical Conditions, Digestibility, and Innocuity of a GAPDH-Related Fish Antimicrobial Peptide and Analogs Thereof. Antibiotics. 2023; 12(9):1410. https://doi.org/10.3390/antibiotics12091410
Chicago/Turabian StyleCashman-Kadri, Samuel, Patrick Lagüe, Ismail Fliss, and Lucie Beaulieu. 2023. "Assessing the Activity under Different Physico-Chemical Conditions, Digestibility, and Innocuity of a GAPDH-Related Fish Antimicrobial Peptide and Analogs Thereof" Antibiotics 12, no. 9: 1410. https://doi.org/10.3390/antibiotics12091410
APA StyleCashman-Kadri, S., Lagüe, P., Fliss, I., & Beaulieu, L. (2023). Assessing the Activity under Different Physico-Chemical Conditions, Digestibility, and Innocuity of a GAPDH-Related Fish Antimicrobial Peptide and Analogs Thereof. Antibiotics, 12(9), 1410. https://doi.org/10.3390/antibiotics12091410







