Effects of Sulforaphene on the Cariogenic Properties of Streptococcus Mutans In Vitro and Dental Caries Development In Vivo
Abstract
:1. Introduction
2. Results
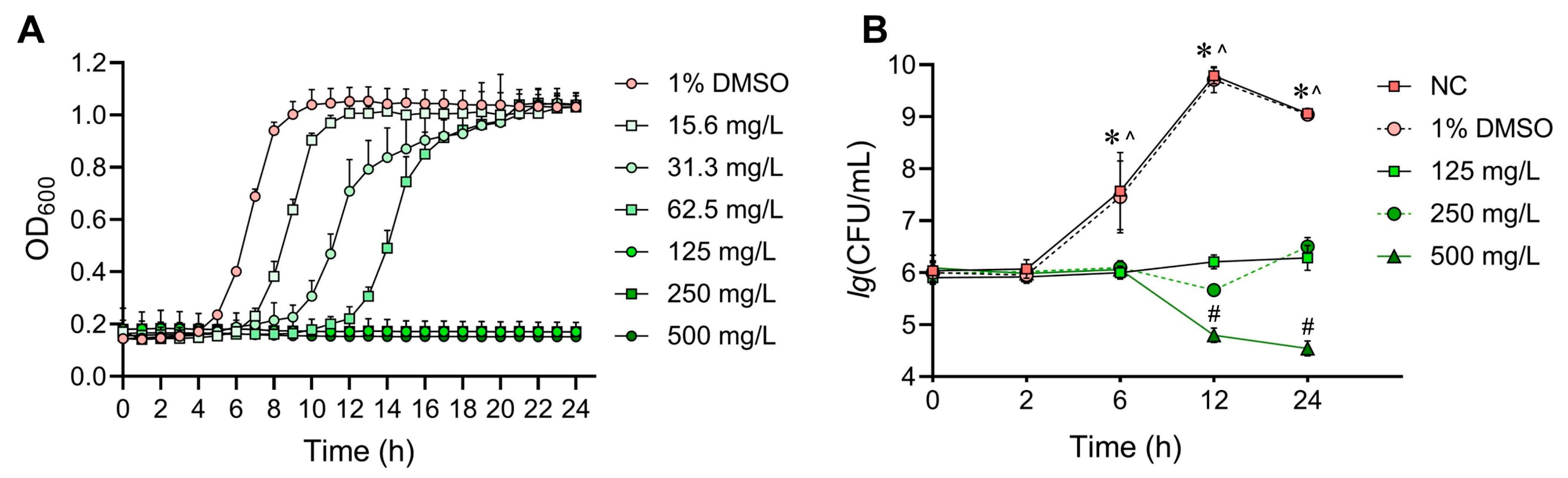
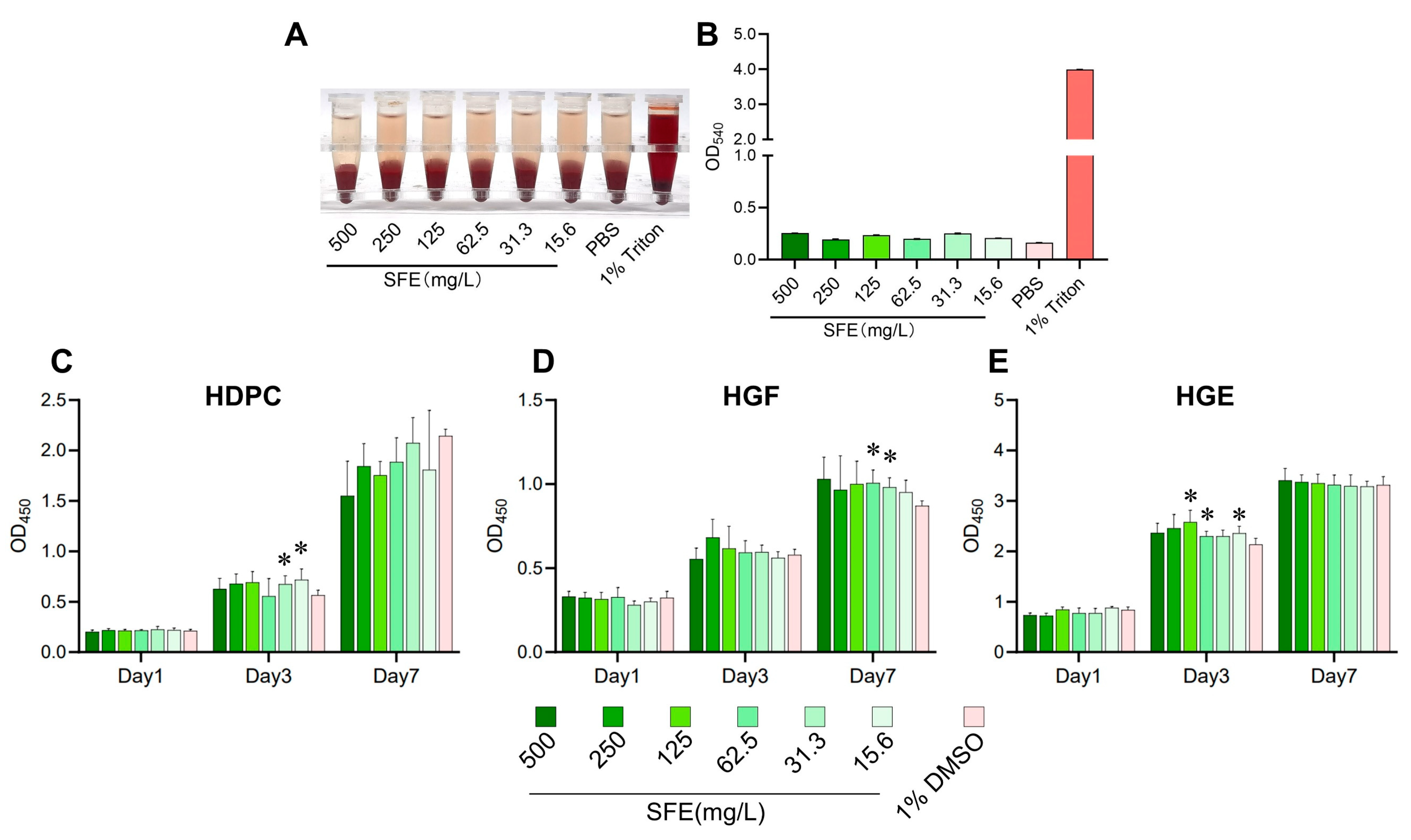
2.1. Evaluation of SFE’s Antibacterial Effects against S. mutans and Its In Vitro Cytotoxicity
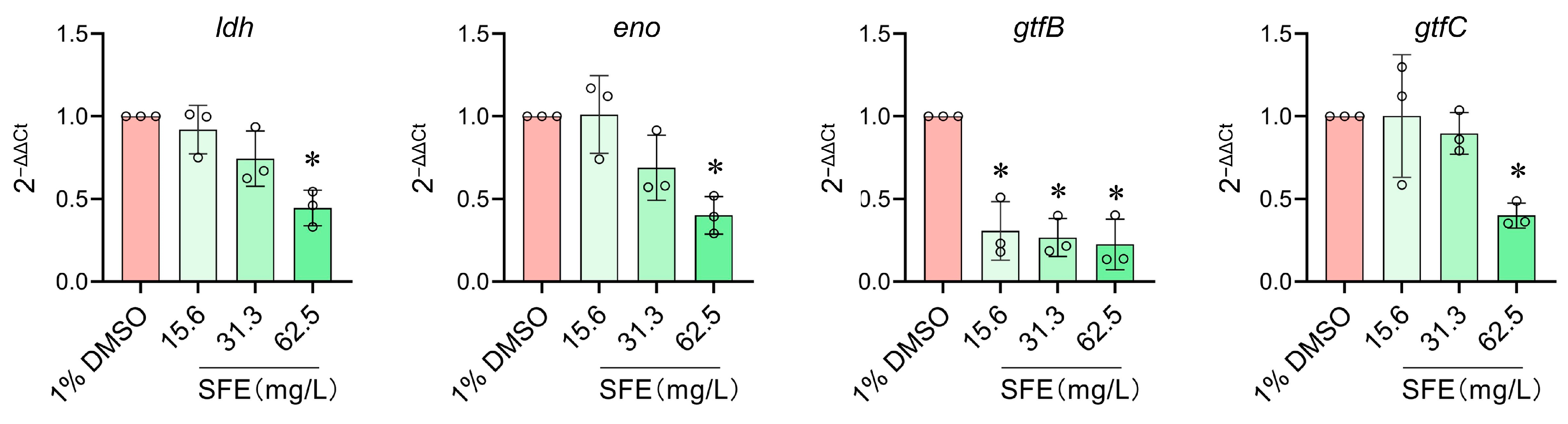
2.2. Evaluation of SFE’s Effects on Cariogenic Virulence Factors and Biofilm Formation of S. mutans
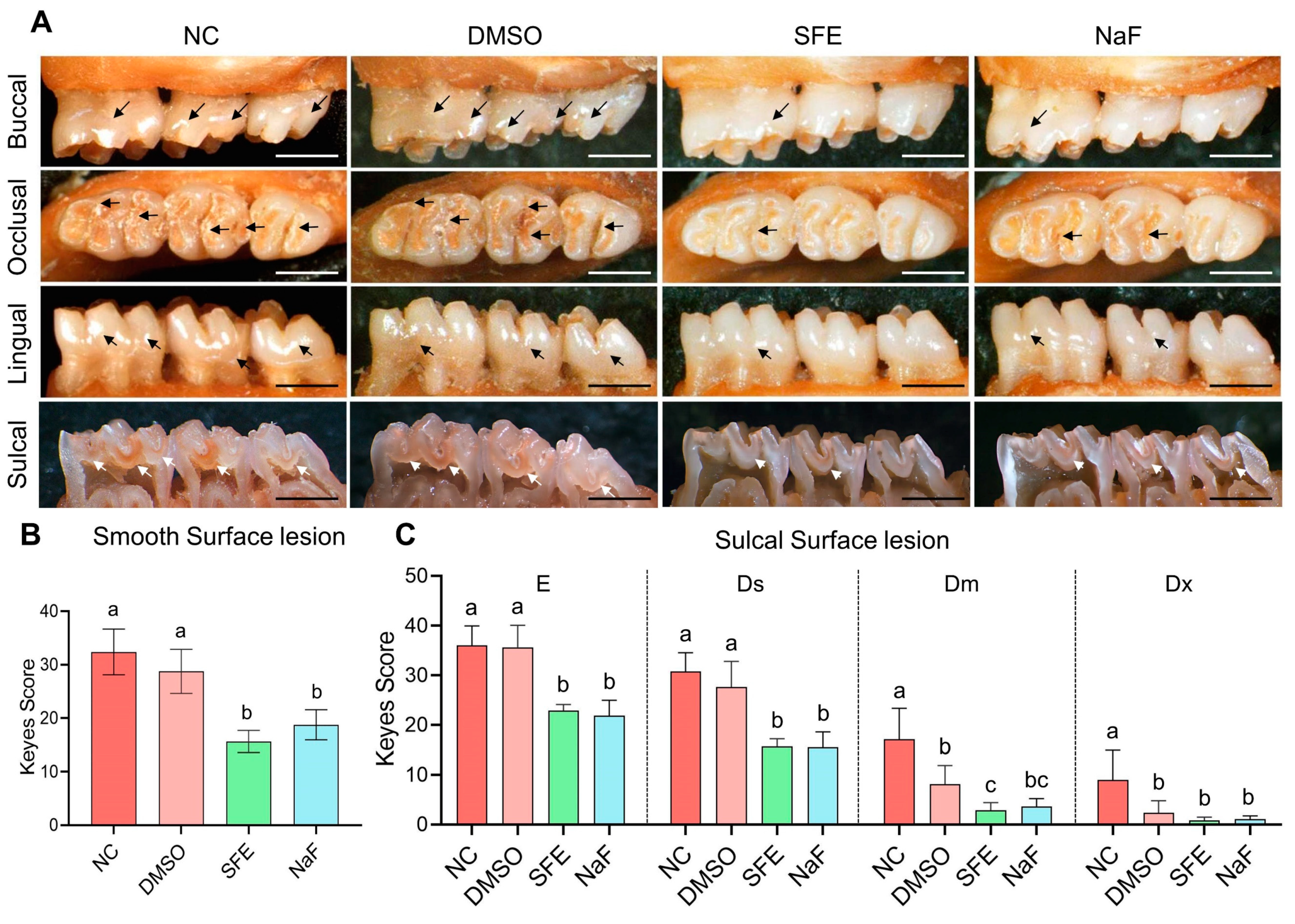
2.3. Evaluation of SFE’s Effects on Carious Lesion Formation and Progression In Vivo
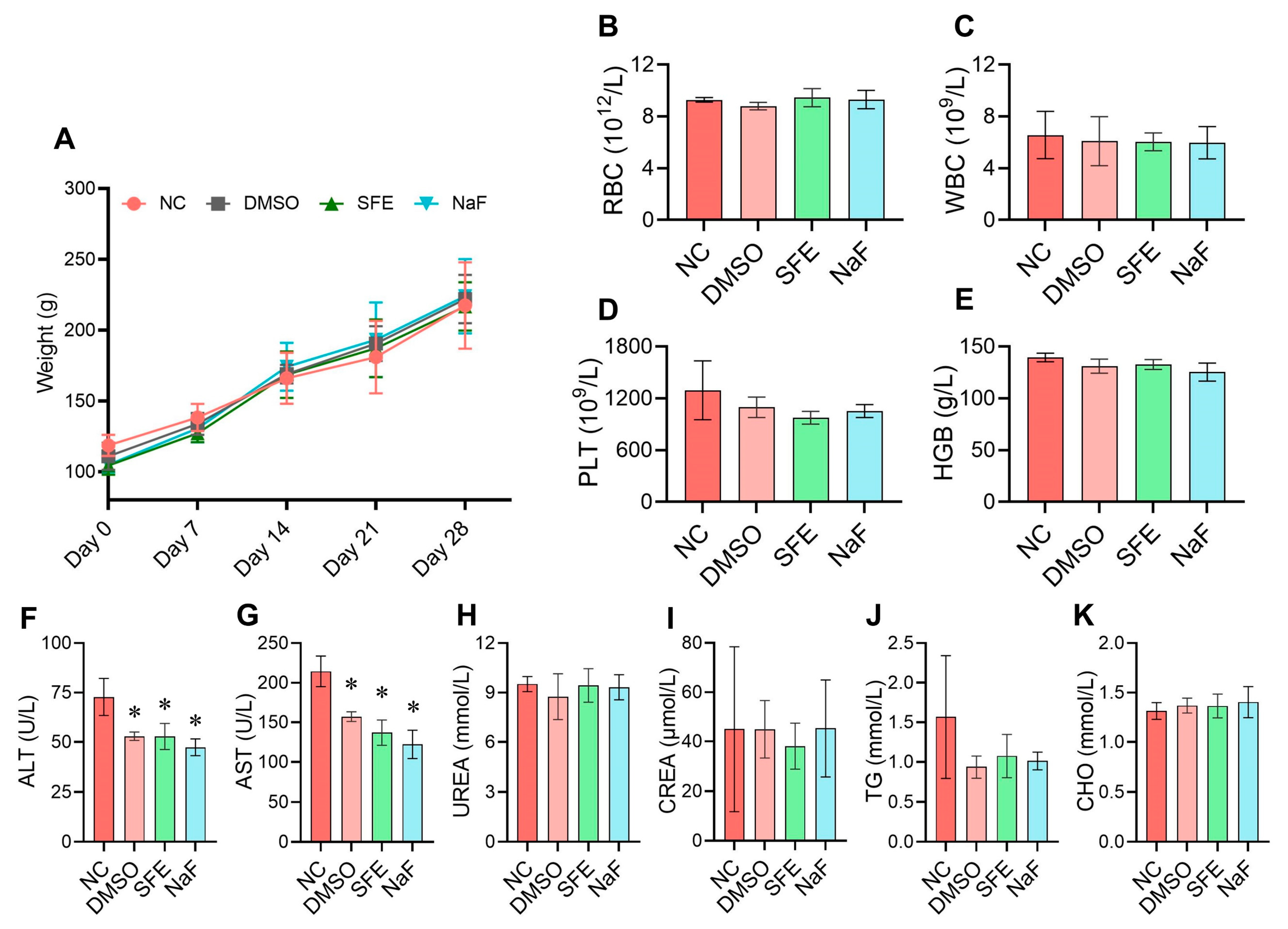
2.4. Evaluation of the In Vivo Biological Safety of SFE
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Cells, and Growth Conditions
4.2. Bacterial Susceptibility Assay
4.3. Kinetic Growth Curve Assays
4.4. In Vitro Biocompatibility Assay
4.5. Experiments of Biofilm Formation Inhibition
4.6. Biofilm Morphology Observations
4.7. Lethal Acid Tolerance Test
4.8. Lactic Acid Measurement
4.9. Water-Insoluble Polysaccharides (WIPs) Measurement
4.10. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR (RT-qPCR)
4.11. Establishment of the Rat Caries Model
4.12. In Vivo Biological Safety Evaluation
4.13. Keyes Score Assessment
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Peng, W.; Zhu, Y. Effects of resveratrol on cariogenic virulence properties of Streptococcus mutans. BMC Microbiol. 2020, 20, 99. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Xu, Y.; Guan, J.; Wang, Q.; Xue, R.; He, Z.; Lu, X.; Fan, J.; Yu, H.; Turghun, C.; Yu, W.; et al. Mussel-Inspired Caries Management Strategy: Constructing a Tribioactive Tooth Surface with Remineralization, Antibiofilm, and Anti-inflammation Activity. ACS Appl. Mater. Interfaces 2023, 15, 15946–15964. [Google Scholar] [CrossRef]
- Lugo-Flores, M.A.; Quintero-Cabello, K.P.; Palafox-Rivera, P.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Ortega-Ramirez, L.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Plant-Derived Substances with Antibacterial, Antioxidant, and Flavoring Potential to Formulate Oral Health Care Products. Biomedicines 2021, 9, 1669. [Google Scholar] [CrossRef]
- Milho, C.; Silva, J.; Guimarães, R.; Ferreira, I.C.F.R.; Barros, L.; Alves, M.J. Antimicrobials from Medicinal Plants: An Emergent Strategy to Control Oral Biofilms. Appl. Sci. 2021, 11, 4020. [Google Scholar] [CrossRef]
- Martelli, A.; Citi, V.; Testai, L.; Brogi, S.; Calderone, V. Organic Isothiocyanates as Hydrogen Sulfide Donors. Antioxid. Redox Signal 2020, 32, 110–144. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Ma, J.; Wang, Z.; Wang, H.-M.D.; Yuan, Q. Sulforaphene inhibits esophageal cancer progression via suppressing SCD and CDH3 expression, and activating the GADD45B-MAP2K3-p38-p53 feedback loop. Cell Death Dis. 2020, 11, 713. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, C.; Zhu, Y. Sulforaphene inhibits the progression of osteosarcoma via regulating FSTL1/NF-κB pathway. Life Sci. 2020, 263, 118485. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Xu, Q.-Q.; Xian, Y.-F.; Lin, Z.-X. Sulforaphene Ameliorates Neuroinflammation and Hyperphosphorylated Tau Protein via Regulating the PI3K/Akt/GSK-3βPathway in Experimental Models of Alzheimer’s Disease. Oxidative Med. Cell Longev. 2020, 2020, 4754195. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Yan, T.; Shen, J.; Shi, X.; Luo, F.; Ren, Y. Sulforaphene targets NLRP3 inflammasome to suppress M1 polarization of macrophages and inflammatory response in rheumatoid arthritis. J. Biochem. Mol. Toxicol. 2023, 37, e23362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bao, C.; Kim, J.T.; Cho, J.S.; Qiu, S.; Lee, H.J. Sulforaphene Inhibition of Adipogenesis via Hedgehog Signaling in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2018, 66, 11926–11934. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kang, M.J.; Hur, G.; Lee, T.K.; Park, I.S.; Seo, S.G.; Yu, J.G.; Song, Y.S.; Park, J.H.Y.; Lee, K.W. Sulforaphene Suppresses Adipocyte Differentiation via Induction of Post-Translational Degradation of CCAAT/Enhancer Binding Protein Beta (C/EBPβ). Nutrients 2020, 12, 758. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.; Armstrong, E.; Moses, A.; Fahlman, R.; Koosha, H.; Yager, J.Y. Broccoli, Kale, and Radish Sprouts: Key Phytochemical Constituents and DPPH Free Radical Scavenging Activity. Molecules 2023, 28, 4266. [Google Scholar] [CrossRef]
- Aires, A.; Mota, V.; Saavedra, M.; Rosa, E. Bennett the antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, J.-E.; Lee, K.W. Sulforaphene Attenuates Cutibacterium acnes-Induced Inflammation. J. Microbiol. Biotechnol. 2022, 32, 1390–1395. [Google Scholar] [CrossRef]
- Ko, M.-O.; Kim, M.-B.; Lim, S.-B. Relationship between Chemical Structure and Antimicrobial Activities of Isothiocyanates from Cruciferous Vegetables against Oral Pathogens. J. Microbiol. Biotechnol. 2016, 26, 2036–2042. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef]
- de La Higuera, A.; Gutiérrez, J.; Liébana, J.; Garcia-Mendoza, A.; Castillo, A. A new biotyping method for Streptococcus mutans with the API ZYM system. Clin. Microbiol. Infect. 1999, 5, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-S.; Hwang, J.H.; Choi, H.; Kim, K.-J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Chen, J.; Zhou, S.; Zhao, Y.; Xu, M.; Xu, H. Dual Mode of Anti-Biofilm Action of G3 against Streptococcus mutans. ACS Appl. Mater. Interfaces 2020, 12, 27866–27875. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Zhou, Z.; Tu, H.; Ren, Q.; Wang, X.; Ding, L.; Zhou, X.; Zhang, L. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch. Oral Biol. 2017, 80, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Fanfoni, L.; Marsich, E.; Turco, G.; Breschi, L.; Cadenaro, M. Development of di-methacrylate quaternary ammonium monomers with antibacterial activity. Acta Biomater. 2021, 129, 138–147. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, S.-J.; Kim, H.-R.; Kim, S.-B. Deodorizing, antimicrobial and glucosyltransferase inhibitory activities of polyphenolics from biosource. Korean J. Chem. Eng. 2017, 34, 1400–1404. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. The Tea Catechin Epigallocatechin Gallate Suppresses Cariogenic Virulence Factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef]
- Hernández, P.; Sánchez, M.C.; Llama-Palacios, A.; Ciudad, M.J.; Collado, L. Strategies to Combat Caries by Maintaining the Integrity of Biofilm and Homeostasis during the Rapid Phase of Supragingival Plaque Formation. Antibiotics 2022, 11, 880. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef]
- Maria Bedoya-Correa, C.; Rincon Rodriguez, R.J.; Tatiana Parada-Sanchez, M. Genomic and phenotypic diversity of Streptococcus mutans. J. Oral Biosci. 2019, 61, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Horiuchi, M.; Yamada, T. Effects of acidification on growth and glycolysis of Streptococcus sanguis and Streptococcus mutans. Oral Microbiol. Immunol. 1997, 12, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Roosterman, D.; Meyerhof, W.; Cottrell, G.S. Proton Transport Chains in Glucose Metabolism: Mind the Proton. Front. Neurosci. 2018, 12, 404. [Google Scholar] [CrossRef]
- Song, D.; Liang, H.; Kuang, P.; Tang, P.; Hu, G.; Yuan, Q. Instability and Structural Change of 4-Methylsulfinyl-3-butenyl Isothiocyanate in the Hydrolytic Process. J. Agric. Food Chem. 2013, 61, 5097–5102. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ma, Q.; Li, Y.; Zou, J. Molecular and regulatory mechanisms of oxidative stress adaptation in Streptococcus mutans. Mol. Oral Microbiol. 2023, 38, 1–8. [Google Scholar] [CrossRef]
- Hajishengallis, G. Illuminating the oral microbiome and its host interactions: Animal models of disease. FEMS Microbiol. Rev. 2023, 47, fuad018. [Google Scholar] [CrossRef]
- Zhang, O.L.; Niu, J.Y.; Yin, I.X.; Yu, O.Y.; Mei, M.L.; Chu, C.H. Bioactive Materials for Caries Management: A Literature Review. Dent. J. 2023, 11, 59. [Google Scholar] [CrossRef]
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; He, W.; Wu, X.; Liu, G.; Huang, H.; Jiang, H.; Zhang, X. Variation in the sexual behavior and blood count parameters induced by sleep deprivation in male rats. Andrology 2022, 10, 800–807. [Google Scholar] [CrossRef]
- Kovaleski, E.S.; Gonçalves, L.K.; Bortolato, G.; Marinho, J.P.; Silva, L.F.L.; Russo, M.K.B.; Agostini, F.; Funchal, C.; Dani, C. Effects of the ingestion of different kinds of white grape juice (Vitis labrusca) during adolescence on body weight, biochemical parameters and oxidative stress in liver of adult Wistar rats. Food Chem. 2019, 291, 110–116. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Jiang, W.; Wang, K.; Luo, J.; Li, W.; Zhou, X.; Zhang, L. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J. Oral Microbiol. 2018, 10, 1442089. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, Y.; Wang, Y. Antimicrobial peptide GH12 targets Streptococcus mutans to arrest caries development in rats. J. Oral Microbiol. 2019, 11, 1549921. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wang, K.; Ren, Q.; Li, H.; Zheng, S.; Niu, Y.; Zhou, X.; Li, W.; Zhang, L. Bifunctional anticaries peptides with antibacterial and remineralizing effects. Oral Dis. 2018, 25, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Korolev, D.; Shumilo, M.; Shulmeyster, G.; Krutikov, A.; Golovkin, A.; Mishanin, A.; Spiridonova, A.; Kulagina, O.; Galagudza, M. Hemolytic Activity, Cytotoxicity, and Antimicrobial Effects of Silver Nanoparticles Conjugated with Lincomycin or Cefazolin. Int. J. Mol. Sci. 2022, 23, 13709. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Feng, Z.; Li, Z.; Jiang, X.; Han, S.; Washio, J.; Takahashi, N.; Zhang, L. Combined Treatment with Fluoride and Antimicrobial Peptide GH12 Efficiently Controls Caries in vitro and in vivo. Caries Res. 2022, 56, 524–534. [Google Scholar] [CrossRef]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Vacca-Smith, A.M.; Bowen, W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, X.D.; Wu, C.D. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch. Oral Biol. 2012, 57, 678–683. [Google Scholar] [CrossRef]
- Sun, M.; Kang, Q.; Li, T.; Huang, L.; Jiang, Y.; Xia, W. Effect of high-fructose corn syrup on Streptococcus mutans virulence gene expression and on tooth demineralization. Eur. J. Oral Sci. 2014, 122, 216–222. [Google Scholar]
- Lopes, A.C.U.d.A.; Lobo, C.I.V.; Ribeiro, S.M.; Colin, J.d.S.; Constantino, V.C.N.; Canonici, M.M.; Barbugli, P.A.; Klein, M.I. Distinct Agents Induce Streptococcus mutans Cells with Altered Biofilm Formation Capacity. Microbiol. Spectr. 2022, 10, e0065022. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Yang, H.; Zou, P.; Chen, J.; Shi, G.; Wu, C.; Wang, M.; Zhou, Q.; Zhou, S. Subclavian Vein Puncture as an Alternative Method of Blood Sample Collection in Rats. J. Vis. Exp. 2018, 141, e58499. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Li, X.; Feng, Z.; Zeng, Y.; Han, S.; Takahashi, N.; Zhang, L. Development and evaluation of a chewing gum containing antimicrobial peptide GH12 for caries prevention. Eur. J. Oral Sci. 2022, 130, e12887. [Google Scholar] [CrossRef] [PubMed]
- Keyes, P.H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J. Dent. Res. 1958, 37, 1088–1099. [Google Scholar]


| Tested Strain | Sulforaphene (SFE) | Chlorhexidine (CHX) | ||
|---|---|---|---|---|
| MIC 1 (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | |
| S. mutans UA159 | 125.00 ± 0.00 | >500 | 2.93 ± 1.07 | 15.63 ± 0.00 |
| S. mutans GS-5 | 145.83 ± 51.03 | >500 | 1.95 ± 0.00 | 11.72 ± 4.28 |
| S. mutans COCC32-3 | 125.00 ± 0.00 | >500 | 2.28 ± 0.80 | 11.72 ± 4.28 |
| S. mutans COCC33-17 | 125.00 ± 0.00 | >500 | 3.25 ± 2.37 | 15.63 ± 0.00 |
| S. mutans COCC31-8 | 125.00 ± 0.00 | >500 | 2.60 ± 1.01 | 15.63 ± 0.00 |
| S. mutans COCC33-4 | 125.00 ± 0.00 | >500 | 2.28 ± 0.80 | 15.63 ± 0.00 |
| S. mutans COCC33-14 | 145.83 ± 51.03 | >500 | 2.93 ± 1.07 | 15.63 ± 0.00 |
| S. mutans COCC33-8 | 125.00 ± 0.00 | >500 | 2.60 ± 1.01 | 15.63 ± 0.00 |
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) | Reference |
|---|---|---|---|
| 16S rRNA | AGCGTTGTCCGGATTTATTG | CTACGCATTTCACCGCTACA | [48] |
| ldh | AAAAACCAGGCGAAACTCGC | CTGAACGCGCATCAACATCA | [49] |
| eno | GTTGAACTTCGCGATGGAGAT | GTCAAGTGCGATCATTGCTTTAT | [50] |
| gtfB | CACTATCGGCGGTTACGAAT | CAATTTGGAGCAAGTCAGCA | [48] |
| gtfC | GATGCTGCAAACTTCGAACA | TATTGACGCTGCGTTTCTTG | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, B.; Wang, Y.; Hu, R. Effects of Sulforaphene on the Cariogenic Properties of Streptococcus Mutans In Vitro and Dental Caries Development In Vivo. Antibiotics 2023, 12, 1359. https://doi.org/10.3390/antibiotics12091359
Zhou Y, Zhang B, Wang Y, Hu R. Effects of Sulforaphene on the Cariogenic Properties of Streptococcus Mutans In Vitro and Dental Caries Development In Vivo. Antibiotics. 2023; 12(9):1359. https://doi.org/10.3390/antibiotics12091359
Chicago/Turabian StyleZhou, Yuehong, Binhan Zhang, Yufei Wang, and Rongdang Hu. 2023. "Effects of Sulforaphene on the Cariogenic Properties of Streptococcus Mutans In Vitro and Dental Caries Development In Vivo" Antibiotics 12, no. 9: 1359. https://doi.org/10.3390/antibiotics12091359
APA StyleZhou, Y., Zhang, B., Wang, Y., & Hu, R. (2023). Effects of Sulforaphene on the Cariogenic Properties of Streptococcus Mutans In Vitro and Dental Caries Development In Vivo. Antibiotics, 12(9), 1359. https://doi.org/10.3390/antibiotics12091359






