High Prevalence of GES-5 Variant and Co-Expression of VIM-2 and GES-45 among Clinical Pseudomonas aeruginosa Strains in Tunisia
Abstract
1. Introduction
2. Results
2.1. Epidemiological Results
2.2. Antimicrobial Susceptibility and Phenotypic Tests
2.3. Characterization of Carbapenem Resistance
2.4. Molecular Typing and Serotypes of CRPA Isolates
2.5. Detection of Virulence Factors
2.6. Detection and Characterization of Integron Structures
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates and Identification
4.2. Antimicrobial Susceptibility Tests
4.3. Molecular Typing
4.4. Serotyping
4.5. Characterization of Class A Carbapenemases, MBLs, ESBL, and Porin OprD
4.6. Detection and Characterization of Integrons
4.7. Detection of Virulence Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Chen, X.-L.; Huang, A.-W.; Liu, S.-L.; Liu, W.-J.; Zhang, N.; Lu, X.-Z. Mortality Attributable to Carbapenem-Resistant Pseudomonas aeruginosa Bacteremia: A Meta-Analysis of Cohort Studies. Emerg. Microbes Infect. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-Negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-Line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- Baughman, R.P. The Use of Carbapenems in the Treatment of Serious Infections. J. Intensiv. Care Med. 2009, 24, 230–241. [Google Scholar] [CrossRef]
- Lee, C.-S.; Doi, Y. Therapy of Infections Due to Carbapenem-Resistant Gram-Negative Pathogens. Infect. Chemother. 2014, 46, 149–164. [Google Scholar] [CrossRef]
- Walsh, T.R. The Emergence and Implications of Metallo-β-Lactamases in Gram-Negative Bacteria. Clin. Microbiol. Infect. 2005, 11, 2–9. [Google Scholar] [CrossRef]
- Tenover, F.C.; Nicolau, D.P.; Gill, C.M. Carbapenemase-Producing Pseudomonas aeruginosa –an Emerging Challenge. Emerg. Microbes Infect. 2022, 11, 811–814. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Castanheira, M.; Mendes, R.E.; Walsh, T.R.; Gales, A.C.; Jones, R.N. Emergence of the Extended-Spectrum β-Lactamase GES-1 in a Pseudomonas aeruginosa Strain from Brazil: Report from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2004, 48, 2344–2345. [Google Scholar] [CrossRef]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.; Ghozzi, R.; Burghoffer, B.; Arlet, G.; Redjeb, S. Mechanisms of Carbapenem Resistance in Non-Metallo-β-Lactamase-Producing Clinical Isolates of Pseudomonas aeruginosa from a Tunisian Hospital. Pathol. Biol. 2009, 57, 530–535. [Google Scholar] [CrossRef]
- Pottier, M.; Gravey, F.; Castagnet, S.; Auzou, M.; Langlois, B.; Guérin, F.; Giard, J.-C.; Léon, A.; Le Hello, S. A 10-Year Microbiological Study of Pseudomonas aeruginosa Strains Revealed the Circulation of Populations Resistant to Both Carbapenems and Quaternary Ammonium Compounds. Sci. Rep. 2023, 13, 2639. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P.; Lagrutta, E.; Cleary, T.; Munoz-Price, L.S. Emergence of KPC-Producing Pseudomonas aeruginosa in the United States. Antimicrob. Agents Chemother. 2010, 54, 3072. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Wang, Q.; Zeng, Y.; Sun, Q.; Shu, L.; Lu, J.; Cai, J.; Wang, S.; Zhang, R.; et al. Emergence and Expansion of a Carbapenem-Resistant Pseudomonas aeruginosa Clone Are Associated with Plasmid-Borne blaKPC-2 and Virulence-Related Genes. mSystems 2021, 6, e00154-21. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Jeong, S.H. Mobile Carbapenemase Genes in Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 614058. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-Lactamase DataBase (BLDB)—Structure and Function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Kotsakis, S.D.; Miriagou, V.; Tzelepi, E.; Tzouvelekis, L.S. Comparative Biochemical and Computational Study of the Role of Naturally Occurring Mutations at Ambler Positions 104 and 170 in GES β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 4864–4871. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, P.; Chang, D.; Mi, Z. A Pseudomonas aeruginosa Isolate Producing the GES-5 Extended-Spectrum Beta-Lactamase. J. Antimicrob. Chemother. 2006, 57, 1261–1262. [Google Scholar] [CrossRef]
- Hishinuma, T.; Tada, T.; Kuwahara-Arai, K.; Yamamoto, N.; Shimojima, M.; Kirikae, T. Spread of GES-5 Carbapenemase-Producing Pseudomonas aeruginosa Clinical Isolates in Japan Due to Clonal Expansion of ST235. PLoS ONE 2018, 13, e0207134. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence Factors of Pseudomonas aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The Increasing Threat of Pseudomonas aeruginosa High-Risk Clones. Drug Resist. Updat. 2015, 21–22, 41–59. [Google Scholar] [CrossRef]
- Sabat, A.J.; Budimir, A.; Nashev, D.; Sá-Leão, R.; van Dijl, J.M.; Laurent, F.; Grundmann, H.; Friedrich, A.W.; ESCMID Study Group of Epidemiological Markers (ESGEM). Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Eur. Commun. Dis. Bull. 2013, 18, 20380. [Google Scholar] [CrossRef]
- Curran, B.; Jonas, D.; Grundmann, H.; Pitt, T.; Dowson, C.G. Development of a Multilocus Sequence Typing Scheme for the Opportunistic Pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 2004, 42, 5644–5649. [Google Scholar] [CrossRef]
- Mansour, W.; Poirel, L.; Bettaieb, D.; Bouallegue, O.; Boujaafar, N.; Nordmann, P. Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa Isolates in Tunisia. Diagn. Microbiol. Infect. Dis. 2009, 64, 458–461. [Google Scholar] [CrossRef]
- Hammami, S.; Gautier, V.; Ghozzi, R.; Da Costa, A.; Ben-Redjeb, S.; Arlet, G. Diversity in VIM-2-Encoding Class 1 Integrons and Occasional blaSHV2a Carriage in Isolates of a Persistent, Multidrug-Resistant Pseudomonas aeruginosa Clone from Tunis. Clin. Microbiol. Infect. 2010, 16, 189–193. [Google Scholar] [CrossRef]
- Ktari, S.; Mnif, B.; Znazen, A.; Rekik, M.; Mezghani, S.; Mahjoubi-Rhimi, F.; Hammami, A. Diversity of β-Lactamases in Pseudomonas aeruginosa Isolates Producing Metallo-β-Lactamase in Two Tunisian Hospitals. Microb. Drug Resist. Larchmt. N 2011, 17, 25–30. [Google Scholar] [CrossRef]
- Hammami, S.; Boutiba-Ben Boubaker, I.; Ghozzi, R.; Saidani, M.; Amine, S.; Ben Redjeb, S. Nosocomial Outbreak of Imipenem-Resistant Pseudomonas aeruginosa Producing VIM-2 Metallo-β-Lactamase in a Kidney Transplantation Unit. Diagn. Pathol. 2011, 6, 106. [Google Scholar] [CrossRef]
- Chairat, S.; Ben Yahia, H.; Rojo-Bezares, B.; Sáenz, Y.; Torres, C.; Ben Slama, K. High Prevalence of Imipenem-Resistant and Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa in the Burns Hospital in Tunisia: Detection of a Novel Class 1 Integron. J. Chemother. 2019, 31, 120–126. [Google Scholar] [CrossRef]
- Ferjani, S.; Maamar, E.; Ferjani, A.; Kanzari, L.; Boubaker, I.B.B. Evaluation of Three Carbapenemase-Phenotypic Detection Methods and Emergence of Diverse VIM and GES Variants among Pseudomonas aeruginosa Isolates in Tunisia. Antibiot. 2022, 11, 858. [Google Scholar] [CrossRef]
- Hmissi, S.; Raddaoui, A.; Frigui, S.; Abbassi, M.S.; Achour, W.; Chebbi, Y.; Thabet, L. Detection of carbapenem resistant Pseudomonas aeruginosa co-harboring blaVIM-2 and blaGES-5 in burn patients. Acta Microbiol. Immunol. Hung. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Del Barrio-Tofiño, E.; López-Causapé, C.; Oliver, A. Pseudomonas aeruginosa Epidemic High-Risk Clones and Their Association with Horizontally-Acquired β-Lactamases: 2020 Update. Int. J. Antimicrob. Agents 2020, 56, 106196. [Google Scholar] [CrossRef]
- Meradji, S.; Barguigua, A.; Zerouali, K.; Mazouz, D.; Chettibi, H.; Elmdaghri, N.; Timinouni, M. Epidemiology of Carbapenem Non-Susceptible Pseudomonas aeruginosa Isolates in Eastern Algeria. Antimicrob. Resist. Infect. Control 2015, 4, 27. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Hammoudi Halat, D.; Akkawi, C.; Nabi, A.; AlSharhan, M.A.; AlDeesi, Z.O.; Peters, C.C.; Celiloglu, H.; Sarkis, D.K. Role of Outer Membrane Permeability, Efflux Mechanism, and Carbapenemases in Carbapenem-Nonsusceptible Pseudomonas aeruginosa from Dubai Hospitals: Results of the First Cross-Sectional Survey. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2019, 84, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Mechergui, A.; Achour, W.; Mathlouthi, S.; Hassen, A.B. Prevalence of Infectious Multi-Drug Resistant Bacteria Isolated from Immunocompromised Patients in Tunisia. Afr. Health Sci. 2019, 19, 2021–2025. [Google Scholar] [CrossRef] [PubMed]
- Basha, A.M.; El-Sherbiny, G.M.; Mabrouk, M.I. Phenotypic Characterization of the Egyptian Isolates “Extensively Drug-Resistant Pseudomonas aeruginosa” and Detection of Their Metallo-β-Lactamases Encoding Genes. Bull. Natl. Res. Cent. 2020, 44, 117. [Google Scholar] [CrossRef]
- Al-Otaibi, F.E.; Bukhari, E.E.; Badr, M.; Alrabiaa, A.A. Prevalence and Risk Factors of Gram-Negative Bacilli Causing Blood Stream Infection in Patients with Malignancy. Saudi Med. J. 2016, 37, 979–984. [Google Scholar] [CrossRef]
- Mikucionyte, G.; Zamorano, L.; Vitkauskiene, A.; López-Causapé, C.; Juan, C.; Mulet, X.; Oliver, A. Nosocomial Dissemination of VIM-2-Producing ST235 Pseudomonas aeruginosa in Lithuania. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 195–200. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, K.-H.; Chen, W.; Yu, Y.; Feng, S.-F. Epidemiology and Risk Factors for Nosocomial Infection in the Respiratory Intensive Care Unit of a Teaching Hospital in China: A Prospective Surveillance during 2013 and 2015. BMC Infect. Dis. 2019, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of Antibiotic Resistance Pseudomonas aeruginosa in Intensive Care Unit; a Critical Review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar] [CrossRef]
- Evans, J.C.; Segal, H. A Novel Insertion Sequence, ISPA26, in OprD of Pseudomonas aeruginosa Is Associated with Carbapenem Resistance. Antimicrob. Agents Chemother. 2007, 51, 3776–3777. [Google Scholar] [CrossRef]
- Rojo-Bezares, B.; Estepa, V.; Cebollada, R.; de Toro, M.; Somalo, S.; Seral, C.; Castillo, F.J.; Torres, C.; Sáenz, Y. Carbapenem-Resistant Pseudomonas aeruginosa Strains from a Spanish Hospital: Characterization of Metallo-Beta-Lactamases, Porin OprD and Integrons. Int. J. Med. Microbiol. 2014, 304, 405–414. [Google Scholar] [CrossRef]
- Bellés, A.; Bueno, J.; Rojo-Bezares, B.; Torres, C.; Javier Castillo, F.; Sáenz, Y.; Seral, C. Characterisation of VIM-2-Producing Pseudomonas aeruginosa Isolates from Lower Tract Respiratory Infections in a Spanish Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kong, W.; Yang, W.; Chen, G.; Liang, H.; Zhang, Y. Multilocus Sequence Typing and Variations in the OprD Gene of Pseudomonas aeruginosa Isolated from a Hospital in China. Infect. Drug Resist. 2018, 11, 45–54. [Google Scholar] [CrossRef]
- Bocharova, Y.; Savinova, T.; Shagin, D.A.; Shelenkov, A.A.; Mayanskiy, N.A.; Chebotar, I.V. Inactivation of the OprD Porin Gene by a Novel Insertion Sequence ISPa195 Associated with Large Deletion in a Carbapenem-Resistant Pseudomonas aeruginosa Clinical Isolate. J. Glob. Antimicrob. Resist. 2019, 17, 309–311. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Viedma, E.; Juan, C.; Acosta, J.; Zamorano, L.; Otero, J.R.; Sanz, F.; Chaves, F.; Oliver, A. Nosocomial Spread of Colistin-Only-Sensitive Sequence Type 235 Pseudomonas aeruginosa Isolates Producing the Extended-Spectrum β-Lactamases GES-1 and GES-5 in Spain. Antimicrob. Agents Chemother. 2009, 53, 4930–4933. [Google Scholar] [CrossRef]
- Hammoudi Halat, D.; Moubareck, C.A.; Sarkis, D.K. Heterogeneity of Carbapenem Resistance Mechanisms Among Gram-Negative Pathogens in Lebanon: Results of the First Cross-Sectional Countrywide Study. Microb. Drug Resist. 2017, 23, 733–743. [Google Scholar] [CrossRef]
- Zafer, M.M.; Al-Agamy, M.H.; El-Mahallawy, H.A.; Amin, M.A.; El Din Ashour, S. Dissemination of VIM-2 Producing Pseudomonas aeruginosa ST233 at Tertiary Care Hospitals in Egypt. BMC Infect. Dis. 2015, 15, 122. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Jeannot, K.; El-Mahdy, T.S.; Samaha, H.A.; Shibl, A.M.; Plésiat, P.; Courvalin, P. Diversity of Molecular Mechanisms Conferring Carbapenem Resistance to Pseudomonas aeruginosa Isolates from Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 4379686. [Google Scholar] [CrossRef] [PubMed]
- Malkoçoğlu, G.; Aktaş, E.; Bayraktar, B.; Otlu, B.; Bulut, M.E. VIM-1, VIM-2, and GES-5 Carbapenemases Among Pseudomonas aeruginosa Isolates at a Tertiary Hospital in Istanbul, Turkey. Microb. Drug Resist. 2017, 23, 328–334. [Google Scholar] [CrossRef]
- Kanayama, A.; Kawahara, R.; Yamagishi, T.; Goto, K.; Kobaru, Y.; Takano, M.; Morisada, K.; Ukimura, A.; Kawanishi, F.; Tabuchi, A.; et al. Successful Control of an Outbreak of GES-5 Extended-Spectrum β-Lactamase-Producing Pseudomonas aeruginosa in a Long-Term Care Facility in Japan. J. Hosp. Infect. 2016, 93, 35–41. [Google Scholar] [CrossRef]
- Treepong, P.; Kos, V.N.; Guyeux, C.; Blanc, D.S.; Bertrand, X.; Valot, B.; Hocquet, D. Global Emergence of the Widespread Pseudomonas aeruginosa ST235 Clone. Clin. Microbiol. Infect. 2018, 24, 258–266. [Google Scholar] [CrossRef]
- Doumith, M.; Alhassinah, S.; Alswaji, A.; Alzayer, M.; Alrashidi, E.; Okdah, L.; Aljohani, S.; NGHA AMR Surveillance Group; Balkhy, H.H.; Alghoribi, M.F. Genomic Characterization of Carbapenem-Non-Susceptible Pseudomonas aeruginosa Clinical Isolates From Saudi Arabia Revealed a Global Dissemination of GES-5-Producing ST235 and VIM-2-Producing ST233 Sub-Lineages. Front. Microbiol. 2022, 12, 765113. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging Broad-Spectrum Resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and Epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Royer, G.; Fourreau, F.; Boulanger, B.; Mercier-Darty, M.; Ducellier, D.; Cizeau, F.; Potron, A.; Podglajen, I.; Mongardon, N.; Decousser, J.-W. Local Outbreak of Extended-Spectrum β-Lactamase SHV2a-Producing Pseudomonas aeruginosa Reveals the Emergence of a New Specific Sub-Lineage of the International ST235 High-Risk Clone. J. Hosp. Infect. 2020, 104, 33–39. [Google Scholar] [CrossRef]
- Cabot, G.; Ocampo-Sosa, A.A.; Domínguez, M.A.; Gago, J.F.; Juan, C.; Tubau, F.; Rodríguez, C.; Moyà, B.; Peña, C.; Martínez-Martínez, L.; et al. Genetic Markers of Widespread Extensively Drug-Resistant Pseudomonas aeruginosa High-Risk Clones. Antimicrob. Agents Chemother. 2012, 56, 6349–6357. [Google Scholar] [CrossRef]
- Elabbadi, A.; Pont, S.; Verdet, C.; Plésiat, P.; Cretin, F.; Voiriot, G.; Fartoukh, M.; Djibré, M. An Unusual Community-Acquired Invasive and Multi Systemic Infection Due to ExoU-Harboring Pseudomonas aeruginosa Strain: Clinical Disease and Microbiological Characteristics. J. Microbiol. Immunol. Infect. 2020, 53, 647–651. [Google Scholar] [CrossRef]
- Foulkes, D.M.; McLean, K.; Haneef, A.S.; Fernig, D.G.; Winstanley, C.; Berry, N.; Kaye, S.B. Pseudomonas aeruginosa Toxin ExoU as a Therapeutic Target in the Treatment of Bacterial Infections. Microorganisms 2019, 7, 707. [Google Scholar] [CrossRef]
- Peña, C.; Cabot, G.; Gómez-Zorrilla, S.; Zamorano, L.; Ocampo-Sosa, A.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A.; et al. Influence of Virulence Genotype and Resistance Profile in the Mortality of Pseudomonas aeruginosa Bloodstream Infections. Clin. Infect. Dis. 2015, 60, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Faghri, J.; Nouri, S.; Jalalifar, S.; Zalipoor, M.; Halaji, M. Investigation of Antimicrobial Susceptibility, Class I and II Integrons among Pseudomonas aeruginosa Isolates from Hospitalized Patients in Isfahan, Iran. BMC Res. Notes 2018, 11, 806. [Google Scholar] [CrossRef]
- Belotti, P.T.; Thabet, L.; Laffargue, A.; André, C.; Coulange-Mayonnove, L.; Arpin, C.; Messadi, A.; M’Zali, F.; Quentin, C.; Dubois, V. Description of an Original Integron Encompassing blaVIM-2, QnrVC1 and Genes Encoding Bacterial Group II Intron Proteins in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2015, 70, 2237–2240. [Google Scholar] [CrossRef]
- De Vos, D.; Lim, A.; Pirnay, J.P.; Struelens, M.; Vandenvelde, C.; Duinslaeger, L.; Vanderkelen, A.; Cornelis, P. Direct Detection and Identification of Pseudomonas aeruginosa in Clinical Samples Such as Skin Biopsy Specimens and Expectorations by Multiplex PCR Based on Two Outer Membrane Lipoprotein Genes, OprI and OprL. J. Clin. Microbiol. 1997, 35, 1295–1299. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0. 2020. Available online: https://eucast.org/clinical_breakpoints/ (accessed on 6 January 2020).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Lee, K.; Chong, Y.; Shin, H.B.; Kim, Y.A.; Yong, D.; Yum, J.H. Modified Hodge and EDTA-Disk Synergy Tests to Screen Metallo-β-Lactamase-Producing Strains of Pseudomonas and Acinetobacter Species. Clin. Microbiol. Infect. 2001, 7, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Bezares, B.; Cavalié, L.; Dubois, D.; Oswald, E.; Torres, C.; Sáenz, Y. Characterization of Carbapenem Resistance Mechanisms and Integrons in Pseudomonas aeruginosa Strains from Blood Samples in a French Hospital. J. Med. Microbiol. 2016, 65, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.E. Pulsed-Field Gel Electrophoresis. Methods Mol. Med. 1998, 15, 33–50. [Google Scholar] [CrossRef]
- Heras, J.; Domínguez, C.; Mata, E.; Pascual, V.; Lozano, C.; Torres, C.; Zarazaga, M. GelJ—A Tool for Analyzing DNA Fingerprint Gel Images. BMC Bioinform. 2015, 16, 270. [Google Scholar] [CrossRef]
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for Rapid Detection of Genes Encoding Acquired Metallo-Beta-Lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Poirel, L.; Le Thomas, I.; Naas, T.; Karim, A.; Nordmann, P. Biochemical Sequence Analyses of GES-1, a Novel Class A Extended-Spectrum β-Lactamase, and the Class 1 Integron In52 from Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 2000, 44, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Briñas, L.; Verlinde, A.; Ide, L.; Nordmann, P. BEL-1, a Novel Clavulanic Acid-Inhibited Extended-Spectrum β-Lactamase, and the Class 1 Integron In120 in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Porres-Osante, N.; Azcona-Gutiérrez, J.M.; Rojo-Bezares, B.; Undabeitia, E.; Torres, C.; Sáenz, Y. Emergence of a Multiresistant KPC-3 and VIM-1 Carbapenemase-Producing Escherichia coli Strain in Spain. J. Antimicrob. Chemother. 2014, 69, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Wolter, D.J.; Hanson, N.D.; Lister, P.D. Insertional Inactivation of OprD in Clinical Isolates of Pseudomonas aeruginosa Leading to Carbapenem Resistance. FEMS Microbiol. Lett. 2004, 236, 137–143. [Google Scholar] [CrossRef]
- Gutiérrez, O.; Juan, C.; Cercenado, E.; Navarro, F.; Bouza, E.; Coll, P.; Pérez, J.L.; Oliver, A. Molecular Epidemiology and Mechanisms of Carbapenem Resistance in Pseudomonas aeruginosa Isolates from Spanish Hospitals. Antimicrob. Agents Chemother. 2007, 51, 4329–4335. [Google Scholar] [CrossRef]
- Estepa, V.; Rojo-Bezares, B.; Torres, C.; Sáenz, Y. Faecal Carriage of Pseudomonas aeruginosa in Healthy Humans: Antimicrobial Susceptibility and Global Genetic Lineages. FEMS Microbiol. Ecol. 2014, 89, 15–19. [Google Scholar] [CrossRef]
- Petit, S.M.-C.; Lavenir, R.; Colinon-Dupuich, C.; Boukerb, A.M.; Cholley, P.; Bertrand, X.; Freney, J.; Doléans-Jordheim, A.; Nazaret, S.; Laurent, F.; et al. Lagooning of Wastewaters Favors Dissemination of Clinically Relevant Pseudomonas aeruginosa. Res. Microbiol. 2013, 164, 856–866. [Google Scholar] [CrossRef]
- Ruiz-Roldán, L.; Bellés, A.; Bueno, J.; Azcona-Gutiérrez, J.M.; Rojo-Bezares, B.; Torres, C.; Castillo, F.J.; Sáenz, Y.; Seral, C. Pseudomonas aeruginosa Isolates from Spanish Children: Occurrence in Faecal Samples, Antimicrobial Resistance, Virulence, and Molecular Typing. BioMed. Res. Int. 2018, 2018, 8060178. [Google Scholar] [CrossRef]
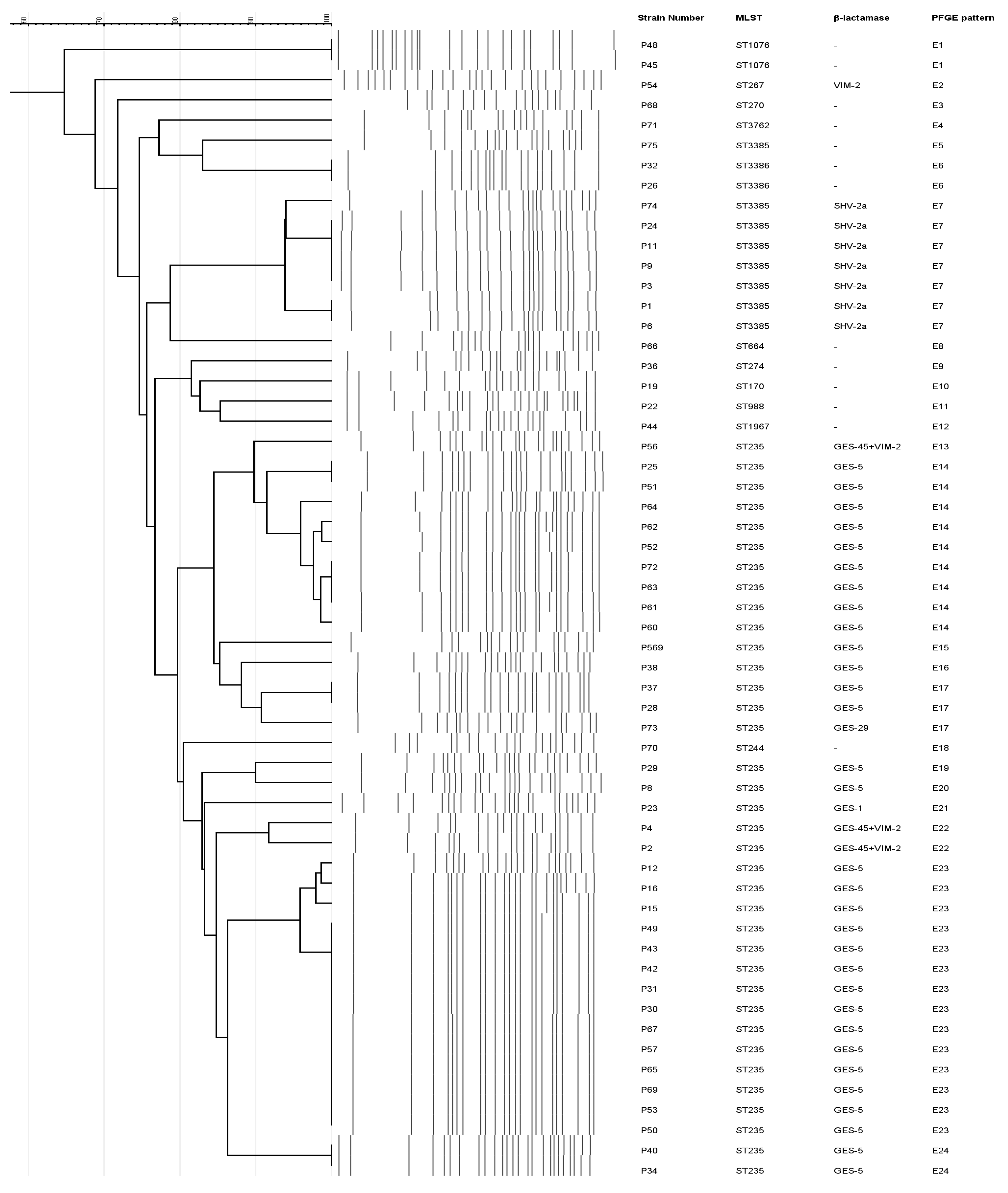
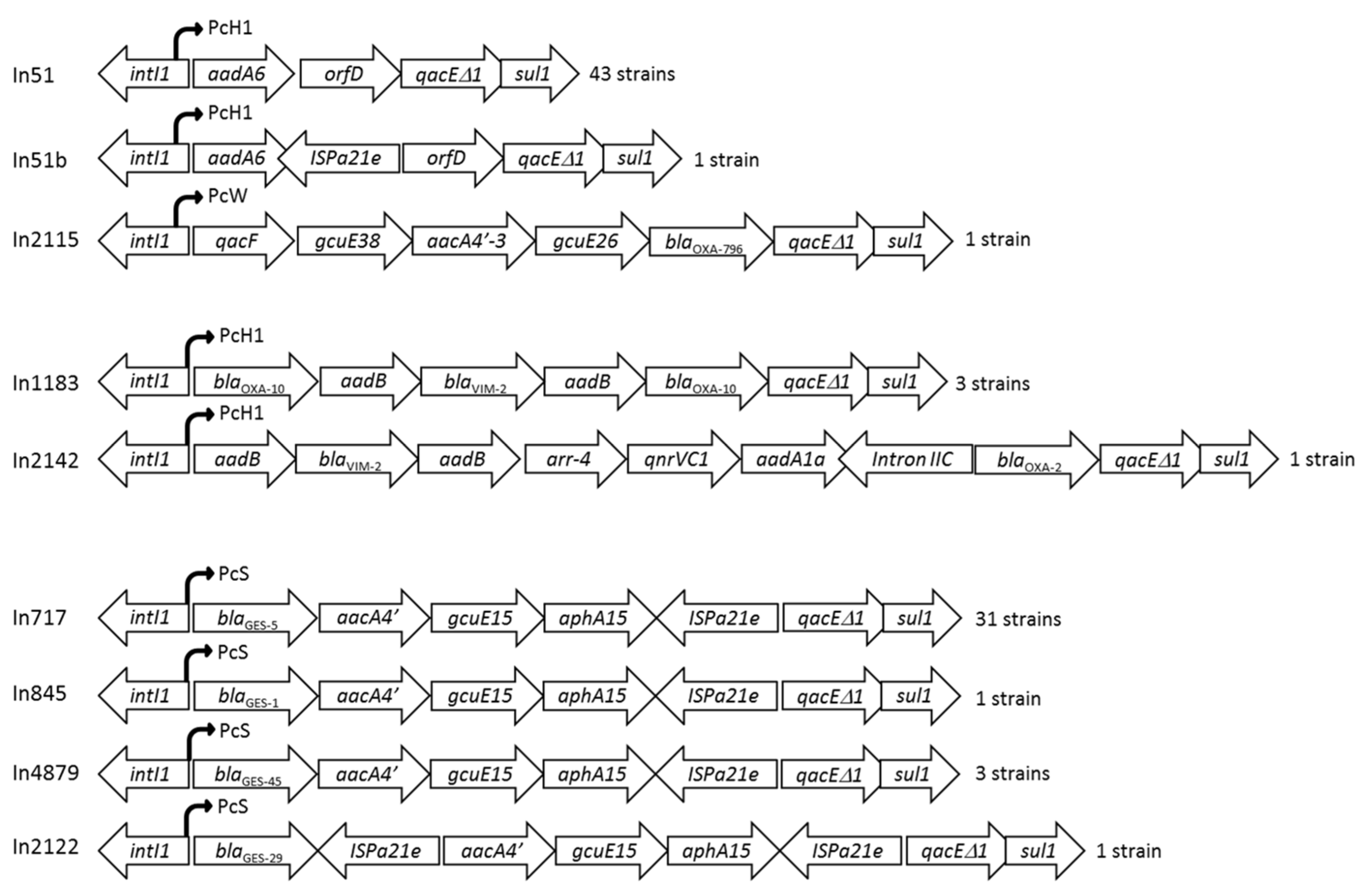
| Characteristic | Number of CRPA (n = 57) | Carbapenemase Producer (n = 35) | Non-Carbapenemase Producer (n = 22) |
|---|---|---|---|
| Patient Gender: | |||
| Number of males (%) | 45 (79%) | 25 (56%) | 20 (44%) |
| Number of females (%) | 12 (21%) | 10 (83%) | 2 (17%) |
| Patient age (year range) | |||
| [20–45] | 16 (28%) | 9 (56%) | 7 (44%) |
| [46–64] | 19 (33%) | 12 (63%) | 7 (37%) |
| [65–80] | 22 (39%) | 14 (64%) | 8 (36%) |
| Hospital admission to: | |||
| Intensive care unit | 47 (82%) | 34 (72%) | 13 (28%) |
| Vascular surgery | 4 (7%) | 0 (0%) | 4 (100%) |
| Cardiothoracic surgery | 2 (3.5%) | 0 (0%) | 2 (100%) |
| Orthopedic surgery | 2 (3.5%) | 1 (50%) | 1 (50%) |
| Urology | 1 (2%) | 0 (0%) | 1 (100%) |
| Bacteriology | 1 (2%) | 0 (0%) | 1 (100%) |
| Type of sample: | |||
| Tracheal aspirate | 23 (40%) | 16 (70%) | 7 (30%) |
| Broncho-alveolar lavage | 5 (9%) | 4 (80%) | 1 (20%) |
| Sputum | 2 (3.5%) | 0 (0%) | 2 (100%) |
| Blood | 13 (23%) | 9 (69%) | 4 (31%) |
| Pus | 7 (12%) | 1 (14%) | 6 (86%) |
| Catheter | 4 (7%) | 3 (75%) | 1 (25%) |
| Urine | 2 (3.5%) | 1 (50%) | 1 (50%) |
| Urethral sample | 1 (2%) | 1 (100%) | 0 (0%) |
| Strains | Resistance Phenotype | MIC (mg/L) | Beta- Lactamases | Molecular Typing | |||
|---|---|---|---|---|---|---|---|
| IPM | MER | MLST | PFGE Pattern (nº. Strains) | Serotype | |||
| P8, P12, P15, P16, P25, P28, P29, P30, P31, P34, P37, P38, P40, P42, P43, P49, P50, P52, P53, P57, P60, P61, P62, P63, P64, P67, P69, P72, P569 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | ≥16 | GES-5 | ST235 | E14 (8), E15 (1), E16 (1), E17 (2), E19 (1), E20 (1), E23 (13), E24 (2) | O:11 |
| P51 | IPM, MEM, CAZ, FEP, TCC, PIP, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | ≥16 | GES-5 | ST235 | E14 (1) | O:11 |
| P65 | IPM, MEM, CAZ, TCC, PIP, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | ≥16 | GES-5 | ST235 | E23 (1) | O:11 |
| P23 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | 8 | GES-1 | ST235 | E21 (1) | O:11 |
| P73 | IPM, MEM, CAZ, FEP, TCC, PIP, ATM, GEN, TOB, CIP, LVX | ≥16 | 4 | GES-29 | ST235 | E17 (1) | O:11 |
| P2, P4, P56 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | ≥16 | VIM-2, GES-45 | ST235 | E13 (1), E22 (2) | O:11 |
| P54 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, GEN, TOB, CIP, LVX | ≥16 | ≥16 | VIM-2 | ST267 | E2 (1) | O:16 |
| P1, P3, P9, P11, P24, P74 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | 4–8 | SHV-2a | ST3385 | E7 (6) | O:11 |
| P6 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, GEN, TOB, CIP, LVX | ≥16 | 8 | SHV-2a | ST3385 | E7 (1) | PA |
| P19 | IPM, MEM, TCC, ATM | ≥16 | 4 | - | ST170 | E10 (1) | Agg- |
| P22 | IPM, MEM, TCC, ATM | ≥16 | 4 | - | ST988 | E11 (1) | O:4 |
| P26 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, CIP, LVX | ≥16 | ≥16 | - | ST3386 | E6 (1) | O:5 |
| P32 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, GEN, CIP, LVX | ≥16 | ≥16 | - | ST3386 | E6 (1) | PA |
| P36 | IPM, MEM, ATM | ≥16 | 4 | - | ST274 | E9 (1) | Agg- |
| P44 | IPM, MEM, TCC, ATM | ≥16 | 4 | - | ST1967 | E12 (1) | O:11 |
| P45, P48 | IPM, MEM, TCC, PIP, ATM | ≥16 | ≥16 | - | ST1076 | E1 (2) | O:11 |
| P66 | IPM, MEM, FEP, TCC, PIP, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | ≥16 | - | ST664 | E8 (1) | O:5 |
| P68 | IPM, MEM, TCC, PIP, ATM, CIP, LVX | ≥16 | ≥16 | - | ST270 | E3 (1) | O:16 |
| P70 | IPM, TCC, ATM | ≥16 | 2 | - | ST244 | E18 (1) | O:2 |
| P71 | IPM, TCC, ATM | ≥16 | 2 | - | ST3762 | E4 (1) | O:11 |
| P75 | IPM, MEM, CAZ, FEP, TCC, PIP, PTZ, ATM, AMK, GEN, TOB, CIP, LVX | ≥16 | ≥16 | - | ST3385 | E5 (1) | O:11 |
| Strains | MLST | OprD Size (Amino Acid) | Amino Acid Changes in OprD Sequence | Insertion/Deletion | MIC (mg/L) | Beta- Lactamase | |
|---|---|---|---|---|---|---|---|
| IPM | MEM | ||||||
| P4 | ST235 | - | - | OprD is truncated by ISPa33 | ≥16 | ≥16 | VIM-2, GES-45 |
| P45 | ST1076 | >443 | T103S, K115T, F170L, E185Q, P186G, V189T, R310E, A315G | insertion of 1 bp (C) at nt 1205 | ≥16 | ≥16 | - |
| P51 | ST235 | 340 | T103S, K115T, F170L, E185Q, P186G, V189T | deletion of 13 bp at nt 836 | ≥16 | ≥16 | GES-5 |
| P56 | ST235 | 283 | T103S, K115T, F170L, E185Q, P186G, V189T, Y282V, T283STOP | deletion of 59 bp at nt 845 | ≥16 | ≥16 | VIM-2, GES-45 |
| P66 | ST664 | 93 | - | deletion of 1 bp (C) at nt 198 | ≥16 | ≥16 | - |
| P569 | ST235 | - | - | OprD is truncated by ISPa26, and lacks ATG (M1) | ≥16 | ≥16 | GES-5 |
| P1, P11, P74, P75 | ST3385 | 237 | T103S, K115T, F170L | deletion of 1 bp (G) at nt 557 | ≥16 | 8 | SHV-2a |
| P19 | ST170 | 345 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S | deletion of 1 bp (C) at nt 825 | ≥16 | 8 | - |
| P26 | ST3386 | 164 | - | deletion of 11 bp at nt 209 | ≥16 | ≥16 | - |
| P36 | ST274 | 348 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K296Q, Q301E, R310G, S349STOP | - | ≥16 | 2 | - |
| P44 | ST1967 | 433 | T103S, K115T, F170L | - | ≥16 | 2 | - |
| P68 | ST270 | 162 | - | deletion of 17 bp at nt 358 | ≥16 | ≥16 | - |
| P71 | ST3762 | 467 | D43N, S57E, S59R, E202Q, I210A, E230K, S240T, N262T, A267S, A281G, K269Q, Q301E, R310G, V359L | Loop7-short a, insertion of 2 bp (TC) at nt 1322 | ≥16 | 2 | - |
| P22 b | ST988 | - | - | - | ≥16 | 2 | - |
| P70 b | ST244 | - | - | - | ≥16 | 2 | - |
| Strains | Virulence Factors | ||||||
|---|---|---|---|---|---|---|---|
| exoS | exoU | exlA | lasI | lasR | rhlI | rhlR | |
| P19, P22, P26, P32, P36, P44, P54, P66, P71 | + | − | − | + | + | + | + |
| P68, P70 | + | − | − | + | + | − | + |
| P8, P12, P15, P16, P23, P25, P28, P29, P30, P31, P34, P37, P40, P42, P43, P45, P48, P49, P50, P51, P52, P53, P57, P60, P61, P62, P63, P64, P65, P67, P69, P72, P73, P569 | − | + | − | + | + | + | + |
| P38 | − | + | − | + | − a | + | + |
| P1, P3, P9, P11, P24 | − | + | − | + | + | + | − |
| P6, P74 | − | + | − | + | − b | + | − |
| P2, P4 | − | + | − | + | + | − | − |
| P56, P75 | − | + | − | + | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fethi, M.; Rojo-Bezares, B.; Arfaoui, A.; Dziri, R.; Chichón, G.; Barguellil, F.; López, M.; El Asli, M.S.; Toledano, P.; Ouzari, H.-I.; et al. High Prevalence of GES-5 Variant and Co-Expression of VIM-2 and GES-45 among Clinical Pseudomonas aeruginosa Strains in Tunisia. Antibiotics 2023, 12, 1394. https://doi.org/10.3390/antibiotics12091394
Fethi M, Rojo-Bezares B, Arfaoui A, Dziri R, Chichón G, Barguellil F, López M, El Asli MS, Toledano P, Ouzari H-I, et al. High Prevalence of GES-5 Variant and Co-Expression of VIM-2 and GES-45 among Clinical Pseudomonas aeruginosa Strains in Tunisia. Antibiotics. 2023; 12(9):1394. https://doi.org/10.3390/antibiotics12091394
Chicago/Turabian StyleFethi, Meha, Beatriz Rojo-Bezares, Ameni Arfaoui, Raoudha Dziri, Gabriela Chichón, Farouk Barguellil, María López, Mohamed Selim El Asli, Paula Toledano, Hadda-Imen Ouzari, and et al. 2023. "High Prevalence of GES-5 Variant and Co-Expression of VIM-2 and GES-45 among Clinical Pseudomonas aeruginosa Strains in Tunisia" Antibiotics 12, no. 9: 1394. https://doi.org/10.3390/antibiotics12091394
APA StyleFethi, M., Rojo-Bezares, B., Arfaoui, A., Dziri, R., Chichón, G., Barguellil, F., López, M., El Asli, M. S., Toledano, P., Ouzari, H.-I., Sáenz, Y., & Klibi, N. (2023). High Prevalence of GES-5 Variant and Co-Expression of VIM-2 and GES-45 among Clinical Pseudomonas aeruginosa Strains in Tunisia. Antibiotics, 12(9), 1394. https://doi.org/10.3390/antibiotics12091394






