SK-03-92 Drug Kills Intracellular Mycobacterium tuberculosis
Abstract
:1. Introduction
2. Results
2.1. SK-03-92 Has Activity against Drug-Resistant M. tuberculosis Strains
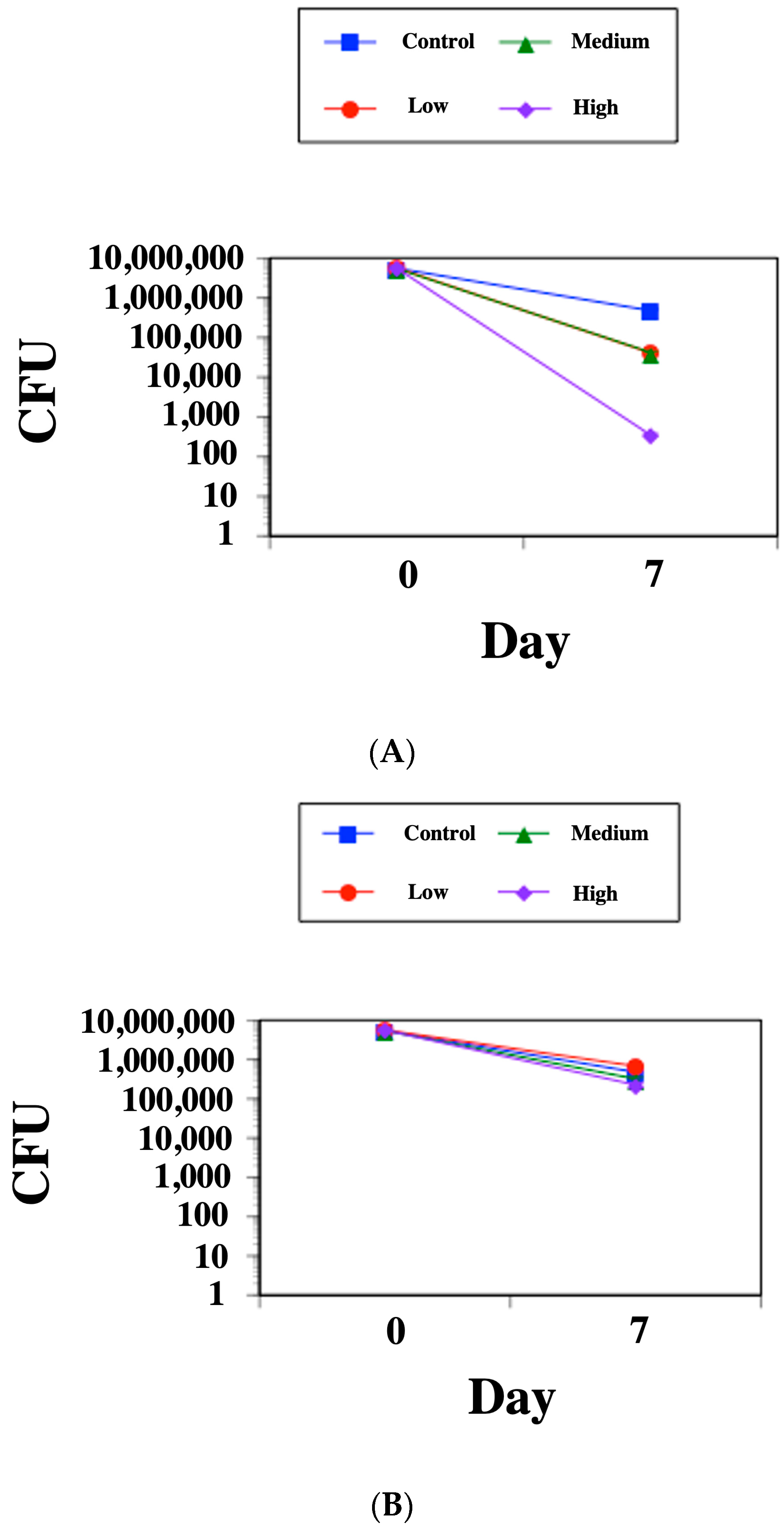
2.2. SK-03-92 Kills M. tuberculosis within Mammalian Macrophages
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Growth Media, and Antibiotics
4.2. Minimum Inhibitory Concentration (MIC)
4.3. Cytotoxicity Testing of SK-03-92
4.4. Intracellular Killing Assay
4.5. Statistics
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Tuberculosis: Key Facts 2022. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 31 July 2023).
- Miramontes, R.; Hill, A.N.; Yelk Woodruff, R.S.; Lambert, L.A.; Navin, T.R.; Castro, K.G.; LoBue, P.A. Tuberculosis infection in the United States: Prevalence estimates from the National Health and Nutrition Examination Survey, 2011–2012. PLoS ONE 2015, 10, e0140881. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The End TB Strategy. Available online: https://www.who.int/tb/strategy/endtb/en/ (accessed on 31 July 2023).
- Peloquin, C.A.; Davies, G.R. The treatment of tuberculosis. Clin. Pharmacol. Ther. 2021, 110, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.J.; Schaaf, H.S.; Mandalakas, A.; Chiappini, E.; Zumla, A.; Marais, B.J. Preventing the spread of multidrug-resistant tuberculosis and protecting contacts of infectious cases. Clin. Microbiol. Infect. 2017, 23, 147–153. [Google Scholar] [CrossRef]
- Falzon, D.; Schünemann, H.J.; Harausz, E.; González-Angulo, L.; Lienhardt, C.; Jaramillo, E.; Weyer, K. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur. Respir. J. 2017, 49, 1602308. [Google Scholar] [CrossRef]
- Shah, N.S.; Pratt, R.; Armstrong, L.; Robison, V.; Castro, K.G.; Cegielski, J.P. Extensively drug-resistant tuberculosis in the United States, 1993–2007. JAMA 2008, 300, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.; Engelbrecht, K.; Polanowski, R.; Krueger, S.M.; Ignasiak, R.; Rott, M.; Schwan, W.R.; Stemper, M.E.; Reed, K.D.; Sherman, D.; et al. New classes of gram-positive selective antibacterial: Inhibitors of MRSA and surrogates of the causative agents of anthrax and tuberculosis. Bioorg. Med. Chem. Lett. 2008, 18, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Kabir, M.S.; Kallaus, M.; Krueger, S.; Monte, A.; Cook, J.M. Synthesis and minimum inhibitory concentrations of SK-03-92 against Staphylococcus aureus and other gram-positive bacteria. J. Infect. Chemother. 2012, 18, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Pym, A.; Grobusch, M.P.; de los Rios, J.M.; Gotuzzo, E.; Vasilyeva, I.; Lemane, V.; Andries, K.; Bakare, N.; De Marez, T.; et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Engl. J. Med. 2014, 371, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, J.P.; Gruber, G.; Dick, T. Re-understanding the mechanisms of action of the anti-mycobacterial drug bedaquiline. Antibiotics 2019, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Haraguchi, Y.; Itotani, M.; Kuroda, H.; Hashizume, H.; Tomishige, T. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b] oxazoles. J. Med. Chem. 2006, 49, 7854–7860. [Google Scholar] [CrossRef]
- Liu, Y.; Matsumoto, M.; Ishida, H.; Ohguro, K.; Yoshitake, M.; Gupta, R.; Geiter, L.; Hafkin, J. Delmamanid: From discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB). Tuberculosis 2018, 111, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.D.; Field, S.K. The struggle to end a millenia-long pandemic: Novel candidate and repurposed drugs for the treatment of tuberculosis. Drugs 2022, 82, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Gutierrez, N.M.; Marzuki, M.B.; Lu, X.; Foreman, T.W.; Paleja, B.; Lee, B.; Balachander, A.; Chen, J.; Tsenova, L.; et al. Host sirtuin 1 regulates mycobacterial immunopathogeneis and represents a therapeutic target against tuberculosis. Sci. Immunol. 2017, 2, eaaj1789. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, A.; Yan, J.; Liu, B.; Liu, Q.; Zhang, J.; Zhang, X.; Ou, C.; Chen, M. Resveratrol ameliorates cardiac dysfunction by inhibiting apoptosis via the PI3K/Akt/FoxO3a pathway in a rat model of diabetic cardiomyopathy. J. Cardiovasc. Pharmacol. 2017, 70, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, J.; Chen, Y.J.; Ge, B. Role of Sirt1 in innate immune mechanisms against Mycobacterium tuberculosis via the inhibition of TAK1 activation. Arch. Biochem. Biophys. 2019, 667, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, J.; Chen, Y.; Jiang, Y.; Ge, B.; Hong, L. Sirtuin inhibits M. tuberculosis-induced aopotosis in macrophage through glycogen synthase kinase-3b. Arch. Biochem. Biophys. 2020, 694, 108612. [Google Scholar] [CrossRef]
- Boland, R.; Heemskerk, M.T.; Forn-Cuni, G.; Korbee, C.J.; Walburg, K.V.; Esselink, J.J.; Carvalho Dos Santos, C.; de Waal, A.M.; van der Hoeven, D.C.M.; van der Sar, E.; et al. Repurposing tamoxifen as potential host-directed therapeutic for tuberculosis. mBio 2023, 14, e0302422. [Google Scholar] [CrossRef]
- Suarez, M.A.; Valencia, J.; Cadena, C.C.; Maiti, R.; Datta, C.; Puerto, G.; Isaza, J.H.; San Juan, H.; Nagaraja, V.; Guzman, J.D. Diarylethanes display in vitro anti-TB activity and are efficient hits targeting the Mycobacterium tuberculosis HU protein. Molecules 2017, 22, 1245. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, S.; Podder, B.; Jyoti, M.A.; Nam, K.W.; Lee, B.E.; Song, H.Y. Anti-mycobacterial activity of tamoxifen against drug-resistant and intra-macrophage Mycobacterium tuberculosis. J. Microbiol. Biotechnol. 2015, 25, 946–950. [Google Scholar] [CrossRef]
- Weng, J.Q.; Rimando, A.M. Synthesis and biological evaluation of 3,5-dimethoxysilbene analogs. Chem. Biodivers. 2016, 13, 1165–1177. [Google Scholar] [CrossRef]
- Pettit, G.R.; Minardi, M.D.; Rosenberg, J.H.; Hamel, E.; Bibby, M.C.; Martin, S.W.; Jung, M.K.; Pettit, R.K.; Cuthbertson, T.J.; Chapuis, J.C. Antineoplastic agents. 509. Synthesis of fluorocombstatin phosphate and related 3-halostilbenes (1). J. Nat. Prod. 2005, 68, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.R.; de Carvalho, G.S.G.; da Silva, A.D.; Leite, C.Q.F. Synthesis and anti-Mycobacterium tuberculosis evaluation of aza-stilbene derivatives. Sci. World J. 2011, 11, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Zank, A.; Schulte, L.; Brandon, X.; Carstensen, L.; Wescott, A.; Schwan, W.R. Mutations of the brpR and brpS genes affect biofilm formation in Staphylococcus aureus. World J. Clin. Infect. Dis. 2022, 12, 20–32. [Google Scholar] [CrossRef]
- Abed, N.; Couvreur, P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int. J. Antimicrob. Agents 2014, 43, 485–496. [Google Scholar] [CrossRef]
- Bongers, S.; Hellebrekers, P.; Leenen, L.P.H.; Koenderman, L.; Hietbrink, F. Intracellular penetration and effects of antibiotics on Staphylococcus aureus inside human neutrophils: A comprehensive review. Antibiotics 2019, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Kolesar, J.M.; Kabir, M.S.; Elder, E.J., Jr.; William, J.B.; Minerath, R.; Cook, J.M.; Witzigmann, C.M.; Monte, A.; Flaherty, T. Pharmacokinetic/toxicity properties of the new anti-staphylococcal lead compound SK-03-92. Antibiotics 2015, 4, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard 2nd Edition M24-A2; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2011. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
| Strain | Drug Tested | ||
|---|---|---|---|
| SK-03-92 | RIF a | INH | |
| M. tuberculosis H37Rv | 6.25 b | 0.049 | 0.02 |
| M. tuberculosis SRI 1369 (INHR) d | 6.25 | 0.039 | NA c |
| M. tuberculosis SRI 1367 (RIFR) | 0.39 | NA | 0.02 |
| M. tuberculosis SRI 4000 (OXNR) | 0.78 | 0.78 | NA |
| Drug | % Viability | % Viability | % Viability |
|---|---|---|---|
| (low conc.) a | (med. conc.) | (high conc.) | |
| SK-03-92 | 98 ± 2 b | 79 ± 3 | <10 |
| Rifampin | 95 ± 1 | 93 ± 2 | 88 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwan, W.R. SK-03-92 Drug Kills Intracellular Mycobacterium tuberculosis. Antibiotics 2023, 12, 1385. https://doi.org/10.3390/antibiotics12091385
Schwan WR. SK-03-92 Drug Kills Intracellular Mycobacterium tuberculosis. Antibiotics. 2023; 12(9):1385. https://doi.org/10.3390/antibiotics12091385
Chicago/Turabian StyleSchwan, William R. 2023. "SK-03-92 Drug Kills Intracellular Mycobacterium tuberculosis" Antibiotics 12, no. 9: 1385. https://doi.org/10.3390/antibiotics12091385
APA StyleSchwan, W. R. (2023). SK-03-92 Drug Kills Intracellular Mycobacterium tuberculosis. Antibiotics, 12(9), 1385. https://doi.org/10.3390/antibiotics12091385







