Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort
Abstract
:1. Background
2. Results
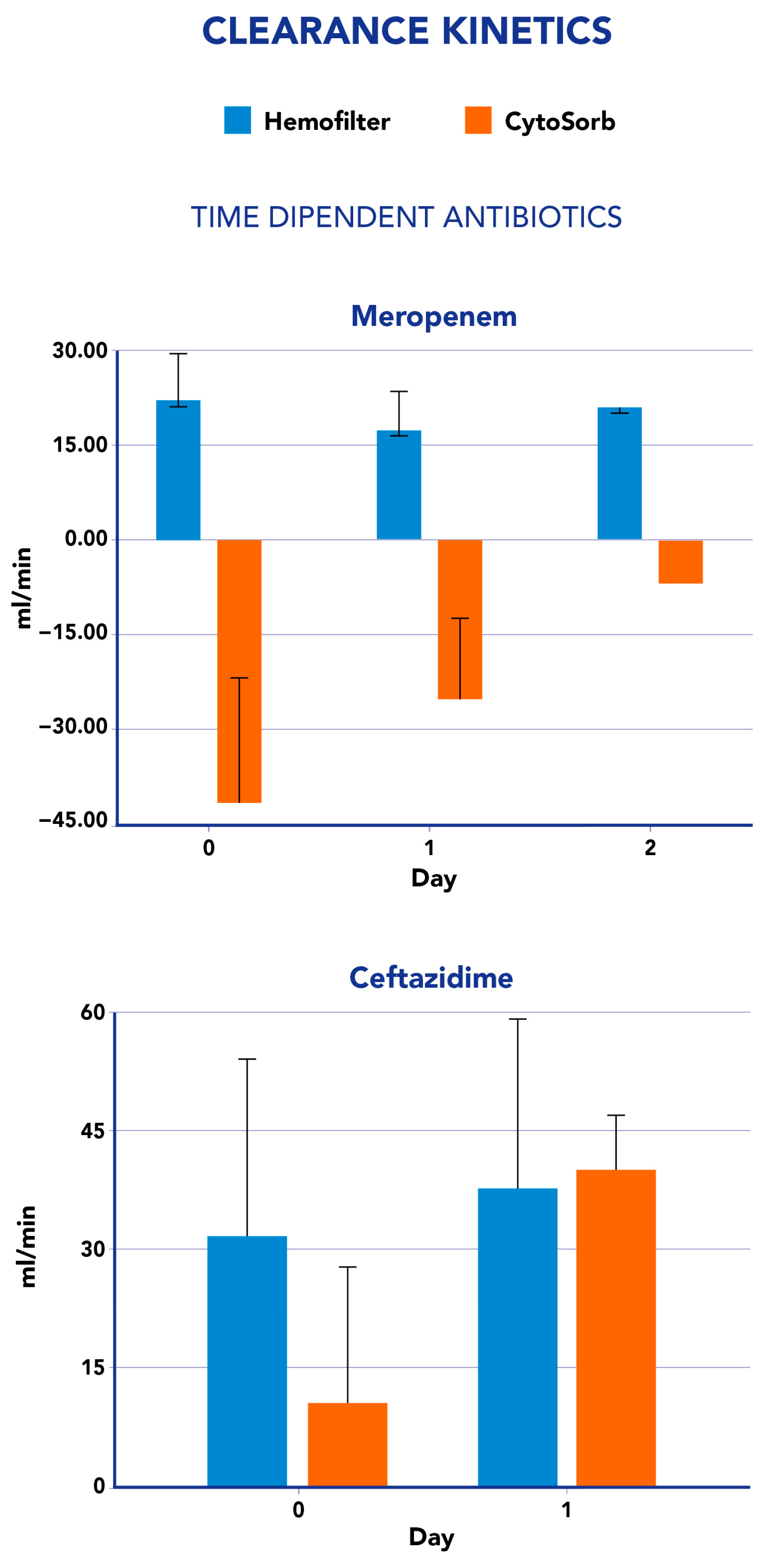
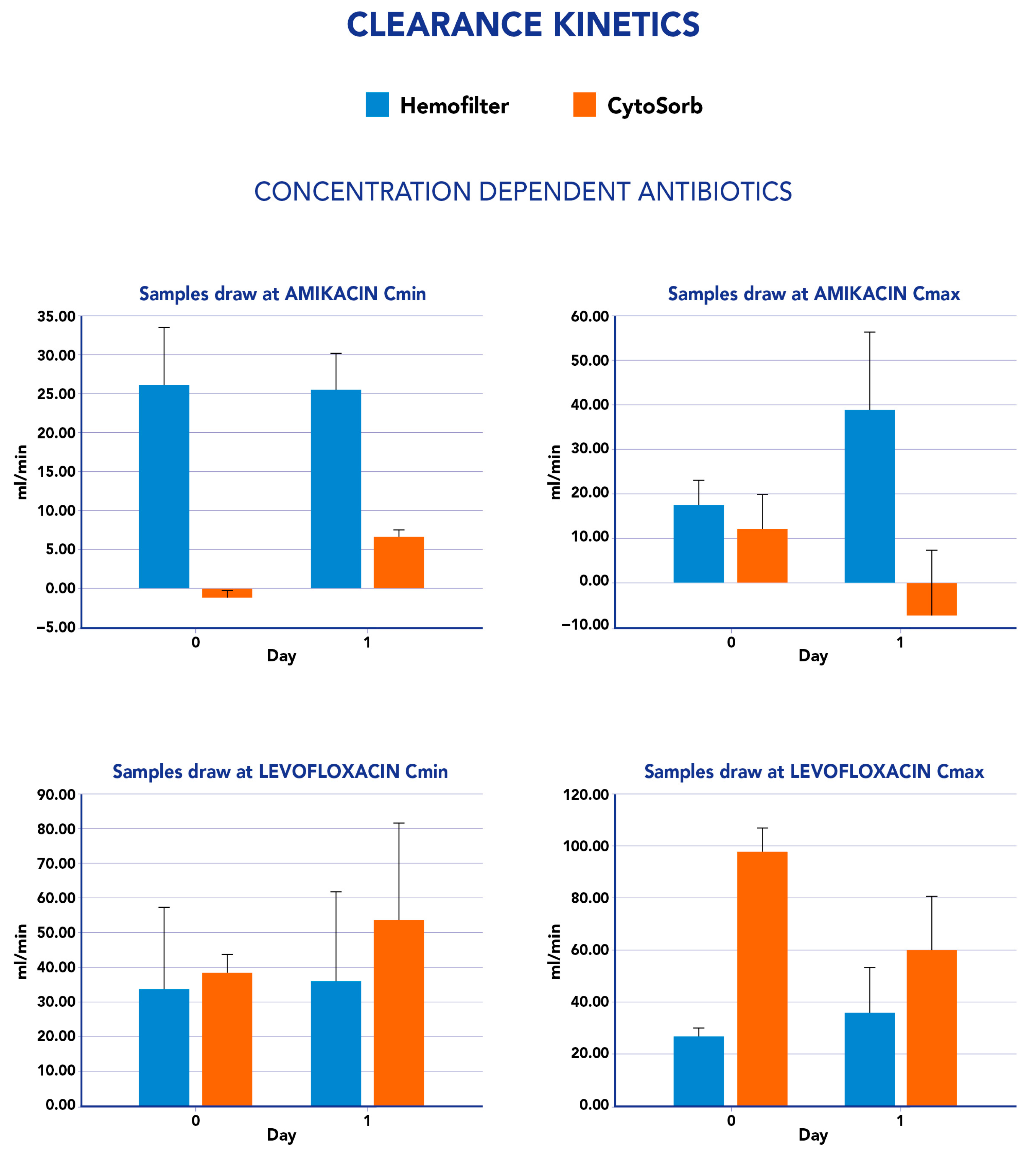
2.1. CytoSorb and CKRT Clearance and Mass Removal
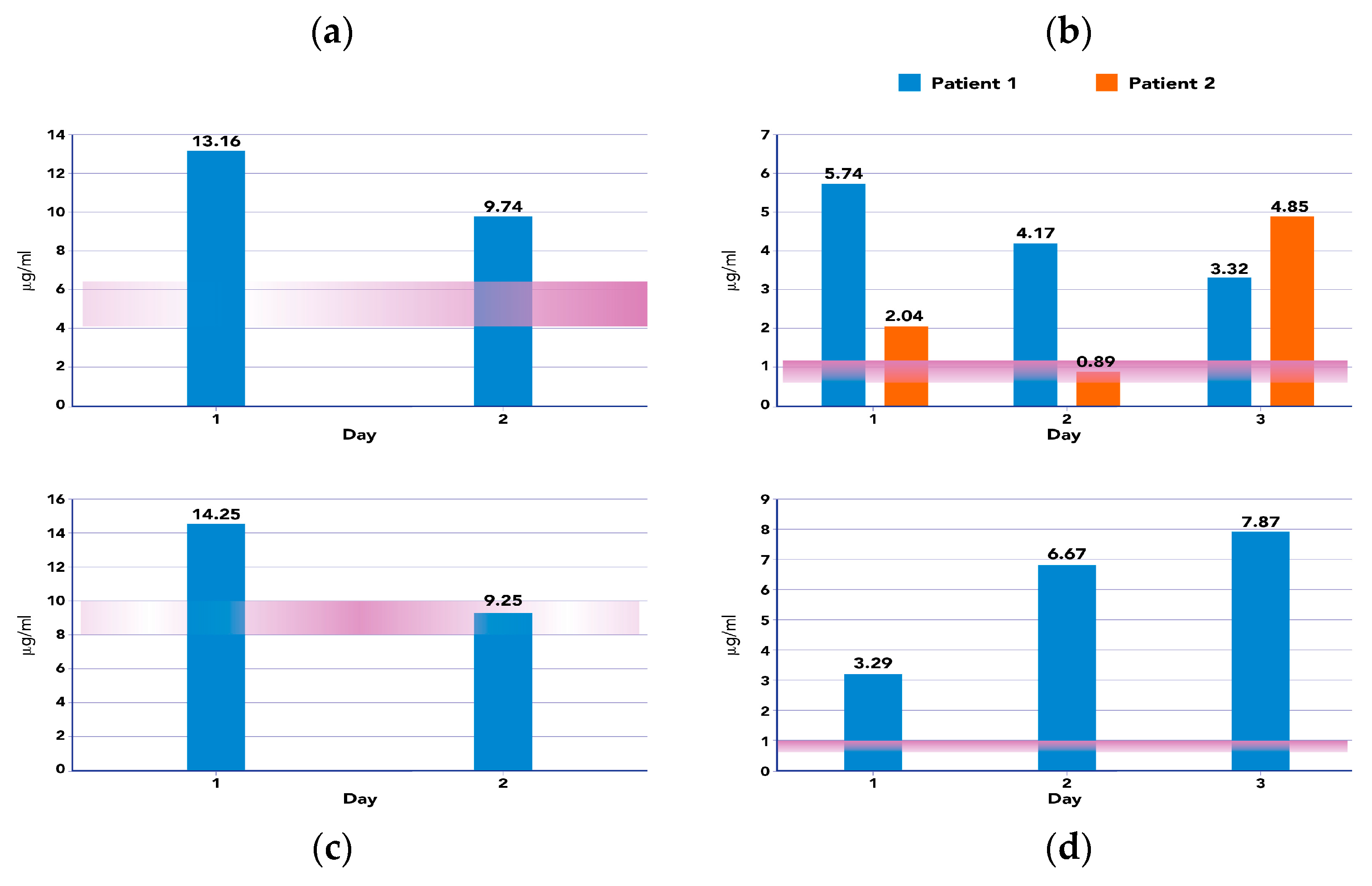
2.2. Clinical Outcomes and AUC during ET Treatments
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Collection and Characteristics of Treatment
4.3. CKRT and Hemoadsorption with CytoSorb
4.4. Blood Samples Protocol
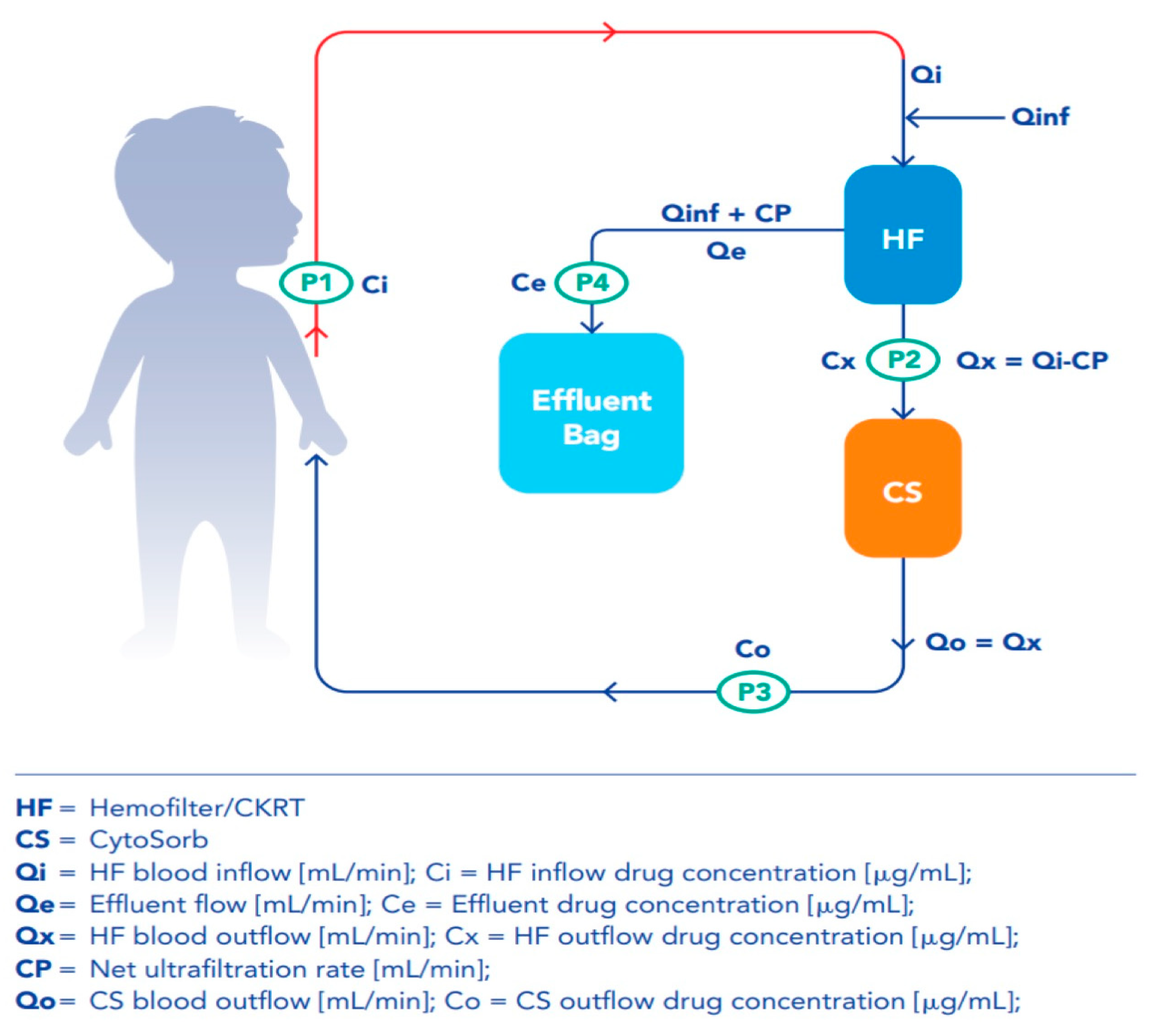
4.5. Clearance and Mass Removal Calculations
4.6. Therapeutic Drug Monitoring
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranieri, V.M.; Brodie, D.; Vincent, J.L. Extracorporeal organ support: From technological tool to clinical strategy supporting severe organ failure. JAMA 2017, 318, 1105–1106. [Google Scholar] [CrossRef]
- Pistolesi, V.; Morabito, S.; Di Mario, F.; Regolisti, G.; Cantarelli, C.; Fiaccadori, E. A guide to under standing antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob. Agents Chemother. 2019, 63, e00583-19. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Xia, Y.; Chu, Y.; Zhong, H.; Li, J.; Liang, P.; Bu, Y.; Zhao, R.; Liao, Y.; et al. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front. Pharmacol. 2020, 11, 786. [Google Scholar] [CrossRef]
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- Jamal, J.-A.; Mueller, B.A.; Choi, G.Y.S.; Lipman, J.; Roberts, J.A. How can we ensure effective antibiotic dosing in critically ill patients receiving different types of renal replacement therapy? Diagn. Microbiol. Infect. Dis. 2015, 82, 92–103. [Google Scholar] [CrossRef]
- Wong, W.-T.; Choi, G.; Gomersall, C.D.; Lipman, J. To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: The eternal doubt. Curr. Opin. Pharmacol. 2015, 24, 68–78. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert clinical pharmacological advice may make an antimicrobial TDM program for emerging candidates more clinically useful in tailoring therapy of critically ill patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.-H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A position paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Poli, E.C.; Rimmele, T.; Schneider, A.G. Hemoadsorption with CytoSorb((R)). Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef]
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12. [Google Scholar] [CrossRef]
- Bottari, G.; Lorenzetti, G.; Severini, F.; Cappoli, A.; Cecchetti, C.; Guzzo, I. Role of Hemoperfusion with CytoSorb Associated with Continuous Kidney Replacement Therapy on Renal Outcome in Critically III Children with Septic Shock. Front. Pediatr. 2021, 9, 718049. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Guzzo, I.; Marano, M.; Stoppa, F.; Ravà, L.; Di Nardo, M.; Cecchetti, C. Hemoperfusion with Cytosorb in pediatric patients with septic shock: A retrospective observational study. Int. J. Artif. Organs 2020, 43, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Liebchen, U.; Scharf, C.; Zoller, M.; Weinelt, F.; Kloft, C.; CytoMero Collaboration Team. No clinically relevant removal of meropenem by cytokine adsorber CytoSorb® in critically ill patients with sepsis or septic shock. Intensive Care Med. 2021, 47, 1332–1333. [Google Scholar] [CrossRef] [PubMed]
- Dimski, T.; Brandenburger, T.; MacKenzie, C.; Kindgen-Milles, D. Elimination of glycopeptide antibiotics by cytokine hemoadsorption in patients with septic shock: A study of three cases. Int. J. Artif. Organs 2020, 43, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Tedeschi, S.; Trapani, F.; Ramirez, S.; Mancini, R.; Giannella, M.; Viale, P.; Pea, F. A Proof of Concept of the Usefulness of a TDM-Guided Strategy for Optimizing Pharmacokinetic/Pharmacodynamic Target of Continuous Infusion Ampicillin-Based Regimens in a Case Series of Patients with Enterococcal Bloodstream Infections and/or Endocarditis. Antibiotics 2022, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Scheier, J.; Nelson, P.J.; Schneider, A.; Colombier, S.; Kindgen-Milles, D.; Deliargyris, E.N.; Nolin, T.D. Mechanistic Considerations and Pharmacokinetic Implications on Concomitant Drug Administration During CytoSorb Therapy. Crit. Care Explor. 2022, 4, e0688. [Google Scholar] [CrossRef]
- Schneider, A.G.; André, P.; Scheier, J.; Schmidt, M.; Ziervogel, H.; Buclin, T.; Kindgen-Milles, D. Pharmacokinetics of anti-infective agents during CytoSorb hemoadsorption. Sci. Rep. 2021, 11, 10493. [Google Scholar] [CrossRef]
- Perrottet, N.; Robatel, C.; Meylan, P.; Pascual, M.; Venetz, J.P.; Aubert, J.D.; Berger, M.M.; Decosterd, L.A.; Buclin, T. Disposition of valganciclovir during continuous renal replacement therapy in two lung transplant recipients. J. Antimicrob. Chemother. 2008, 61, 1332–1335. [Google Scholar] [CrossRef]
- Reiter, K.; Bordoni, V.; Dall’Olio, G.; Ricatti, M.G.; Soli, M.; Ruperti, S.; Soffiati, G.; Galloni, E.; D’Intini, V.; Bellomo, R.; et al. In vitro removal of therapeutic drugs with a novel adsorbent system. Blood Purif. 2002, 20, 380–388. [Google Scholar] [CrossRef]
- König, C.; Röhr, A.C.; Frey, O.R.; Brinkmann, A.; Roberts, J.A.; Wichmann, D.; Braune, S.; Kluge, S.; Nierhaus, A. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int. J. Artif. Organs 2019, 42, 57–64. [Google Scholar] [CrossRef]
- Leteurtre, S.; Duhamel, A.; Salleron, J.; Grandbastien, B.; Lacroix, J.; Leclerc, F.; Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP). PELOD-2: An update of the PEdiatric logistic organ dysfunction score. Crit. Care Med. 2013, 41, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Gaies, M.G.; Jeffries, H.E.; Niebler, R.A.; Pasquali, S.K.; Donohue, J.E.; Yu, S.; Gall, C.; Rice, T.B.; Thiagarajan, R.R. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: An analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr. Crit. Care Med. 2014, 15, 529–537. [Google Scholar] [CrossRef] [PubMed]
- KDIGO KDIGO AKI Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Zellos, A.; Debray, D.; Indolfi, G. Proceedings of ESPGHAN monothematic conference 2020: “acute liver failure in children”: Diagnosis and initial management. J. Pediatr. Gastroenterol. Nutr. 2022, 74, e45–e56. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Bartolini, E.; Calvo, P.L.; Cananzi, M.; Cirillo, F.; Della Corte, C.; Dionisi-Vici, C.; Indolfi, G.; Iorio, R.; Maggiore, G.; et al. Diagnostic Approach to Acute Liver Failure in Children: A Position Paper by the SIGENP Liver Disease Working Group. Dig. Liver Dis. 2021, 53, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A.; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef]
- European Medicines Agency. Guidelines on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-methodvalidation_en.pdf (accessed on 4 October 2022).
- Cairoli, S.; Simeoli, R.; Tarchi, M.; Dionisi, M.; Vitale, A.; Perioli, L.; Dionisi-Vici, C.; Goffredo, B.M. A new HPLC-DAD method for contemporary quantification of 10 antibiotics for therapeutic drug monitoring of critically ill pediatric patients. Biomed. Chromatogr. 2020, 34, e4880. [Google Scholar] [CrossRef]
| N = 10 | |
|---|---|
| Age | 11.5 (6.5–14.75) |
| Male, n | 2 (10) |
| Weight | 42 (26.25–71.25) |
| Diagnosis on Admission | |
| Severe sepsis, n | 1 (10) |
| Septic shock, n | 7 (10) |
| HLH, n | 2 (10) |
| Comorbidities | |
| Liver disease, n | 1 (10) |
| Pulmonary disease, n | 1 (10) |
| Cardiovascular disease, n | 1(10) |
| Hematologic disease, n | 3 (10) |
| Neoplasia, n | 2(10) |
| Other, n | 4 (10) |
| Etiologies of Infection | |
| Gram positive, n | 4 (10) |
| Gram negative, n | 6 (10) |
| Virus, n | 6 (10) |
| Fungus, n | 1 (10) |
| Not detected, n | 4 (10) |
| Source of infection | |
| Blood stream, n | 2 (10) |
| Pulmonary, n | 5 (10) |
| Abdominal, n | 2 (10) |
| Other, n | 2 (10) |
| AKI stage | |
| Stage III, n | 10 (10) |
| Acute Liver Failure | |
| Yes, n | 4(10) |
| Not, n | 6 (10) |
| PELOD-2 | 9.5 (7.75–13.25) |
| VIS | 75 (23.23–91.5) |
| Clearance Hemofilter (L/h) Qef × Ce/Ci × (Qi/ (Qi + Qe − CP) | Clearance CytoSorb (L/h): Qx × (Cx − Co)/Cx | Mass removal Hemofilter (mg/h) Qi × Ci – Qx × Cx | Mass Removal CytoSorb (mg/h) Qo × (Cx − Co) | |
|---|---|---|---|---|
| Meropenem | 1.1 (SD 0.3) | −1.5 (SD 1.5) | 11.5 (SD 19) | −7.6 (SD 10) |
| Ceftazidime | 2 (SD 1.3) | 1.5 (SD 1.1) | 61.8 (SD 52) | −4.1(SD 18.1) |
| Amikacine Cmax | 1.5 (SD 0.9) | 0.2 (SD 0.8) | 26 (SD 43.7) | 2.5 (SD 18.9) |
| Amikacine Cmin | 1.5 (SD 0.3) | 0.1 (SD 0.5) | 1.7 (SD 1.4) | 0.3 (SD 0.6) |
| Levofloxacine Cmax | 1.8 (SD 0.8) | 4.7 (SD 1.5) | 22.5 (SD 3.1) | 26 (SD 15.5) |
| Levofloxacine Cmin | 1.7 (SD 1) | 1.9 (SD 1.2) | 0.6 (SD 5.9) | 4.5 (SD 3.5) |
| n = 10 | ||
|---|---|---|
| Number of Patients per Drug Type | Drug | Dosing |
| 5 | Meropenem | 40 mg/kg q 8 h |
| 3 | Cefatazidime | 50 mg/kg q 12 h |
| 3 | Levofloxacin | 10 mg/kg q 12 h |
| 2 | Amikacin | 15 mg/kg q 12 h |
| Parameter | ET CONTRIBUTIONS | ||
|---|---|---|---|
| Total ET | CKRT | CS | |
| CL | Qef × Ce/Ci × (Qi/(Qi + Qe − CP) + Qx × (Cx − Co)/Cx | Qef × Ce/Ci × (Qi/(Qi + Qe − CP) | Qx × (Cx − Co)/Cx |
| MR | Qo × Co − Qi × Ci | Qx × Cx – Qi × Ci | Qo × (Co − Cx) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottari, G.; Goffredo, B.M.; Marano, M.; Maccarrone, C.; Simeoli, R.; Bianco, G.; Vallesi, L.; Beetham, J.C.C.; Mazzeo, A.T.; Cappoli, A.; et al. Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort. Antibiotics 2023, 12, 1395. https://doi.org/10.3390/antibiotics12091395
Bottari G, Goffredo BM, Marano M, Maccarrone C, Simeoli R, Bianco G, Vallesi L, Beetham JCC, Mazzeo AT, Cappoli A, et al. Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort. Antibiotics. 2023; 12(9):1395. https://doi.org/10.3390/antibiotics12091395
Chicago/Turabian StyleBottari, Gabriella, Bianca Maria Goffredo, Marco Marano, Cristina Maccarrone, Raffaele Simeoli, Giuseppe Bianco, Leonardo Vallesi, Joseph Charles Charlie Beetham, Anna Teresa Mazzeo, Andrea Cappoli, and et al. 2023. "Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort" Antibiotics 12, no. 9: 1395. https://doi.org/10.3390/antibiotics12091395
APA StyleBottari, G., Goffredo, B. M., Marano, M., Maccarrone, C., Simeoli, R., Bianco, G., Vallesi, L., Beetham, J. C. C., Mazzeo, A. T., Cappoli, A., Cairoli, S., Labbadia, R., Cecchetti, C., Bernaschi, P., Corsetti, T., Morabito, S., Taccone, F. S., & Guzzo, I. (2023). Impact of Continuous Kidney Replacement Therapy and Hemoadsorption with CytoSorb on Antimicrobial Drug Removal in Critically Ill Children with Septic Shock: A Single-Center Prospective Study on a Pediatric Cohort. Antibiotics, 12(9), 1395. https://doi.org/10.3390/antibiotics12091395








