Effects of Dietary Supplementation of Lacticaseibacillus Chiayiensis AACE3 on Hepatic Antioxidant Capacity, Immune Factors and Gut Microbiology in Nandan Yao Chicks
Abstract
1. Introduction
2. Results
2.1. Growth Performance
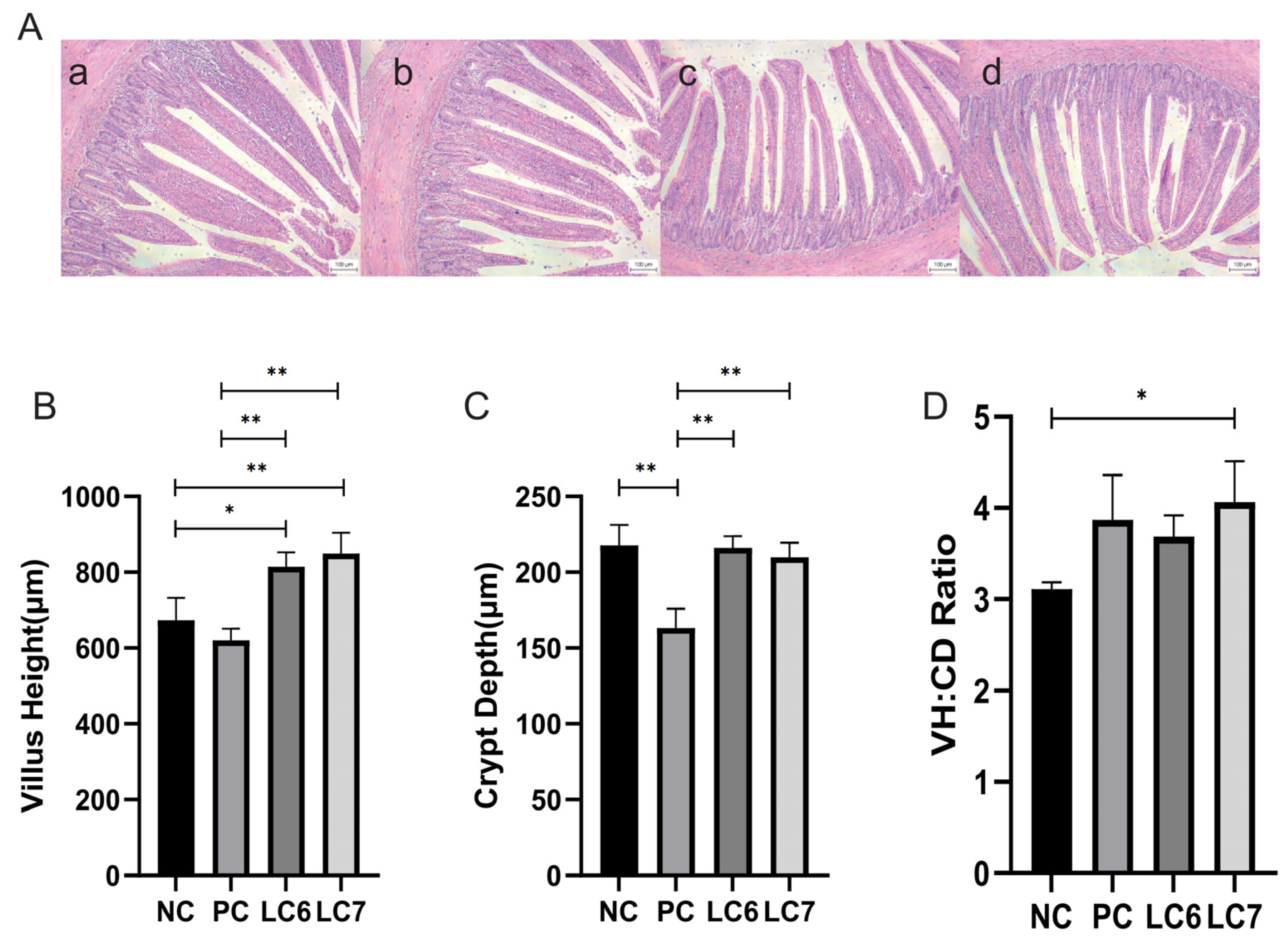
2.2. Effects of L. chiayiensis AACE3 on the Gut Morphology of Chickens
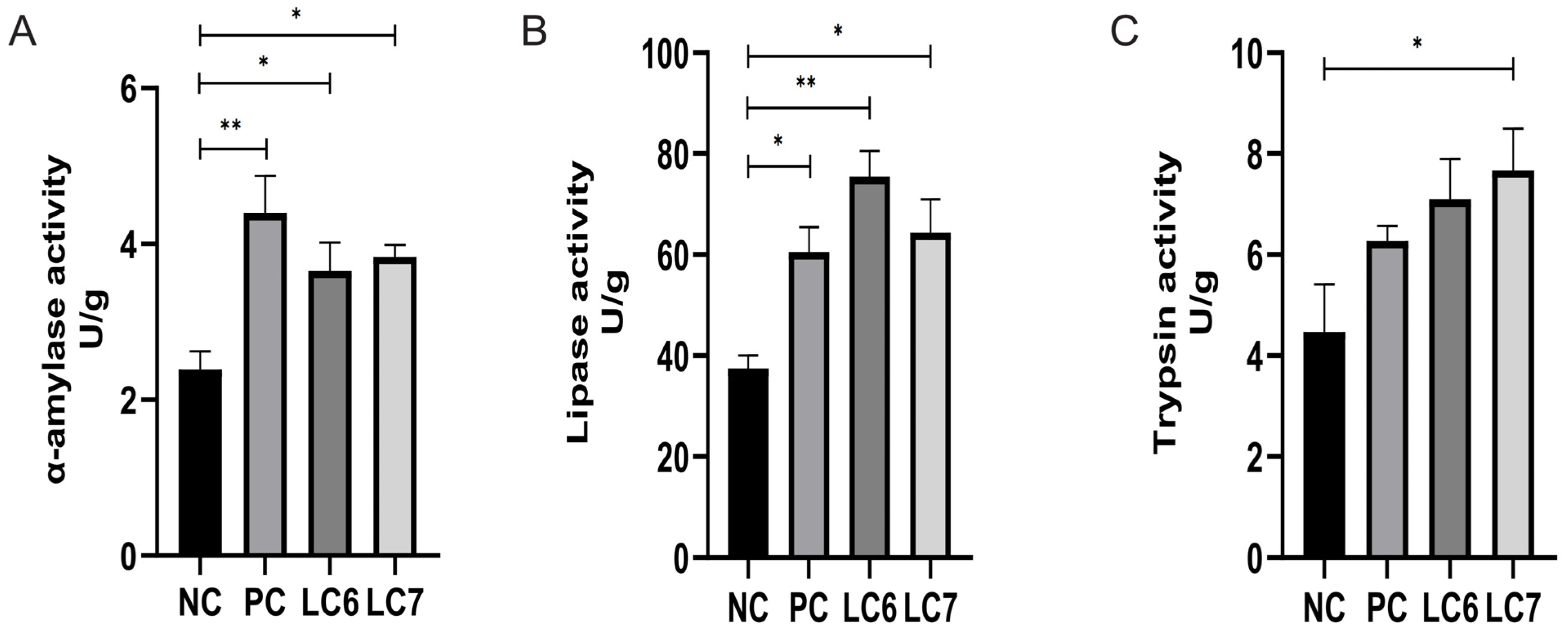
2.3. The Effect of L. chiayiensis AACE3 on Digestion Properties
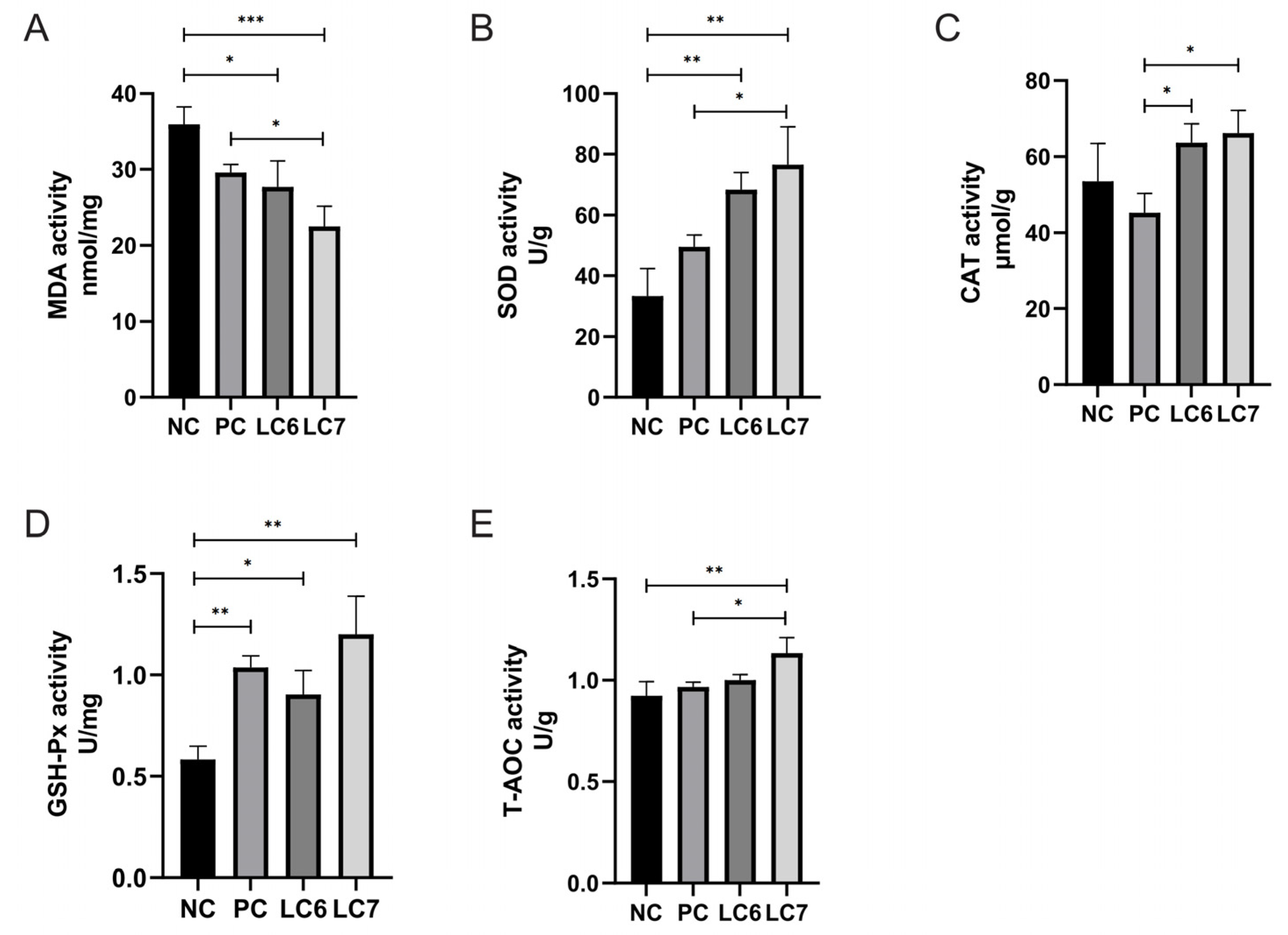
2.4. Effects of L. chiayiensis AACE3 Supplementation on Hepatic Function
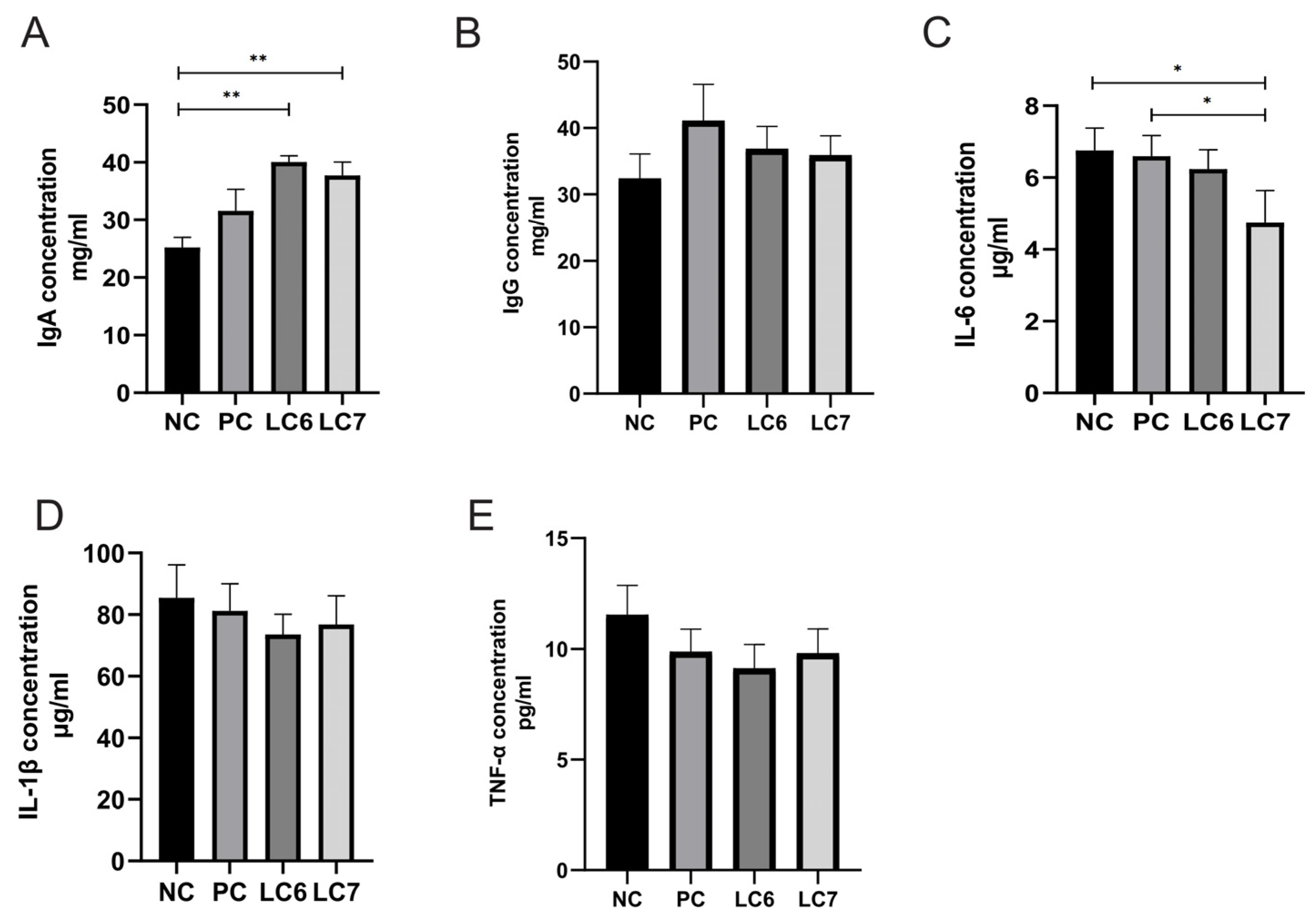
2.5. Effects of L. chiayiensis AACE3 on Immune Factors
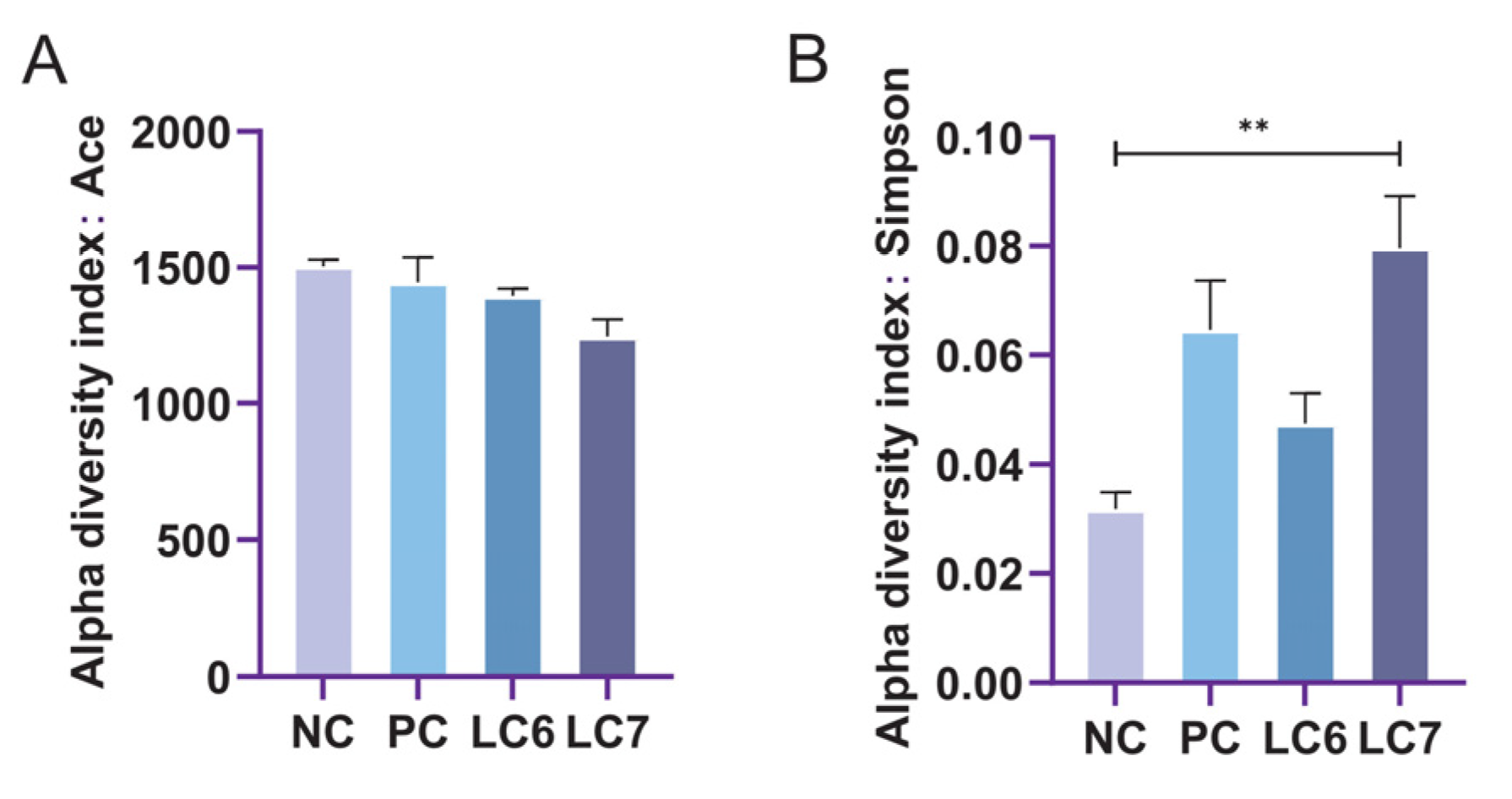
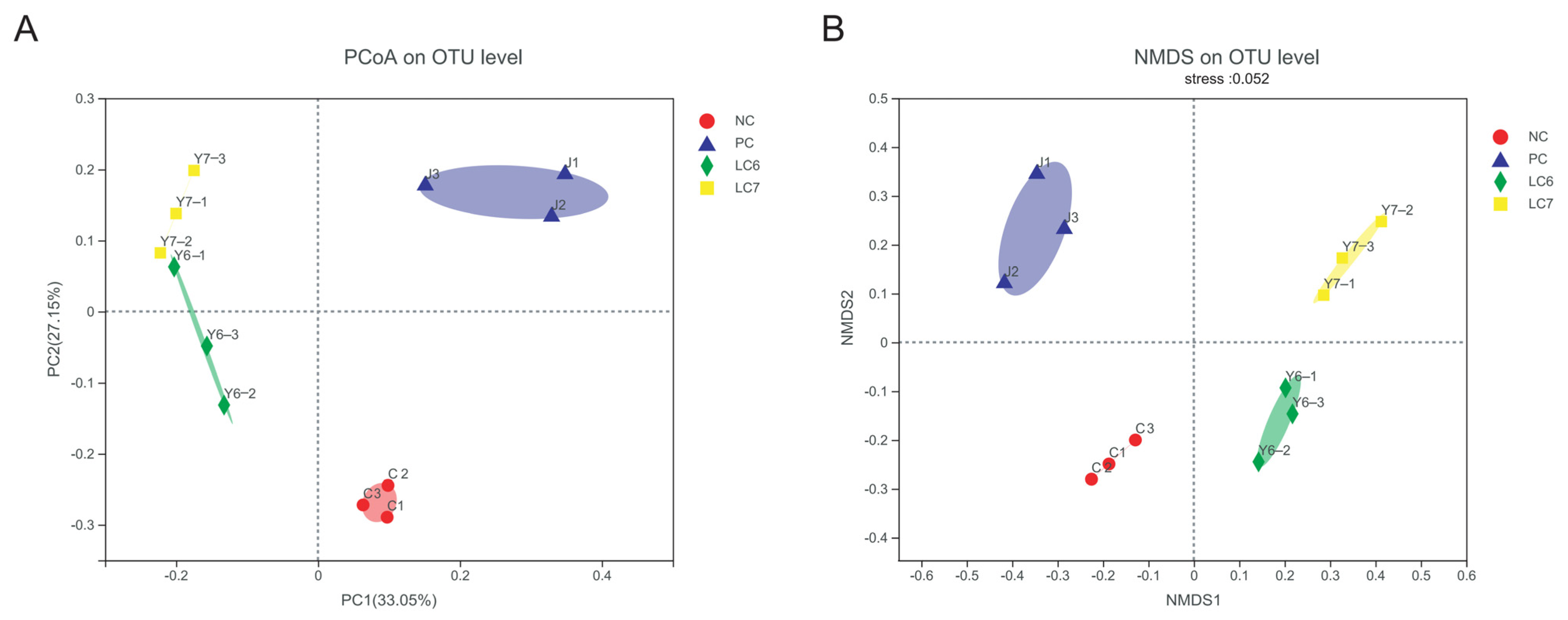
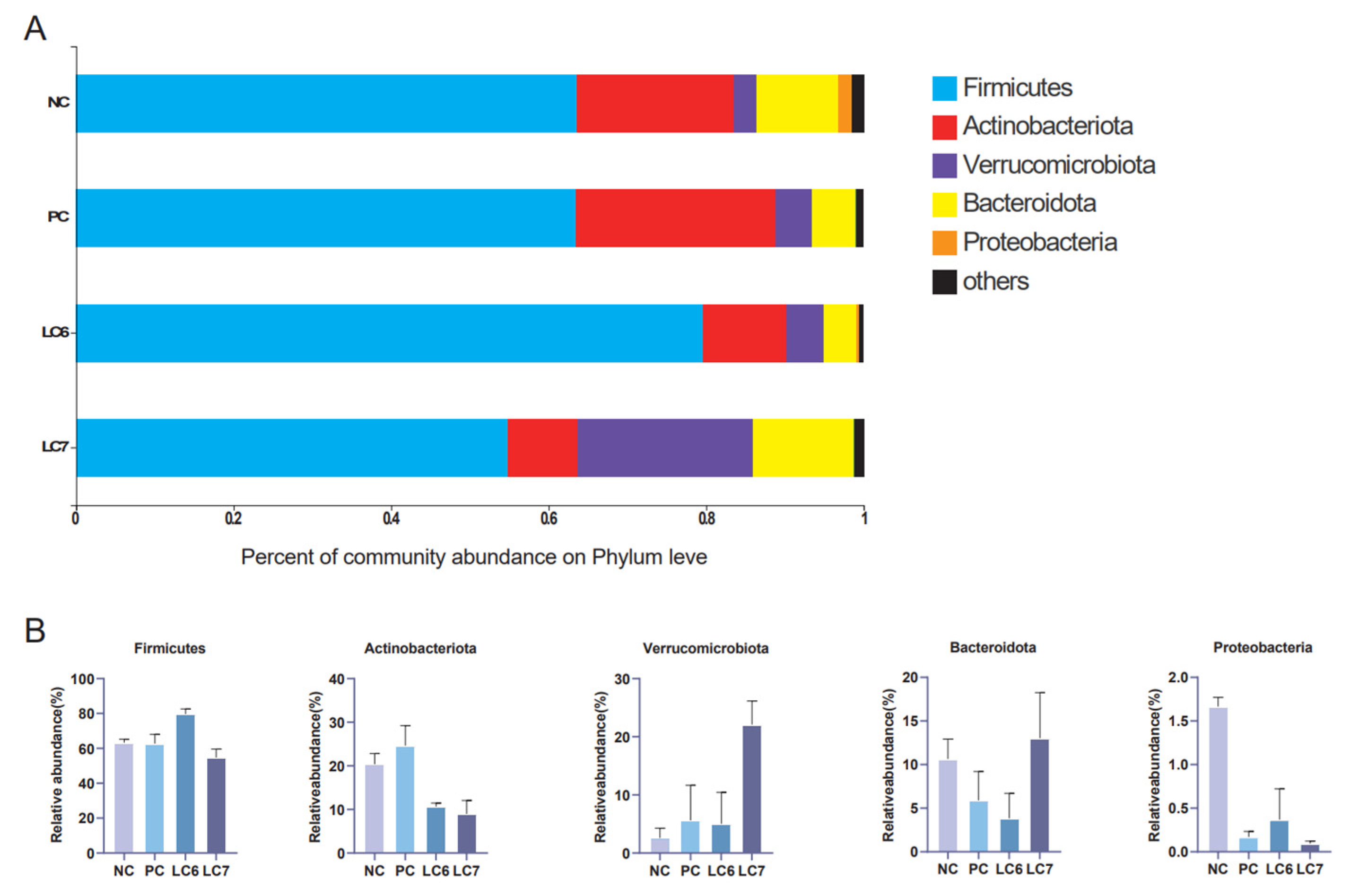
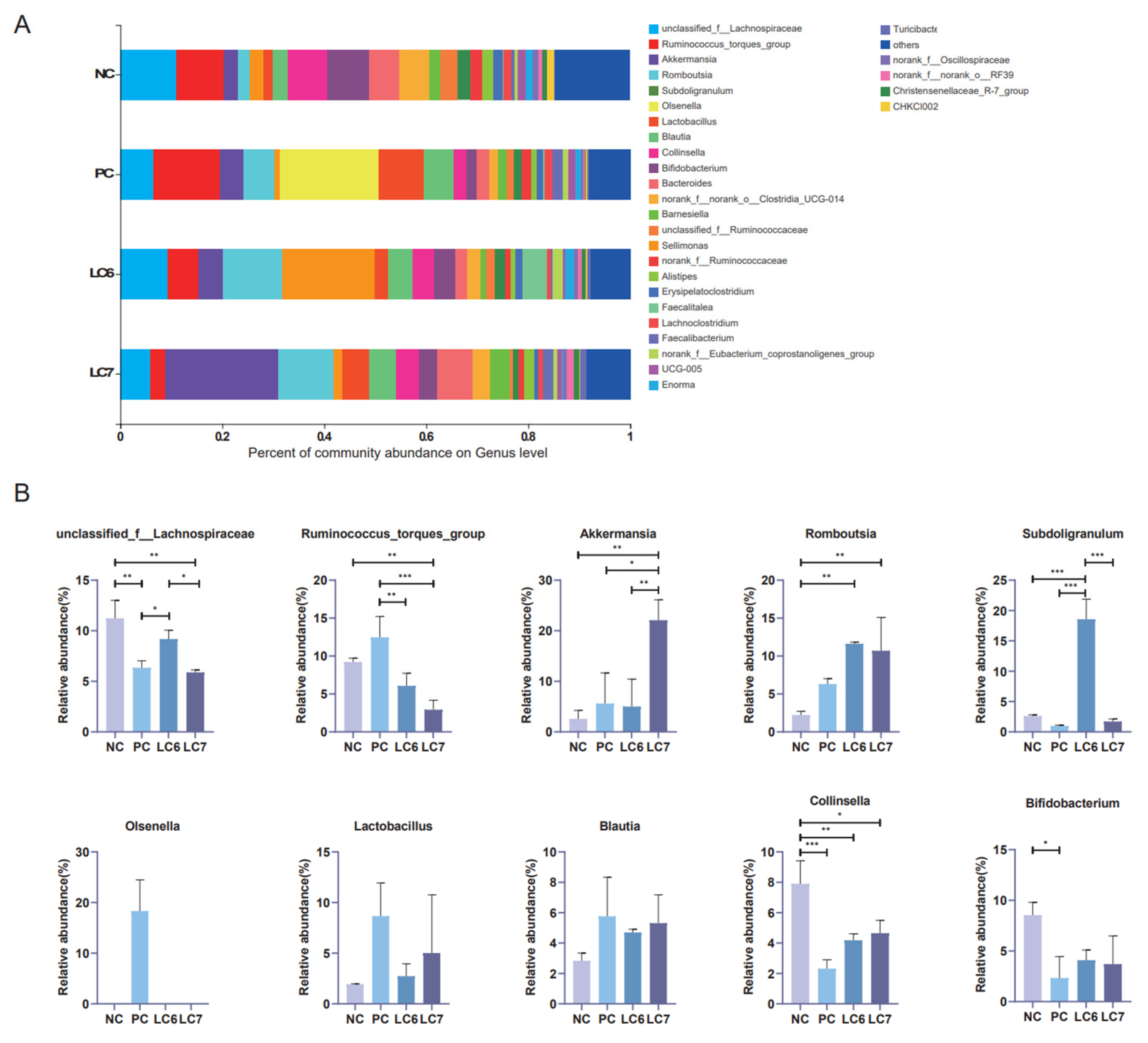
2.6. Effects of L. chiayiensis AACE3 on the Chicken Gut Microbiota
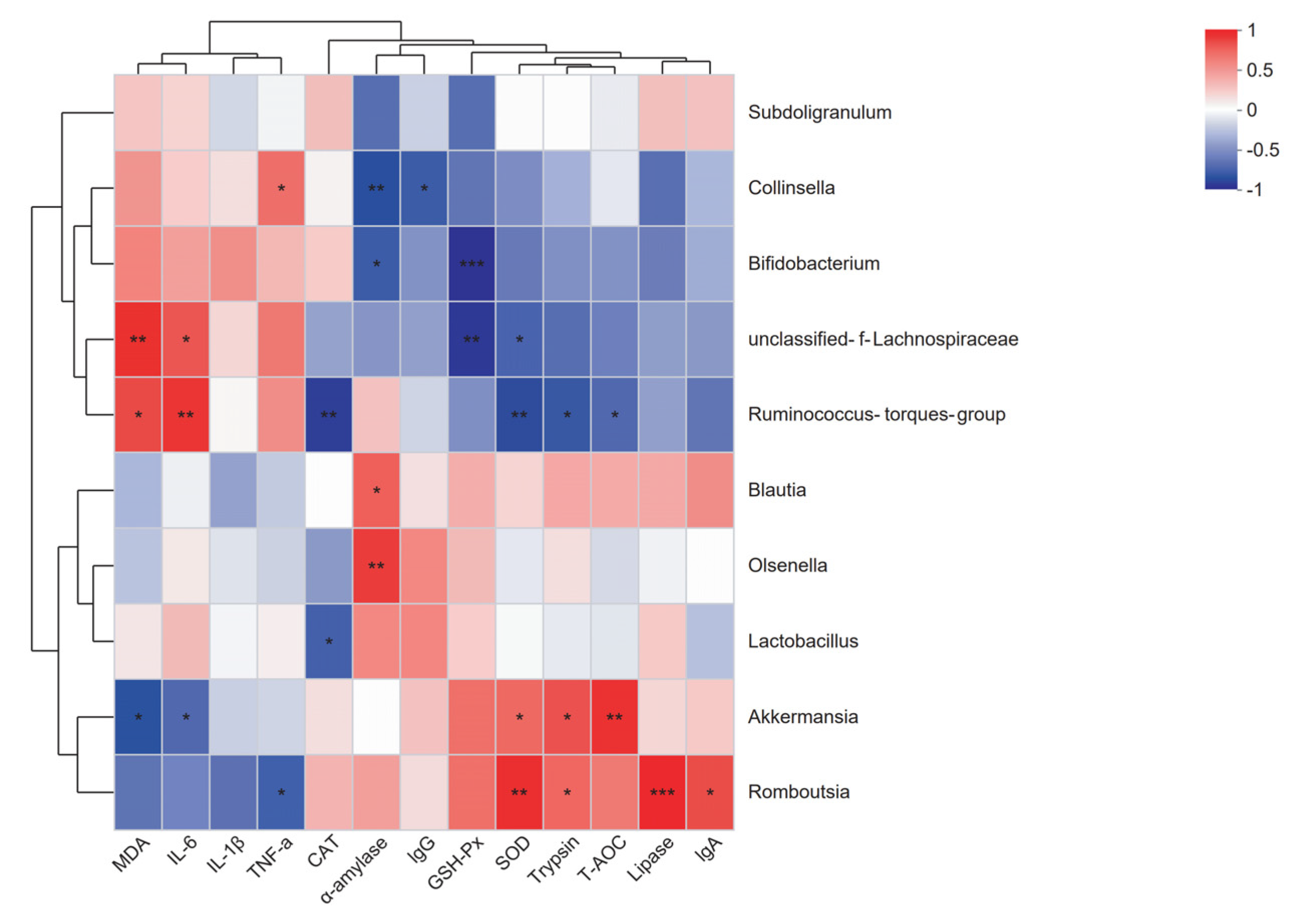
2.7. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Birds, Diet, and Experimental Design
4.3. Sample Collection
4.4. Analysis of the Gut Morphostructure
4.5. Measurement of Physiological Indicators
4.6. Analyses of the Cecal Microbiota
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monteverde, V.; Congiu, F.; Vazzana, I.; Dara, S.; Di Pietro, S.; Piccione, G. Serum lipid profile modification related to polyunsaturated fatty acid supplementation in thoroughbred horses. J. Appl. Anim. Res. 2017, 45, 615–618. [Google Scholar] [CrossRef]
- Armato, L.; Gianesella, M.; Morgante, M.; Fiore, E.; Rizzo, M.; Giudice, E.; Piccione, G. Rumen volatile fatty acids × dietary supplementation with live yeast and yeast cell wall in feedlot beef cattle. Acta Agric. Scand. Sect. A-Anim. Sci. 2016, 66, 119–124. [Google Scholar] [CrossRef]
- Shi, Z.H.; Rothrock, M.J.; Ricke, S.C. Applications of Microbiome Analyses in Alternative Poultry Broiler Production Systems. Front. Vet. Sci. 2019, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A. Broiler production without antibiotics: United States field perspectives. Anim. Feed. Sci. Technol. 2019, 250, 93–98. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mandal, G.P.; Patra, A.K. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed. Sci. Technol. 2018, 236, 86–97. [Google Scholar] [CrossRef]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Bak, H.; Rathkjen, P.H. Reduced use of antimicrobials after vaccination of pigs against porcine proliferative enteropathy in a Danish SPF herd. Acta Vet. Scand. 2009, 51, 1. [Google Scholar] [CrossRef]
- Eseceli, H.; Demir, E.; Degirmencioglu, N.; Bilgic, M. The Effects of Bio-Mos (R) Mannan Oligosaccharide and Antibiotic Growth Promoter Performance of Broilers. J. Anim. Vet. Adv. 2010, 9, 392–395. [Google Scholar] [CrossRef][Green Version]
- Deepa, K.; Purushothaman, M.R.; Vasanthakumar, P.; Sivakumar, K. Effect of Sodium Butyrate as an Antibiotic Substitute on Production Performance, Carcass Characteristics and Economics in Broiler Chicken. Anim. Nutr. Feed. Technol. 2018, 18, 377–387. [Google Scholar] [CrossRef]
- Ahiwe, E.U.; Dos Santos, T.T.T.; Graham, H.; Iji, P.A. Can probiotic or prebiotic yeast (Saccharomyces cerevisiae) serve as alternatives to in-feed antibiotics for healthy or disease-challenged broiler chickens?: A review. J. Appl. Poult. Res. 2021, 30, 100164. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, C.; Chen, M.; Ya, T.; Huang, W.; Gao, P.; Zhang, H. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int. Immunopharmacol. 2015, 29, 901–907. [Google Scholar] [CrossRef]
- Deng, Z.; Han, D.; Wang, Y.; Wang, Q.; Yan, X.; Wang, S.; Liu, X.; Song, W.; Ma, Y. Lactobacillus casei protects intestinal mucosa from damage in chicks caused by Salmonella pullorum via regulating immunity and the Wnt signaling pathway and maintaining the abundance of gut microbiota. Poult. Sci. 2021, 100, 101283. [Google Scholar] [CrossRef]
- Bahule, C.E.; Silva, T.N.S. Probiotics as a Promising Additive in Broiler Feed: Advances and Limitations. In Advances in Poultry Nutrition Research; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Huang, C.H.; Liou, J.S.; Lee, A.Y.; Tseng, M.; Miyashita, M.; Huang, L.; Watanabe, K. Polyphasic characterization of a novel species in the Lactobacillus casei group from cow manure of Taiwan: Description of L. chiayiensis sp nov. Syst. Appl. Microbiol. 2018, 41, 270–278. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.-M.; Kim, D.; Kim, H.-Y. Complete Genome Sequencing and Comparative Genomics of Three Potential Probiotic Strains, Lacticaseibacillus casei FBL6, Lacticaseibacillus chiayiensis FBL7, and Lacticaseibacillus zeae FBL8. Front. Microbiol. 2021, 12, 794315. [Google Scholar] [CrossRef] [PubMed]
- Almeida Paz, I.C.d.L.; de Lima Almeida, I.C.; de La Vega, L.T.; Milbradt, E.L.; Borges, M.R.; Chaves, G.H.C.; dos Ouros, C.C.; Lourenço da Silva, M.I.; Caldara, F.R.; Andreatti Filho, R.L. Productivity and Well-Being of Broiler Chickens Supplemented With Probiotic. J. Appl. Poult. Res. 2019, 28, 930–942. [Google Scholar] [CrossRef]
- Song, Y.; Cui, Y.; Wang, Y.; Yu, J.; Wang, B.; Wen, Q.; Zheng, X. Donor selection for fecal bacterial transplantation and its combined effects with inulin on early growth and ileal development in chicks. J. Appl. Microbiol. 2023, 134, lxad099. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, C.; Deng, J.; Yang, Z.; Zou, L.; Du, W.; Li, S.; Huo, X.; Zeng, L.; Yang, X. Transcriptome analysis reveals key genes and pathways associated with egg production in Nandan-Yao domestic chicken. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100889. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, Q.H.; Xiao, C.; Li, J.N.; Yang, X.R. Correlation analysis of crown and reproductive traits in Nandan Yao chickens (Scallopus nandans). China Poult. 2023, 45, 117–119. [Google Scholar] [CrossRef]
- Niewold, T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007, 86, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef]
- Rehman, A.; Arif, M.; Sajjad, N.; Al-Ghadi, M.Q.; Alagawany, M.; Abd El-Hack, M.E.; Alhimaidi, A.R.; Elnesr, S.S.; Almutairi, B.O.; Amran, R.A.; et al. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci. 2020, 99, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Fellenberg, M.A.; Speisky, H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. Worlds Poult. Sci. 2006, 62, 53–70. [Google Scholar] [CrossRef]
- Sagada, G.; Gray, N.; Wang, L.; Xu, B.Y.; Zheng, L.; Zhong, Z.W.; Ullah, S.; Tegomo, A.F.; Shao, Q.J. Effect of dietary inactivated lactobacillus plantarum on growth performance, antioxidative capacity, and intestinal integrity of black sea bream (Acanthopagrus schlegelii) fingerlings. Aquaculture 2021, 535, 736370. [Google Scholar] [CrossRef]
- Xin, W.G.; Li, X.D.; Lin, Y.C.; Jiang, Y.H.; Xu, M.Y.; Zhang, Q.L.; Wang, F.; Lin, L.B. Whole genome analysis of host-associated Lactobacillus salivarius and the effects on hepatic antioxidant enzymes and gut microorganisms of Sinocyclocheilus grahami. Front. Microbiol. 2022, 13, 1014970. [Google Scholar] [CrossRef]
- Ebeid, T.A.; Al-Homidan, I.H. Organic acids and their potential role for modulating the gastrointestinal tract, antioxidative status, immune response, and performance in poultry. Worlds Poult. Sci. 2022, 78, 83–101. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from Lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Jia, H.; Zhu, Z.; Li, H.; Ma, Y. Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life Sci. 2021, 16, 311–322. [Google Scholar] [CrossRef]
- Fathi, M.M.; Ebeid, T.A.; Al-Homidan, I.; Soliman, N.K.; Abou-Emera, O.K. Influence of probiotic supplementation on immune response in broilers raised under hot climate. Br. Poult. Sci. 2017, 58, 512–516. [Google Scholar] [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of linoleic acid by human gut bacteria: Different routes for biosynthesis of conjugated linoleic acid. J. Bacteriol. 2007, 189, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.T.; Zhan, X.A.; Zhang, L.L.; Zeng, X.F.; Chen, A.G.; Yang, C.M. Modulation of broilers’ caecal microflora and metabolites in response to a potential probiotic Bacillus amyloliquefaciens. J. Anim. Physiol. Anim. Nutr. 2018, 102, e909–e917. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Yang, W.-Y.; Chou, C.-H.; Wang, C. The effects of feed supplementing Akkemansia muciniphila on incidence, severity, and gut microbiota of necrotic enteritis in chickens. Poult. Sci. 2022, 101, 101751. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A. Akkermansia muciniphila. A new target to control obesity, type-2 diabetes and inflammation? Med. Sci. 2014, 30, 125–127. [Google Scholar] [CrossRef]
- Gerritsen, J. The Genus Romboutsia: Genomic and Functional Characterization of Novel Bacteria Dedicated to Life in the Intestinal Tract. Doctoral Dissertation, Wageningen University and Research, Wageningen, The Netherlands, 2015. [Google Scholar]
- Council, N.R. Nutrient Requirements of Poultry: 1994; National Academies Press: Washington, DC, USA, 1994. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
| Variables | NC | PC | LC6 | LC7 | SEM |
|---|---|---|---|---|---|
| BW (g) | 135.12 ± 4.86 a | 146.40 ± 10.32 a | 139.68 ± 3.14 a | 149.81 ± 6.37 a | 3.302 |
| ADFI (g) | |||||
| d 1 to d 21 | 12.19 ± 0.16 a | 13.59 ± 0.60 b | 12.81 ± 0.27 ab | 13.57 ± 0.47 ab | 0.719 |
| ADG (g) | |||||
| d 1 to d 21 | 4.72 ± 0.07 a | 5.67 ± 0.09 b | 5.08 ± 0.15 ac | 5.61 ± 0.12 b | 0.226 |
| FCR (g:g) | |||||
| d 1 to d 21 | 2.58 ± 0.03 a | 2.40 ± 0.05 a | 2.52 ± 0.06 a | 2.41 ± 0.04 a | 0.044 |
| Ingredients, % | 1–21 d |
|---|---|
| Corn | 55.41 |
| Soybean meal | 31.50 |
| Palm oil | 5.00 |
| Phosphorus | 3.60 |
| Calcium | 1.30 |
| Salt | 0.34 |
| Lysine HCL | 1.40 |
| Methionine | 0.22 |
| Arginine | 0.03 |
| Vitamin–mineral premix | 0.50 |
| Limestone | 0.60 |
| Sodium carbonate | 0.10 |
| Metabolizable energy (MJ·kg−1) | 14.01 |
| Crude protein | 20.0 |
| Calcium | 1.00 |
| Total phosphorus | 0.55 |
| Lysine total | 1.41 |
| Methionine | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, X.; Li, X.-D.; Luo, C.-Y.; Xin, W.-G.; Zhou, H.-Y.; Wang, F.; Lin, L.-B. Effects of Dietary Supplementation of Lacticaseibacillus Chiayiensis AACE3 on Hepatic Antioxidant Capacity, Immune Factors and Gut Microbiology in Nandan Yao Chicks. Antibiotics 2023, 12, 1356. https://doi.org/10.3390/antibiotics12091356
Kang X, Li X-D, Luo C-Y, Xin W-G, Zhou H-Y, Wang F, Lin L-B. Effects of Dietary Supplementation of Lacticaseibacillus Chiayiensis AACE3 on Hepatic Antioxidant Capacity, Immune Factors and Gut Microbiology in Nandan Yao Chicks. Antibiotics. 2023; 12(9):1356. https://doi.org/10.3390/antibiotics12091356
Chicago/Turabian StyleKang, Xin, Xin-Dong Li, Cheng-Ying Luo, Wei-Gang Xin, Huan-Yu Zhou, Feng Wang, and Lian-Bing Lin. 2023. "Effects of Dietary Supplementation of Lacticaseibacillus Chiayiensis AACE3 on Hepatic Antioxidant Capacity, Immune Factors and Gut Microbiology in Nandan Yao Chicks" Antibiotics 12, no. 9: 1356. https://doi.org/10.3390/antibiotics12091356
APA StyleKang, X., Li, X.-D., Luo, C.-Y., Xin, W.-G., Zhou, H.-Y., Wang, F., & Lin, L.-B. (2023). Effects of Dietary Supplementation of Lacticaseibacillus Chiayiensis AACE3 on Hepatic Antioxidant Capacity, Immune Factors and Gut Microbiology in Nandan Yao Chicks. Antibiotics, 12(9), 1356. https://doi.org/10.3390/antibiotics12091356




