Microbiological Profile of Fracture Related Infection at a UK Major Trauma Centre
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metsemakers, W.J.; Morgenstern, M.; McNally, M.A.; Moriarty, T.F.; McFadyen, I.; Scarborough, M.; Athanasou, N.A.; Ochsner, P.E.; Kuehl, R.; Raschke, M.; et al. Fracture-related infection: A consensus on definition from an international expert group. Injury 2018, 49, 505–510. [Google Scholar]
- Metsemakers, W.J.; Smeets, B.; Nijs, S.; Hoekstra, H. Infection after fracture fixation of the tibia: Analysis of healthcare utilization and related costs. Injury 2017, 48, 1204–1210. [Google Scholar] [PubMed]
- Dudareva, M.; Barrett, L.K.; Morgenstern, M.; Atkins, B.L.; Brent, A.J.; McNally, M.A. Providing an Evidence Base for Tissue Sampling and Culture Interpretation in Suspected Fracture-Related Infection. J. Bone Jt. Surg. Am. 2021, 103, 977–983. [Google Scholar]
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [PubMed]
- Bezstarosti, H.; Metsemakers, W.J.; van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; McNally, M.A.; Marais, L.C.; Verhofstad, M.H.J. Management of critical-sized bone defects in the treatment of fracture-related infection: A systematic review and pooled analysis. Arch. Orthop. Trauma Surg. 2021, 141, 1215–1230. [Google Scholar] [PubMed]
- Depypere, M.; Kuehl, R.; Metsemakers, W.J.; Senneville, E.; McNally, M.A.; Obremskey, W.T.; Zimmerli, W.; Atkins, B.L.; Trampuz, A. Recommendations for Systemic Antimicrobial Therapy in Fracture-Related Infection: A Consensus from an International Expert Group. J. Orthop. Trauma 2020, 34, 30–41. [Google Scholar]
- Vijayakumar, A.B.; Reddy, Y.P.; Suphala, B.; Gopalakrishnan, A.; Vinod Kumar, C.S. Microbiological and antibiotic profile of osteomyelitis in tertiary care hospital. Int. J. Surg. 2021, 8, 910–914. [Google Scholar]
- Wang, B.; Xiao, X.; Zhang, J.; Han, W.; Hersi, S.A.; Tang, X. Epidemiology and microbiology of fracture-related infection: A multicenter study in Northeast China. J. Orthop. Surg. Res. 2021, 16, 490. [Google Scholar] [PubMed]
- Yusuf, E.; Steinrucken, J.; Buchegger, T.; Trampuz, A.; Borens, O. A descriptive study on the surgery and the microbiology of Gustilo type III fractures in an university hospital in Switzerland. Acta Orthop. Belg. 2015, 81, 327–332. [Google Scholar]
- Ardehali, B.; Geoghegan, G.; Khajuria, A.; Reissis, D.; Lawton, G.; Jain, A.; Simmons, J.; Naique, S.; Bhattacharya, R.; Pearse, M.; et al. Microbiological and functional outcomes after open extremity fractures sustained overseas: The experience of a UK level I trauma centre. JPRAS Open 2018, 15, 36–45. [Google Scholar] [PubMed]
- Mthethwa, P.; Marais, L.C. The microbiology of chronic osteomyelitis in a developing world setting. S. Afr. Orthop. J. 2017, 16, 39–45. [Google Scholar] [CrossRef]
- Gonzalez, A.; Suva, D.; Dunkel, N.; Nicodeme, J.D.; Lomessy, A.; Lauper, N.; Rohner, P.; Hoffmeyer, P.; Uckay, I. Are there clinical variables determining antibiotic prophylaxis-susceptible versus resistant infection in open fractures? Int. Orthop. 2014, 38, 2323–2327. [Google Scholar]
- Otchwemah, R.; Grams, V.; Tjardes, T.; Shafizadeh, S.; Bathis, H.; Maegele, M.; Messler, S.; Bouillon, B.; Probst, C. Bacterial contamination of open fractures—Pathogens, antibiotic resistances and therapeutic regimes in four hospitals of the trauma network Cologne, Germany. Injury 2015, 46 Suppl 4, S104–S108. [Google Scholar]
- Burns, T.C.; Stinner, D.J.; Mack, A.W.; Potter, B.K.; Beer, R.; Eckel, T.T.; Possley, D.R.; Beltran, M.J.; Hayda, R.A.; Andersen, R.C.; et al. Microbiology and injury characteristics in severe open tibia fractures from combat. J. Trauma Acute Care Surg. 2012, 72, 1062–1067. [Google Scholar] [CrossRef]
- Glass, G.E.; Barrett, S.P.; Sanderson, F.; Pearse, M.F.; Nanchahal, J. The microbiological basis for a revised antibiotic regimen in high-energy tibial fractures: Preventing deep infections by nosocomial organisms. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 375–380. [Google Scholar]
- Rupp, M.; Baertl, S.; Walter, N.; Hitzenbichler, F.; Ehrenschwender, M.; Alt, V. Is There a Difference in Microbiological Epidemiology and Effective Empiric Antimicrobial Therapy Comparing Fracture-Related Infection and Periprosthetic Jt. Infection? A Retrospective Comparative Study. Antibiotics 2021, 10, 921. [Google Scholar]
- Corrigan, R.A.; Sliepen, J.; Dudareva, M.; Govaert, G.; Atkins, B.L.; Rentenaar, R.; Wouthuyzen-Bakker, M.; McNally, M.A. Causative Pathogens Do Not Differ between Early, Delayed or Late Fracture-Related Infections. Antibiotics 2022, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Sliepen, J.; Onsea, J.; Debaveye, Y.; Govaert, G.; Ijpma, F.; Zimmerli, W.; Metsemakers, W.-J. The microbiological etiology of fracture-related infection. Front. Cell Infect. Microbiol. 2022, 12, 934485. [Google Scholar] [PubMed]
- BOAST. British Orthopaedic Association Standards for Trauma (BOAST) Guidelines for Open Fractures; British Orthopaedic Association: London, UK, 2017. [Google Scholar]
- World Health Organisation. WHO AMR Global Priorities 2022; World Health Organization: Genève, Switzerland, 2022.
- Dudareva, M.; Hotchen, A.J.; Ferguson, J.; Hodgson, S.; Scarborough, M.; Atkins, B.L.; McNally, M.A. The microbiology of chronic osteomyelitis: Changes over ten years. J. Infect. 2019, 79, 189–198. [Google Scholar] [PubMed]
- Sheehy, S.H.; Atkins, B.A.; Bejon, P.; Byren, I.; Wyllie, D.; Athanasou, N.A.; Berendt, A.R.; McNally, M.A. The microbiology of chronic osteomyelitis: Prevalence of resistance to common empirical anti-microbial agents. J. Infect. 2010, 60, 338–343. [Google Scholar] [CrossRef]
- Ferreira, N.; Reddy, K.; Venter, R.G.; Centner, C.M.; Laubscher, M. Antibiogram profiles and efficacy of antibiotic regimens of bacterial isolates from chronic osteomyelitis of the appendicular skeleton: A developing-world perspective. S. Afr. Med. J. 2021, 111, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, F.A.; Medoff, G.; Swartz, M.N. Osteomyelitis: A review of clinical features, therapeutic considerations and unusual aspects. N. Engl. J. Med. 1970, 282, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, R.N.; Natoli, R.M.; O’Hara, N.N.; Schoonover, C.; Berger, P.Z.; Reahl, G.B.; Shirtliff, M.E.; Manson, T.T.; Torbert, J.T.; O’Toole, R.V.; et al. Variations in the organisms causing deep surgical site infections in fracture patients at a level 1 trauma center (2006–2015). J. Orthop. Trauma 2018, 32, e475–e481. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [PubMed]
- Morgenstern, M.; Erichsen, C.; von Rüden, C.; Metsemakers, W.J.; Kates, S.L.; Moriarty, T.F.; Hungerer, S. Staphylococcal orthopaedic device-related infections in older patients. Injury 2016, 47, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Spellman, B.; Lipsky, B. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar]
- Patel, P.; Iliadis, A.-D.; Vris, A.; Heidari, N.; Trompeter, A. Intramedullary application of local antibiotic bullets for the treatment of long bone fracture related infection. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 385–391. [Google Scholar] [PubMed]
- McNally, M.A.; Ferguson, J.Y.; Scarborough, M.; Ramsden, A.; Stubbs, D.; Atkins, B. Mid- to long-term results of single-stage surgery for patients with chronic osteomyelitis using a bioabsorbable gentamicin-loaded ceramic carrier. Bone Jt. J. 2022, 104, 1095–1100. [Google Scholar] [CrossRef]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [PubMed]
- Flurin, L.; Greenwood-Quaintance, K.E.; Patel, R. Microbiology of polymicrobial prosthetic Jt. infection. Diagn. Microbiol. Infect. Dis. 2019, 94, 255–259. [Google Scholar] [CrossRef]
- Onsea, J.; Van Lieshout, E.M.M.; Zalavras, C.; Sliepen, J.; Depypere, M.; Noppe, N.; Ferguson, J.; Verhofstad, M.H.J.; Govaert, G.A.M.; IJpma, F.F.A.; et al. Validation of the diagnostic criteria of the consenus definition of fracture-related infection. Injury 2022, 53, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, M.; Kühl, R.; Eckardt, H.; Acklin, Y.; Stanic, B.; Garcia, M.; Baumhoer, D.; Metsemakers, W.J. Diagnostic challenges and future persepctives in fracture-realted infection. Injury 2018, 49, S83–S90. [Google Scholar] [CrossRef] [PubMed]
- Minassian, A.M.; Osmon, D.R.; Berendt, A.R. Clinical guidelines in the management of prosthetic Jt. infection. J. Antimicrob. Chemother. 2014, 69 Suppl 1, i29–i35. [Google Scholar] [CrossRef]
- Schäfer, P.; Fink, B.; Sandow, D.; Margull, A.; Berger, I.; Frommelt, L. Prolonged bacterial culture to identify late periprosthetic Jt. infection: A promising strategy. Clin. Infect. Dis. 2008, 47, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Onsea, J.; Depypere, M.; Govaert, G.; Kuehl, R.; Vandendriessche, T.; Morgenstern, M.; McNally, M.; Trampuz, A.; Metsemakers, W.J. Accuracy of tissue and sonication fluid sampling for the diagnosis of fracture-related infection: A systematic review and critical appraisal. J. Bone Jt. Infect. 2018, 3, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, M.; Athanasou, N.A.; Ferguson, J.Y.; Metsemakers, W.J.; Atkins, B.L.; McNally, M.A. The value of quantitative histology in the diagnosis of fracture-related infection. Bone Jt. J. 2018, 100, 966–972. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Higuera, C.; Deirmengian, G.; Parvizi, J.; Austin, M.S. Swab cultures are not as effective as tissue cultures for diagnosis of periprosthetic Jt. infection. Clin. Orthop. Relat. Res. 2013, 471, 3196–3203. [Google Scholar] [CrossRef]
- Mackowiak, P.A.; Jones, S.R.; Smith, J.W. Diagnostic value of sinus-tract cultures in chronic osteomyelitis. JAMA 1978, 239, 2772–2775. [Google Scholar] [CrossRef] [PubMed]
- Govaert, G.A.M.; Kuehl, R.; Atkins, B.L.; Trampuz, A.; Morgenstern, M.; Obremskey, W.T.; Verhofstad, M.H.J.; McNally, M.A.; Metsemakers, W.J. Fracture-Related Infection (FRI) Consensus Group. Diagnosing Fracture-Related Infection: Current Concepts and Recommendations. J. Orthop. Trauma 2020, 34, 8–17. [Google Scholar] [CrossRef] [PubMed]
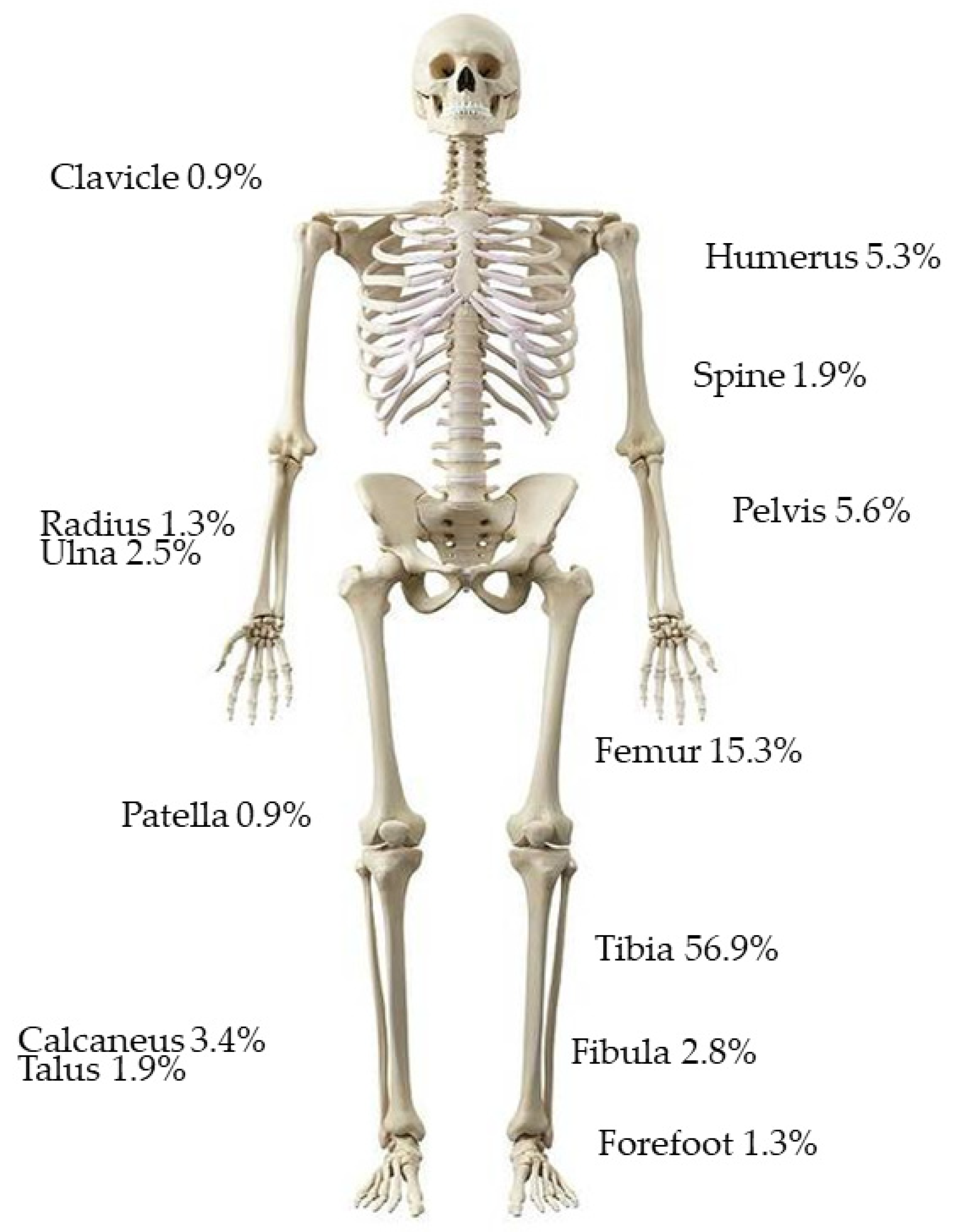
| Demographics (%) | |
| Mean age at diagnosis (years) | 48 |
| Male | 212 (72) |
| Female | 82 (28) |
| Injury (%) | |
| Open | 110 (37.4) |
| Closed | 184 (62.6) |
| Co-morbidities (%) | |
| Diabetes | 8 (1.6) |
| HIV | 2 (0.6) |
| Drug/alcohol abuse | 15 (4.7) |
| Isolated Organisms | |||
|---|---|---|---|
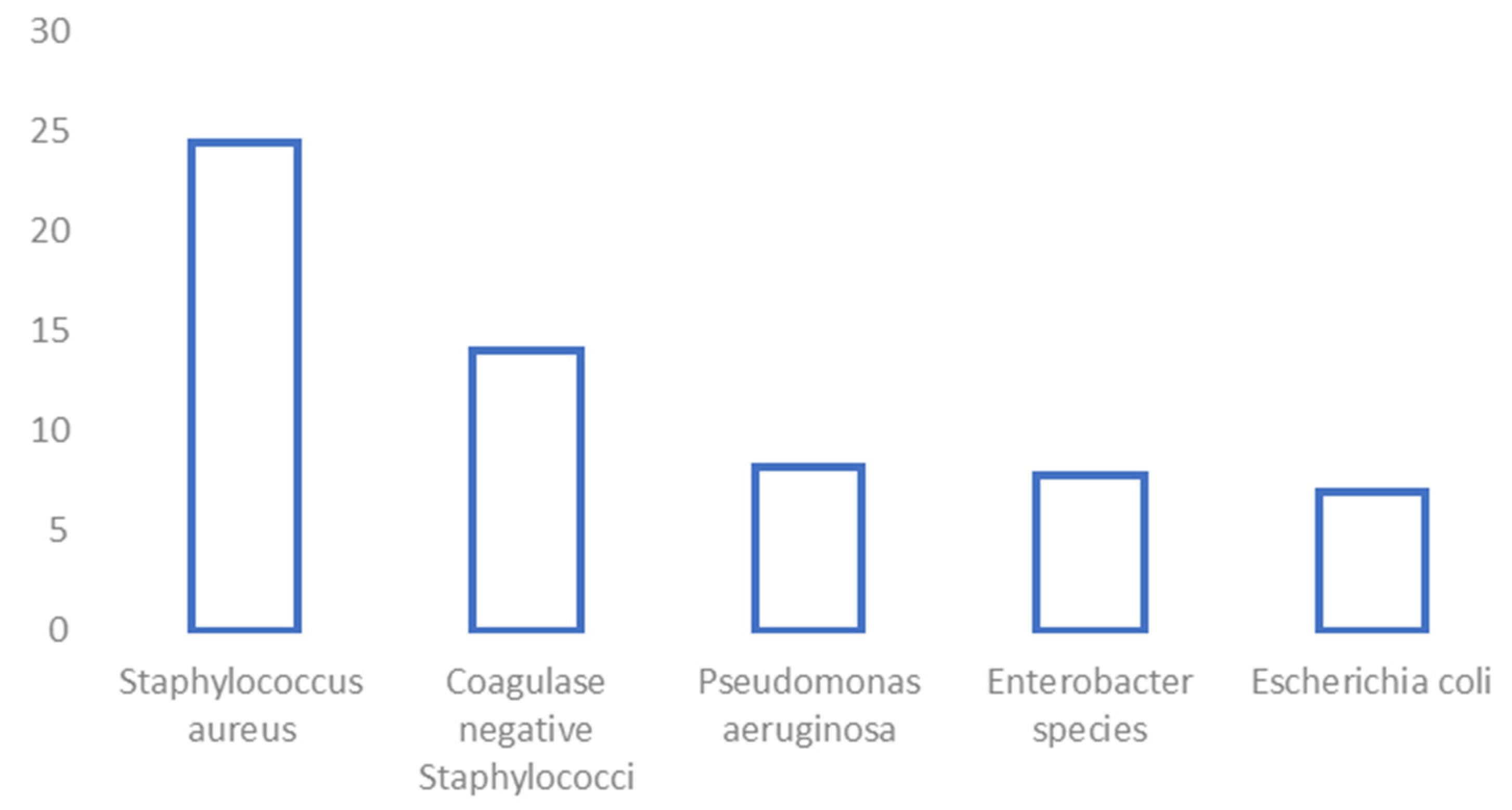
| Staphylococci | 186 (40.2%) | Enterobacterales | 124 (26.8%) |
| Staphylococcus aureus | 113 (24.4%) | Enterobacter species | 36 (7.8%) |
| Coagulase-negative staphylococci | 65 (14.0%) | Escherichia coli | 32 (6.9%) |
| Klebsiella species | 19 (4.1%) | ||
| Streptococci | 21 (4.5%) | Proteus species | 14 (3.0%) |
| Group B streptococci | 8 (1.7%) | Citrobacter species | 9 (1.9%) |
| Strep. dysgalactiae | 8 (1.7%) | Morganella morganii | 5 (1.1%) |
| Viridans streptococci | 5 (1.1%) | Serratia species | 4 (0.9%) |
| Providencia species | 3 (0.6%) | ||
| Enterococci | 28 (6%) | Salmonella typhimurium | 1 (0.2%) |
| Enterococcus faecalis | 17 (3.7%) | Pantoea species | 1 (0.2%) |
| Enterococcus faecium | 7 (1.5%) | ||
| Other Enterococcus species | 4 (0.9%) | Non-fermenting gram-negative bacilli | 42 (9.1%) |
| Pseudomonas aeruginosa | 38 (8.2%) | ||
| Other gram-positive aerobic organisms | 11 (2.4%) | Acinetobacter baumannii | 3 (0.6%) |
| Corynebacterium species | Stenotrophomonas maltophilia | 1 (0.2%) | |
| 11 (2.4%) | |||
| Gram-positive anaerobes | Gram-negative anaerobes | 7 (1.5%) | |
| Peptostreptococcus species | 18 (3.9%) | Bacteroides species | 6 (1.3%) |
| Finegoldia magna | 8 (1.7%) | Prevotella species | 1 (0.2%) |
| Actinomyces species | 4 (0.9%) | ||
| Clostridium species | 3 (0.6%) | Fungal | 8 (1.7%) |
| Cutibacterium acnes | 2 (0.4%) | Candida albicans | 4 (0.9%) |
| 1 (0.2%) | Candida dublinensis | 1 (0.2%) | |
| Candida glabrata | 1 (0.2%) | ||
| Aspergillus fumigatus | 1 (0.2%) | ||
| Rhizopus arrhizus | 1 (0.5) | ||
| Total Infection Episodes = 325 | |
|---|---|
| No significant growth (on cultures or PCR) | 57 (17.5) |
| Single-organism growth | 157 (48.3) |
| Polymicrobial | 111 (34.2) |
| Total potentially pathogenic organisms isolated = 463 | |
| Gram +ve | 265 (57.2) |
| Gram −ve | 184 (39.7) |
| Gram-variable | 1 (0.2) |
| Mixed anaerobes | 5 (1.1) |
| Fungal | 8 (1.7) |
| Polymerase Chain Reaction (PCR) samples = 126 | |
| Organism identified | 44 (34.9) |
| Organism identified on PCR alone (culture negative) | 19 (15.1) |
| Superficial swabs = 96 | |
| Same organism as deep culture/PCR | 20 (20.8) |
| No similarity to deep culture/PCR | 44 (45.8) |
| Methicillin-resistant Staphylococcus aureus | 8 (2.4) 113 7.1% of S. aureus isolated |
| Vancomycin-resistant Enterococci | 3 (0.9) 10.7% of 28 Enterococcus species isolated |
| Extended spectrum beta-lactamases | 14 (4.2) |
| ampC | 29 (8.8) |
| Carbapenemase-producing organisms | 3 (0.9) |
| Other multi-drug resistant organisms | 1 (0.3) |
| Meropenem-resistant gram-negatives | 5 (1.5%) |
| Gentamicin-resistant gram-negatives | 16 (4.8%) |
| Teicoplanin-resistant gram-positives | 1 (0.3%) |
| Vancomycin-resistant gram-positives | 3 (0.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, K.H.; Gill, L.I.; Tissingh, E.K.; Galanis, A.; Hadjihannas, I.; Iliadis, A.D.; Heidari, N.; Cherian, B.; Rosmarin, C.; Vris, A. Microbiological Profile of Fracture Related Infection at a UK Major Trauma Centre. Antibiotics 2023, 12, 1358. https://doi.org/10.3390/antibiotics12091358
Patel KH, Gill LI, Tissingh EK, Galanis A, Hadjihannas I, Iliadis AD, Heidari N, Cherian B, Rosmarin C, Vris A. Microbiological Profile of Fracture Related Infection at a UK Major Trauma Centre. Antibiotics. 2023; 12(9):1358. https://doi.org/10.3390/antibiotics12091358
Chicago/Turabian StylePatel, Kavi H., Laura I. Gill, Elizabeth K. Tissingh, Athanasios Galanis, Ioannis Hadjihannas, Alexis D. Iliadis, Nima Heidari, Benny Cherian, Caryn Rosmarin, and Alexandros Vris. 2023. "Microbiological Profile of Fracture Related Infection at a UK Major Trauma Centre" Antibiotics 12, no. 9: 1358. https://doi.org/10.3390/antibiotics12091358
APA StylePatel, K. H., Gill, L. I., Tissingh, E. K., Galanis, A., Hadjihannas, I., Iliadis, A. D., Heidari, N., Cherian, B., Rosmarin, C., & Vris, A. (2023). Microbiological Profile of Fracture Related Infection at a UK Major Trauma Centre. Antibiotics, 12(9), 1358. https://doi.org/10.3390/antibiotics12091358






