Abstract
Drain-associated intracerebral infections are life-threatening emergencies. Their treatment is challenging due to the limited penetration of antibiotics to the site of infection, resulting in potentially inadequate exposure. The emergence of multidrug-resistant pathogens might force the use of off-label intrathecal (IT) doses of antibiotics. We reviewed the literature on general aspects determining intrathecal dosing regimen, using pharmacometric knowledge. We summarised clinical experience with IT doses of antibiotics that are usually not used intrathecally, as well as the outcome of the cases and concentrations reached in the cerebrospinal fluid (CSF). Factors determining the IT regimen are the size of the ventricle system and the CSF drainage volume. With regard to pharmacometrics, pharmacokinetic/pharmacodynamic indices are likely similar to those in non-cerebral infections. The following number (N) of cases were described: benzylpenicillin (>50), ampicillin (1), ceftazidime (2), cephaloridine (56), ceftriaxone (1), cefotiam (1), meropenem (57), linezolid (1), tigecycline (15), rifampicin (3), levofloxacin (2), chloramphenicol (3) and daptomycin (8). Many side effects were reported for benzylpenicillin in the 1940–50s, but for the other antibiotics, when administered correctly, all side effects were minor and reversible. These data might help when choosing an IT dosing regimen in case there is no alternative option due to antimicrobial resistance.
1. Introduction
The incidence of community-acquired meningitis caused by multi-resistant micro-organisms is still low; however, these infections after neurosurgery might become more prevalent due to the overall rise in antibacterial resistance [1,2]. Infections after neurosurgery are also frequently complicated by the presence of intracerebral devices, such as external ventricular drains (EVD) and external lumbar drains (ELD), used in the treatment of elevated intracranial pressure, and may present a life-threatening emergency.
Both the blood–brain barrier and the blood–CSF barrier protect the central nervous system (CNS) from toxic compounds present in the systemic circulation. However, in the treatment of cerebral infections, both barriers complicate the achievement of adequate concentrations of antibiotics at the site of infection. Therefore, in some cases, extremely high intravenous doses have been administered, such as 24 g/24 h cefotaxime, 15 g/24 h meropenem or 8 g/24 h imipenem [3,4,5]. Another option to overcome this obstacle might be to bypass the blood–brain barrier by administering the antibiotics intrathecally. The term intrathecal (IT) is a general term including both intraventricular (IVT) and intralumbar (IL) injection in, respectively, the cerebral ventricles and the lumbar thecal sac.
Currently, the number of antibiotics that are frequently administered, intrathecally, is limited. Only polymyxin B is registered by the FDA, and the EMA recommends maximum IVT doses of colistin of 125,000 IU [6,7]. However, the increasing rates of antimicrobial resistance and the limited penetration of antibiotics through the blood–brain barrier (BBB), might force the use of IT administration of antibiotics that are usually not used intrathecally to achieve an effective concentration at the site of infection.
For several antibiotics, IT use has been reviewed [8,9,10]. The range of doses administered intrathecally is quite broad. For vancomycin, for example, IT doses reported in the literature range from 5 to 50 mg q24h [8]. This broad range indicates that potentially patient-related factors might be involved in determining the dose for an individual patient and that the dosing regimen might be optimised based on pharmacometric knowledge. This includes information on the pharmacokinetics at the site of infection and on the exposure–response relationship in the CNS. We therefore aimed to describe the factors involved in the designing of an IT dosing regimen in general and give an overview of available data on the IT use of antibiotics that are normally not administered via this route. Information on IT dosing of antibiotics more regularly used intrathecally can be found elsewhere [8,9,11,12,13,14].
2. Results
2.1. Physiological Factors Influencing Intrathecal Pharmacokinetics
As antibacterial efficacy is dependent on a certain antibiotic exposure that needs to be reached at the site of infection, the amount of antibiotics reaching the CSF as well as the resulting concentration–time profiles in the CSF are of importance. Several factors might be taken into consideration in choosing a dosing regimen for IT administration. Figure 1 shows a summary of the factors involved.
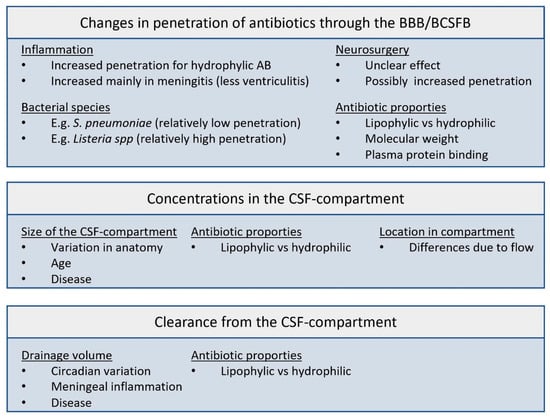
Figure 1.
Factors involved in the pharmacokinetics of antibiotics in the CSF compartment. BBB: blood–brain barrier; BCSFB: blood–cerebrospinal fluid barrier; AB: antibiotic.
2.1.1. Blood–Brain Barrier and the Blood–CSF Barrier
Both the blood–brain barrier (BBB) and the blood–CSF barrier (BCSFB) are lipid layers surrounding the CNS aiming to protect the brain from toxic agents present in the systemic circulation. These barriers thereby limit the penetration of antibiotics to the site of infection when treating meningitis or ventriculitis. For example, the penetration of meropenem into the CSF in patients with (suspected) ventriculitis has a mean of only 9–18% [15,16], and a high interpatient variability has been reported. This high interpatient variability is in line with observations for vancomycin [17]. The low and unpredictable penetration into the CSF puts several patients at risk for insufficient exposure at the site of infection.
Meningeal inflammation during infection leads to the opening of tight junctions in the BBB. The level of inflammation, which is more pronounced in meningitis as compared to that in ventriculitis might, for some antibiotics, lead to an increased penetration into the CSF [14,18]. This increased penetration mainly applies to hydrophylic antibiotics [19]. It has also been suggested that the BBB might be disrupted during neurosurgery, resulting in an increased penetration of systemically administered antibiotics. However, for vancomycin, it has been shown that the penetration in the CSF in patients after neurosurgery was similar to the penetration in patients with uninflamed meninges [20], making this statement questionable. Generally, though inflammation during infection and disruption of the BBB during surgery might both increase the potential penetration into the CSF, the true penetration into the CSF will still be highly variable and unpredictable.
From experimental animal models, it is also suggested that the infecting bacterial species might influence the permeability of the BBB. The presence of Streptococcus pneumoniae and Escherichia coli might result in a relatively low permeability, while the penetration into the CSF in infections caused by Haemophilus spp. and Listeria monocytogenes is higher [21,22]. For ceftriaxone, for example, the CSF/serum penetration was found to be 2.7% in case of a S. pneumoniae infection, while it was 9.0% in a H. influenzae infection in the same study [22]. Also, when the mezlocillin penetration into the CSF was compared between an infection with E. coli and Listeria monocytogenes, different values for the penetration were found of 6–11% and 16–20%, respectively [21].
2.1.2. Size of the CSF System
The size of the CSF compartment is important to determine the volume of distribution for intrathecal dosing (VCSF). After penetrating the BBB, the size of the CSF compartment is one of the determinants for the concentrations reached locally. VCSF consists of four ventricles, an aqueduct, basal cisterns and the subarachnoid space over the convexities and in the spinal cord. There is considerable interindividual variability in the size of the VCSF, depending on age, widths of the ventricles and cerebral subarachnoidal space, length and width of the spinal canal and underlying disease [8]. Several studies estimated the size of the CSF for healthy adults with mean values between 250 mL and 326 mL [23,24,25], patients with communicating hydrocephalus of mean 488 mL [25] and patients with non-communicating hydrocephalus of mean 593 mL [25]. The size of VCSF might be reduced by the presence of blot clots in the ventricles of the basal cisterns.
Although the CSF compartment is convoluted, the distribution cannot be considered homogenous [8,26]. Overall, about two-thirds of the CSF is produced by the choroid plexus, and the remaining one-third originates from the extracellular space of the brain and spinal cord. The equilibration in the CSF is facilitated by the oscillations in the CSF based on the heartbeat and respiration. The net flow of the CSF is from the ventricles to the cisterna magna and from there to the cerebral convexities and into the spinal canal. As a result, antibiotic concentrations in CSF sampled by lumbar puncture might differ from those taken from an extraventricular drain.
Once the antibiotics reach the CSF, they can enter the extracellular fluid of the brain and spinal cord, since there is not a tight barrier between the CSF and extracellular fluid of nervous tissue. The diffusion from the CSF into the extracellular space of the brain and spinal cord occurs against the gradient of the CSF bulk flow (directed from nervous tissue into the CSF) [8].
2.1.3. Location of Antibiotic Administration
As the CSF compartment is not homogenous and there is a net flow of CSF out of the ventricles, the location where the IT doses are administered makes a difference. Differences in concentrations can occur between the ventricular, cisternal and lumbar part of the CSF compartment [27]. As IT doses are most frequently prescribed to treat ventriculitis the antibiotic exposure needs to be optimal in the ventricles and the location of administration is therefore preferable intraventricular. For most drugs, achieved concentrations in the lumbar CSF are higher as compared to those in the ventricular CSF after IV administration [14]. Intraventricular dosing results in distribution throughout the CSF compartment, unless the system is blocked by, for example, bleeding. Due to the net flow of CSF out of the ventricles, antibiotic dosing intralumbar or intraspinal will result in lower concentrations in the ventricular CSF then after intraventricular dosing [28] and the concentrations will be unevenly distributed in the CSF space, more variable and might therefore be inadequate in the ventricles. Pharmacokinetic data therefore suggest that intraventricular dosing is preferred over intralumbar dosing, especially in the treatment of ventriculitis. However, clinically there is no evidence to support this [19].
2.1.4. Drainage Volume
The drainage volume is the volume (in mL/day) that is eliminated from the CSF compartment per day. The drainage volume is therefore important for the clearance of antibiotics from the CSF. The external part of the drain is usually kept at a standard level, and the CSF fluid will be excreted when the intracranial pressure exceeds the pressure in the drain. Changing the level of the drain might therefore alter the drainage volume. For vancomycin as well as for meropenem, it has been shown that the drainage volume is a crucial factor for the determination of the IT dose [29,30,31].
The drainage volume also depends on the CSF production, which is not constant over time. Both in healthy volunteers and in patients with external ventriculostomies, the CSF production appeared to have a circadian variation with the minimum production of 12 ± 7 mL/h around 6 p.m. and the nightly peak around 2 a.m. of 42 ± 2 mL/h [32,33]. Meningeal inflammation might result in a reduced production of CSF, thereby limiting the drainage volume and the clearance of antibiotics from the CSF [14]. The timing of the IT dose might therefore also be of importance.
2.2. Other Factors Influencing the Intrathecal Pharmacokinetics
2.2.1. Antibiotic Properties
The clearance of drugs from the CSF to the blood (CLCSF to Blood) depends on several processes, and these are of importance depending on the properties of the drugs. The processes important for the CLCSF to Blood are as follows: 1. bulk flow (equals the CSF production rate); 2. retrograde diffusion across the blood–CSF barrier and the blood–brain barrier; and 3. active transport. Large hydrophylic molecules, such as aminoglycosides, are mainly cleared from the CSF by the CSF bulk flow. As a result, these drugs have a reduced clearance in hydrocephalic patients [8,34]. On the other hand, small moderately lipophilic molecules, such as quinolones, are mainly cleared through passive elimination via retrograde diffusion across both barriers [35].
Differences in the permeability of the BBB and the VCSF are also partly explained by the difference in properties of hydrophylic or lipophylic antibiotics. The hydrophylic nature of antibiotics limits the penetration into the CSF. Their VCSF equals the volume of VCSF plus the fraction of the extracellular space of the brain that easily equilibrates with the CSF [36,37]. Since lipophilic drugs in the CSF equilibrate more easily with adjacent spaces and are able to bind to lipid membranes, the VCSF of lipophilic drugs is usually larger as compared to hydrophylic drugs. Penetration through the BBB caused by inflammation during meningitis is increased for hydrophilic antibiotics, while for lipophilic antibiotics, this has a minimal effect [19].
Both molecular weight and protein binding also influence the penetration into the CSF. In the presence of an intact barrier, only the free fraction can penetrate into the CSF, since binding proteins pass the barriers only in a small degree [38]. For drugs with a low protein binding such as ceftazidime, the unbound fraction available for CNS penetration is therefore high. Protein binding in CSF is believed to be lower as compared to serum/plasma. For ceftriaxone, for example, serum/plasma protein binding is in the range of 80–95%, while in the CSF, it is found to be 18% ± 6% [39]. The molecular weight also influences the potential to penetrate the BBB, and drugs with large molecular weight, such as vancomycin and daptomycin, have a poor penetration.
2.2.2. Simultaneous Systemic Dosing
As the central volume of distribution is connected with the volume of distribution of the CSF concentrations in both compartments, they might influence each other (Figure 2). It is therefore important to consider the effect on the CSF concentrations after multimodal treatment using both IV and IT administration of antibiotics. Combined dosing of IV with IT administration is likely to result in slightly higher concentrations in the CSF. In a population PK model with meropenem in both serum and CSF, it has been shown that in order to achieve adequate CSF concentrations, relatively low IV doses of 500 mg q12h are needed for pathogens with MICs up to 4 mg/L and a drainage volume up to 200 mL/day [30]. This illustrates that regular IV dosing regimen are not always required simultaneously with the IT doses. However, it is questionable whether simultaneous IV dosing has a relevant effect on the PK in the CSF for all antibiotics [8]. Since most patients are treated with the combination of IV plus IT doses, there is not enough evidence to reduce the regimens to IT only.
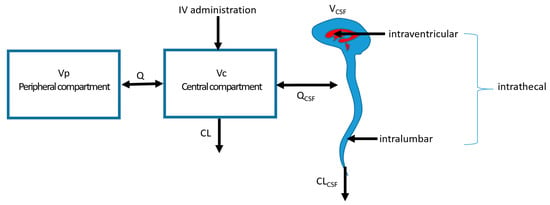
Figure 2.
Multicompartment system with different locations of administration. Vp: peripheral volume of distribution; Vc: central volume of distribution; VCSF: volume of distribution of the CSF compartment; Q: intercompartmental clearance; CL: clearance; IV: intravenous; QCSF: intercompartmental clearance between Vc and VCSF; CLCSF: clearance from the CSF compartment.
2.3. Pharmacodynamics of Antibiotics in the Cerebrospinal Fluid
The penetration of antibiotics into the CSF should be considered in the context that the principal determinant of the antibacterial efficacy is the antibiotic exposure related to the minimal inhibitory concentration (MIC) of the pathogen. Conclusions based on a single concentration in CSF after an IV dose can be incorrect, since the peak of the concentration–time curve in CSF is often delayed compared to the peak in serum. Ratios between CSF and plasma concentrations, frequently reported in the literature, can therefore be misleading. To best describe the penetration into the CSF the ratio between the area under the concentration–time curve (AUC)CSF and AUCserum or the %fT > MIC should be determined. After an intraventricular dose, the peak in the ventricular CSF will be reached immediately, but to describe distribution and clearance, multiple CSF concentrations are needed. As IT dosing is usually accompanied by IV dosing, the timing of the IV dose might also be of influence on the concentration–time profile in the CSF. In patients with an external ventriculostomy, CSF concentrations can be determined repeatedly, but sampling is limited by the fact that every manipulation of the ventriculostomy is an infectious risk.
Despite the fact that penetration into the CSF is best described by the AUCCSF/AUCserum ratio, the interpretation of these penetration ratio values is not straightforward. For the selected antibiotics for which data on IT dosing are available, the values after IV administration are presented in Table 1. A low penetration ratio indicates that the amount of antibiotic entering the CSF is relatively limited. However, a low penetration ratio after IV dosing does not automatically mean that the antibiotic cannot be used in the treatment of meningitis. This is illustrated by the very low penetration ratio of ceftriaxone of 0.007 [40]. Ceftriaxone is frequently used to treat meningitis with IV dosing. This low penetration ratio is likely caused by the high serum protein binding of ceftriaxone [41], and also, the relatively low MIC values in the wild-type distribution of the pathogens might contribute to its effectivity in the treatment of meningitis. The penetration ratio by itself therefore cannot be used to decide whether an antibiotic is suitable in the treatment of meningitis after IV dosing or that additional IT doses are required. Other factors, such as the serum protein binding, the range in MIC values in the wild-type distribution of the pathogen and the pharmacodynamics, should also be taken into account [42].

Table 1.
AUCCSF/AUCserum ratios for the selected antibiotics.
To predict the antibacterial efficacy in the CSF, knowledge on the pharmacodynamics (PD) in the CSF is needed. For most antibiotics, the PK/PD index in serum is known; however, limited data are available on the PD in the CSF. Due to the restricted nutritional supply and acidic pH of the CSF, bacteria therein multiply less rapidly as compared to those in blood [8]. Also, because of the absence of complement and antibodies, the PD in CSF may differ from those in other body sites [19], but there is no indication that the concepts of time-dependent versus concentration-dependent efficacy are not applicable [19]. The PK/PD indices that are best correlated with efficacy should also be taken into account in the design of the dosing regimen.
The magnitude of the PK/PD indices correlated with antibacterial efficacy in the CSF is largely unknown, and the concentration–time profile of most antibiotics in the CSF is also unknown. To link concentrations to efficacy, a practical approach is used, correlating a single antibiotic concentration to the MIC or minimal bactericidal concentration (MBC) of the pathogen. A CSF concentration of at least 10 times that of the MIC or MBC has been correlated to the efficacy of beta-lactams and quinolones [54,55,56] and a pneumococcal meningitis model suggested a vancomycin-CSF-peak-to-MBC ratio >4 to be adequate [57]. The IDSA guideline recommend concentrations of 10–20× MIC [58].
Although little is known on the magnitude of the PK/PD indices needed for an antibacterial effect in the CSF, clinical data suggest an effect after IT dosing. In a study on the IT dosing of post-neurosurgical patients with meningitis (N = 30) or ventriculitis (N = 4) with persistent positive CSF culture despite IV treatment, the results showed an overall mean time to sterilise the CSF after IT administration of 2.9 ± 2.7 days (range of 1–12 days) [59]. In 50% of patients, the CSF cultures were negative <24 h, and in an additional 18%, they were negative within 48 h after the IT dose. The average time to sterilisation in the ventriculitis patients was 6.5 days.
2.4. General Aspects of IT Treatment
2.4.1. Micro-Organisms
All micro-organisms have the potential to cause drain-associated infections. But, the most frequently reported micro-organisms are coagulase-negative staphylococci (especially Staphylococcus epidermidis), S. aureus, P. acnes and Gram-negative bacilli (including Escherichia coli, Klebsiella species, Enterobacter species, Citrobacter species, Serratia species, Acinetobacter spp. and Pseudomonas aeruginosa) [58,60].
A. baumannii is an important pathogen in this patient category. Of all healthcare-associated meningitis, 3.6–11.2% of cases are caused by A. baumannii [13]. It is a difficult-to-treat pathogen, for which the IDSA guidelines even recommend treating meningitis or ventriculitis with a combination of IV and IVT treatment with polymyxins [58].
The distribution in the MICs between various species for a specific antibiotic can be quite different. Even for a single antibiotic–pathogen combination, there is a considerable amount of variation that needs to be taken into account when determining the MIC [61]. Therefore, the epidemiological cut-off value (ECOFF) is usually taken into account when designing a dosing regimen for susceptible pathogens or when performing therapeutic drug monitoring. This ECOFF value is the highest value of the wildtype distribution and can be found on the EUCAST website [62].
2.4.2. Therapeutic Drug Monitoring
The choice whether to perform therapeutic drug monitoring (TDM) or not depends on several factors. There needs to be information on how to interpret the concentration. Interpretation of the concentration in the CSF is complex, since very little is known regarding the PD and the target concentrations. In a retrospective cohort study of 105 patients with vancomycin or an aminoglycoside administered via IVT, there is a higher proportion of the survivors that had TDM on CSF concentrations as compared to the non-survivors [11], suggesting a potential benefit of TDM.
On the other hand, it could be used to avoid toxicity by detecting extremely high concentrations, although CSF concentrations associated with toxicity are also not known. For vancomycin, for example, serious toxicity and adverse events do not appear to be correlated with the CSF concentration [63]. Systematic monitoring of CSF concentrations and correlating them with neurotoxicity could increase our knowledge and predict toxicity in the future. In general, it could be useful to detect IT doses that are administered as bolus or as short infusions and to determine the concentration–time profile in individual patients when multiple samples are needed.
2.4.3. Clinical Outcome
To study the clinical outcome after IT administration compared to IV administration of antibiotics, comparative studies are needed. Obviously, for antibiotics that are not frequently used with IT administration, those studies are not available. Only for meropenem is there one retrospective study in combination with vancomycin [64], showing the beneficial effect of IL administration as compared to IV only. A review on cephalosporin cephaloridine concluded that IT dosing had a positive effect on the outcome in the treatment of bacterial meningitis [65].
Only for colistin 2 has a meta-analysis been performed. In 2019, Hu et al. published a review with a meta-analysis to compare IT/IVT administration with IV administration in patients with post-neurosurgical intracranial infection due to multidrug-resistant Gram-negative bacteria [9]. The vast majority of the studies included in the meta-analysis for the outcome ‘mortality’ included the use of colistin. The use of IT/IVT antibiotics was associated with a lower risk on mortality (nine studies were included; pooled OR 0.15; CI 0.08–0.28; p < 0.001; pooled number of deaths: in the IVT group, 22 of 127 cases, and in the IV group, 95 of 152 cases) and with a high microbiological clearance rate (two studies were included; pooled OR 0.02; CI 0.01–0.1; p < 0.001; pooled number of bacterial clearance: in the IVT group, 30 of 32 cases, and in the IV group, 10 of 47 cases.) [9]. Another meta-analysis was performed on IT plus IV colistin versus IV colistin only for Acinetobacter baumannii infections in post-neurosurgical patients [66]. They included five studies, and four of those were also included in the meta-analysis of Hu et al. The overall conclusion was that patients treated with a combination of IT and IV colistin had an 84% lower risk of dying due to an A. baumannii infection [66].
Gower et al. published a case series of 39 adults with a Gram-negative bacillary meningitis [67]. The study included various micro-organisms, but also many different antibiotic regimen. IT gentamicin, tobramycin, amikacin, polymyxin B and cefamandole were used. The overall mortality was 36%, and when the groups were divided into subgroups based on IT therapy, the mortality rates were as follows: 36.4% in patients without IT therapy (N = 22), 37.5% in patients with a short course of IT therapy (N = 8) and 26.6% in patients with a full course of IT therapy. Although this is in line with the results of Hu et al., the results must be interpreted with caution since there is no detailed information on the antibiotic combinations nor on the reasons behind the choice of a specific regimen [67].
Although several studies reported a beneficial effect of IT administration of antibiotics on the outcome as compared to conventional dosing, there is also a study on IT gentamicin in 52 children, reporting an increased mortality rate in the IT group [9,63,67,68]. Another study found that for carbapenem-resistant isolates the outcome was better in the patient group treated with IT dosing, but this beneficial effect was not found for other micro-organisms [69]. This is in line with the studies on the polymyxins, which are usually prescribed in the treatment of carbapenem-resistant Gram-negatives. This underlines the previous conclusion that IT dosing should only be used in patients when there is no reliable alternative.
In general, studies on clinical outcome compare standard treatment to IT dosing. Since the group of patients that is treated for meningitis or ventriculitis is severely ill, this might result in larger variability in PK than usual [70]. For the standard IV dosing regimens used, there might therefore be a risk of underdosing. When patients with the standard IV regimens are indeed underdosed, this might increase the difference in outcome with IT dosing, while part of the difference might be explained by underdosing of the IV group. The actual benefit of IT dosing could therefore be smaller than that reported in studies.
2.4.4. Adverse Events and Risks of IT Administration
Most reported side effects of IT administration are chemical ventriculitis or meningitis, seizures or local adverse events or infection [60,71]. A meta-analysis including 23 studies (229 patients) reported that the overall complication rate was 13%; chemical meningitis and seizures represented the majority of the complications, with an occurrence rate of 11% and 7%, respectively [71]. However, since patients in the need for IT treatment are usually very sick, there might be underreporting of side effects. On the other hand, side effects as described for IT dosing have also been described after IV administration only. For colistin, the prevalence of side effects after IT was similar to those after IV dosing [72].
Neurotoxic side effects of beta-lactam antibiotics are known to occur when there is high exposure what can be caused by very high doses or in the presence of renal failure. They can induce confusion, encephalopathy, myoclonus and epileptic seizures particularly in patients with underlying neurological disorders [73,74]. The potency to induce seizures is relatively high for cefazolin, cefepime, benzylpenicillin and imipenem, whereas this is much lower for ampicillin, ceftazidime and meropenem [74].
An important side effect of IT dosing is chemical ventriculitis or meningitis, usually mild and reversible. Clinically, it resembles bacterial meningitis, presenting with fever, altered mental state, elevation of white blood cell count in the CSF and low glucose concentrations [13]. It is difficult to distinguish a chemical reaction on an antibiotic from the reappearance of signs of meningitis, which can be caused by a relapse of the existing infection or an infection with a new pathogen due to the multiple manipulations [13].
The IT administration of antibiotics often involves a drain system. Every manipulation of the system increases the risk on drain/device infection. Though the antibiotics might be indicated for a specific pathogen, there is a risk of introducing another pathogen through manipulation of the system, such as irrigation [75]. Therefore, preparation of the antibiotics administered intrathecally must be performed under sterile circumstances, and precautions must be taken to avoid causing an infection.
2.5. Clinical Experience with IT Administration of Selected Antibiotics
The literature search revealed for most antibiotics only case reports. Only for meropenem 2 population pharmacokinetic are models available. The data found will be described below. For fosfomycin, ciprofloxacin, moxifloxacin, piperacillin, cefotaxime, imipenem, ertapenem, metronidazole, co-trimoxazole, tetracycline and doxycycline, no data were found. Data for the cases of several antibiotics are shown in Table 2.

Table 2.
Cases with IT doses of miscellaneous antibiotics.
2.5.1. Penicillins
In the 1940s and 1950s, the use of IT penicillin was quite common. In 1941, it was concluded that IT administration of penicillin was safe, as after the administration of penicillin in the cisterna magna of rabbits there were no histological disturbances [86]. The usual dose for adults was 6 mg (10,000 units) [87], and it was used for pneumococcal meningitis, which used to be a fulminating and rapidly fatal disease in those days [88]. Many case reports have been published describing the serious side effects of IT administration of penicillin, such as generalised flaccid paralysis, flaccid paraplegia or death [89,90,91,92]. In several cases, doses administered intrathecally were higher than the usual dose of 10,000 unit, as described by Wood [87]; the described effects are very serious, but it was also mentioned that the pneumococcal meningitis itself might in part contribute to the side effects. Sweet et al. described 16 patients with pneumococcal meningitis, 9 of whom died, and of the other 7 patients, the side effects of IT penicillin were reported in 4 patients [91]. Due to the side effects and the optimized IV dosing regimen, IT administration of penicillin is no longer used.
One case on the use of IT ampicillin [84] is described in the literature of a patient with E. coli meningitis. He started with a regimen of 20 mg q12h IT ampicillin with 500 mg q6h oral doses. But, due to microbiological failure the IT doses were increased to 40 mg q12h. The patient was cured and experienced no side effects.
2.5.2. Cephalosporins
Several cephalosporins, such as ceftriaxone and ceftazidime are commonly used in the treatment of bacterial meningitis and are usually not administered intrathecally. There are some data on the IT administration of cephaloridine, a first-generation cephalosporin that is no longer available for therapeutic use possibly due to renal toxicity. Furthermore, two cases on the use of ceftazidime have been described, as well as two cases in which the cephalosporin was dosed extremely high after an error in the preparation of the solution or the administration [65,85,93].
In 1975, a review was published on the use of cephaloridine [94]. They found that patients receiving concomitant IT and IV cephaloridine (N = 56) responded significantly better (p < 0.005) in the treatment of bacterial meningitis, as compared to those treated only with IV cephaloridine (N = 16). The IT doses administered varied from 5 to 100 mg/day.
Two cases have been described who were treated with intraventricular ceftazidime in doses of 10–20 mg twice per week. One patient with P. aeruginosa meningitis, who was treated as an out-patient for nearly two years, died after an attempted withdrawal of the intraventricular treatment [85]. The other patient was cured from a ventriculitis with an unknown pathogen after 16 IT doses of ceftazidime. Both patients did not have irreversible side effects [85].
Furthermore, there are two case reports on accidental high doses via IT administration of cephalosporins. In the first case, 800 mg ceftriaxone was administered intrathecally as a consequence of a dilution error (instead of the intended 8 mg) [93]. A solution of 50 mg ceftriaxone/mL was injected in a 74-year-old patient treated for pneumococcal meningitis and pansinusitis. After the injection, the patient experienced severe burning pain in the lumbosacral region radiating into both extremities. Since the pain did not respond to analgesics, 240 mL of CSF was exchanged. At 1 h after the injection, the CSF concentration was 4387 mg/L, and after the exchange of 240 mL CSF, the concentration decreased to 3384 mg/L. There were no permanent side effects. The second case was in a 66-year-old patient accidentally receiving 1.5 g cefotiam via an intrathecal port system put in place for pain management [65]. After the injection, the patient experienced muscular cramps, abdominal pain, massive general myoclonic jerks, massive pain and dyspnoea. Approximately 20 h after the injection, the CSF concentration was 198.8 mg/L. On day 5, the CSF concentration was 10.1 mg/L. The patient was intubated and dialysed, but afterwards, he returned to his previous neurological state, with no signs of permanent damage due to the cefotiam.
2.5.3. Carbapenems
Meropenem is frequently used to treat meningitis with a regimen of 2 g q8h. However, this IV regimen might not be sufficient for infections caused by difficult-to-treat micro-organisms. Therefore, the dosing regimen is sometimes increased up to even 15 g meropenem per day [4]. The need for a higher dosing regimen of meropenem in the treatment of Pseudomonas aeruginosa cerebral infections was also suggested by Konig et al. [95], who based their recommendations on a PK/PD model and a target in the CSF of 100% fT > 2× ECOFF of Pseudomonas aeruginosa. Instead of very high IV doses, IT administration might be an option. For meropenem, three studies are available. One is a retrospective study comparing the clinical outcome and the occurrence of side effects of vancomycin and meropenem [64]. Two studies determined concentrations in CSF as well as in plasma and analysed the data using population pharmacokinetic modelling [30,31]. In the retrospective study, 86 patients were included with an infection after cranial trauma surgery [64]. Two groups were compared. In both groups CSF was released by lumbar cistern drainage, and the control group received vancomycin and meropenem IV, whereas the experimental group was treated with vancomycin and meropenem intrathecally (20 mg q12h) via the drain. The recovery rate in the experimental group was 95% versus 72% in the control group (p < 0.05). Also the cure time was lower in the experimental group (11 ± 5 days and 23 ± 9 days for the experimental and control group, respectively; p < 0.001). Side effects occurred less in the experimental group (p < 0.05). While these dosing regimens are frequently used in clinical practice, vancomycin is usually guided based on TDM. This study did not mention the use of TDM. Since this patient group of the critically ill is known to have a large variability in the PK, the possibility of underdosing should be taken into account in the interpretation of the data. No data were found on imipenem and ertapenem.
The two other studies measured concentrations in plasma as well as in the CSF and analysed the data with a population pharmacokinetic analysis to be able to describe the time course over time, find important covariates and use the population model to predict the most optimal dosing regimen [30,31]. In both studies, a group of patients (9 patients with an aneurysm and 15 patients after neurosurgery) meropenem was dosed at 1990 mg IV q12h and 10 mg IT q12h, and after the intrathecal dose, the drain was clamped for 15 min. Meropenem was administered via IL injection in the first study and via IVT injection in the second study. An important factor that determines the exposure to meropenem in the CSF is the drainage volume per day. Based on simulations with the final model of the first study, the IV doses needed to be increased in patients with a drainage volume of >250 mL/day [31] for regimens designed from MIC values of 4 mg/L and 8 mg/L. In the second study, an increase in the IV as well as in the IVT dose was recommended for patients with a drainage volume of 200–300 mL/day and an MIC of 4, 8 or 16 mg/L and a second increase in both dosages in patients with a drainage volume of 300–400 mL/day [30]. Dosing regimens suggested are based on a target value of 100% fT > MIC. The suggested regimens are quite complex and based on mathematical modelling. Overall, MIC values of micro-organisms that are reported susceptible to meropenem using EUCAST breakpoints [96] will include MIC values up to 4 mg/L. Assuming, based on available data, that IT doses of 10 mg meropenem are well tolerated, and based on the pharmacokinetic models, an overall dosing regimen of 2 g IV and 10 mg IT q12h might be an option. In case the aim is to treat micro-organisms with MIC values higher than the wild-type distribution, especially in patients with a drainage volume > 200 mL/day, higher doses might be needed. Given the elimination rate of meropenem, an IT dosing regimen of q24h is likely not feasible. In both studies, a high variability between concentrations in the CSF was found.
For meropenem, it is also important to mention the study of Hosmann et al. [97]. This study highlighted the importance of measuring concentrations at the site of infection. They measured meropenem concentrations after IV dosing in plasma, CSF as well as in the cerebral tissue via microdialysis. Concentrations in cerebral microdialysate were more than three times higher than those in CSF, showing that measuring CSF concentrations would highly underestimate brain tissue concentrations. Since the magnitude of the PK/PD indices needed for bacterial efficacy might also differ between an infection in the CSF and in brain tissue, it is not possible to interpret these values. The study clearly shows that designing optimal dosing regimen to treat intracerebral infection is very complex.
2.5.4. Linezolid
Little is known on the use of linezolid for the treatment of intracerebral infections. It has a bioavailability of 100%, and penetration in the central nervous system is variable, ranging from 28 to 70% [98]. Since this is relatively high [99], linezolid is normally not administered intrathecally.
Only one case report on IT administration has been described in the literature, and concentrations in the CSF were not determined [80]. The IT doses of 10 mg were administered once daily in a concentration of 2 mg/mL for 15 days in total. No side effects were reported.
2.5.5. Tigecycline
Tigecycline is currently approved for three indications: complicated skin and skin structure infections, complicated intra-abdominal infections and community-acquired bacterial pneumonia. Within the label, there is a box warning that there was an unexplained increased all-cause mortality in patients treated with tigecycline as compared to the comparators in a meta-analysis of clinical trials. It should therefore be reserved for use in situations when alternative treatments are not suitable [100].
Due to the poor BBB permeability of tigecycline, it is not recommended in the treatment of intracerebral infections with IV administrations. Tigecycline CSF concentrations after the usual 100 mg IV dose per day are only 0.035–0.048 mg/L, and increasing the daily dose to 200 mg is not well tolerated [101,102]. Several case reports (N = 14) have been described in the literature of IVT tigecycline in the treatment of infections caused by A. baumannii, K. pneumoniae and K. oxytoca (Table 3) [103,104,105,106,107,108,109,110,111,112,113,114,115]. The dosing regimen using IVT ranged from 1 mg q12h to 10 mg q12h. About half of the regimen used an IVT tigecycline dosing of q24h. In only two patients were concentrations determined in the CSF [103,112]. In the first patient, several concentrations were determined after an IVT dose of 1 mg and after a 2 mg IVT dose. The AUC0–12h was calculated to be 230 h·mg/L and 1132 h·mg/L after the 1 mg and 2 mg IVT dose, respectively. Based on the reported concentrations in another patient after a 5 mg IVT dose, the AUC0–12h can be estimated to be 887–1166 h·mg/L [112]. The magnitude of the PK/PD index correlated with bacteriostasis in a neutropenic thigh infection mouse model for E. coli and K. pneumoniae is a fAUC/MIC of median 5–6 h·mg/L [116]. As the AUCs in the CSF reached in these two patient are much higher than the PK/PD target value, high IVT doses might not be necessary. However, in both patients, the tigecycline was cleared from the CSF quite rapidly, resulting in undetectable or very low concentrations 12 h after the dose. It seems therefore to indicate the administration of IVT doses at q12h. Some patients failed on the initial regimen and were cured after the IVT dose was increased: in one patient, the dose was increased from 3 mg q24h to 4 mg q12h [104]; in the second patient, from 2 mg q24h to 2 mg q12h [105]; and from 2 mg q12h to 4 mg q12h in the third case [110].

Table 3.
Cases with IT tigecycline.
In four cases, potential side effects were reported [105,110,111,113]. As tigecycline is used in these cases for the treatment of multidrug-resistant micro-organisms, it is often difficult to determine whether the observed side-effect is attributable to the tigecycline or to other co-administered drugs. In one case, a ventriculitis and a holocord myelitis were reported [105]. This patient also received colistin via IVT injection, and since this is a known side-effect of IVT colistin, this could well be caused by the colistin [13,117]. This is supported by the fact that in this patient, the IVT administration of colistin and tigecycline were stopped, and because the meningitis reoccurred, IVT tigecycline was restarted after one day, without further problems. One patient had a myoclonic seizure 8 h after the first IVT tigecycline dose [110]. This was treated, and due to a lack of alternative antibiotic options, the IVT tigecycline was continued while the patient used maintenance doses of phenytoin.
Without further seizures, the patient completed the IVT tigecycline treatment of 14 days. For two other patients receiving IVT doses of 5 mg, the potential side effects were described: spinal arachnoiditis (after nine IVT doses); and reduced liver function after 7 days of IVT treatment [111,113].
2.5.6. Rifampicin
Rifampicin is used as part of the combination regimen in the treatment of tuberculosis or as additive antibiotic to flucloxacillin or vancomycin to increase penetration in a biofilm. Two cases have been described in which rifampicin was switched to IT administration because of the failing efficacy of IV-administered combinations of antibiotics in the treatment of intracerebral tuberculosis infections (Table 2) [81,82]. The targeted concentration in the CSF was 15 mg/L [81]. In both cases, IT doses of 5 mg were used without side effects. In addition, Senbaga et al. described a case in which a 41-year-old patient accidentally received a dose of 600 mg rifampicin infusion intrathecally over 4 h [83]. The intention was to administer vancomycin IT and rifampicin IV, but the infusion systems were swapped. No adverse events occurred in this case. It is noteworthy to mention that intravenous preparations of rifampicin have trace doses of formaldehyde [83].
2.5.7. Quinolones
Quinolones are known to have a potent seizure-inducing activity [118,119]. Overall, the incidence of central and peripheral nervous system reactions is estimated to be 0.9–2.1% [118]. A history of epilepsy, cerebral trauma and alcohol abuse are risk factors. Though severe reactions, such as hallucinations, depression and even convulsive seizures, are rare, intraventricular use of quinolones might not be a tempting option. But, since fluoroquinolones enter the CSF readily, IT use might not be necessary.
Two cases of intraventricular administration of levofloxacin have been described and summarised in Table 2 [78,79]. In both cases, patients were treated with an antibiotic regimen consisting of several drugs to treat meningitis due to multidrug-resistant Mycobacterium tuberculosis. Intraventricular doses of 1–2 mg per dose were administered for a prolonged duration without causing serious side effects. In one case, the levofloxacin dose was calculated assuming a CSF volume of 120 mL and a target CSF concentration of 8–10 mg/L [78]. During the first 1–2 months of treatment, there were minor adverse events ascribed to the levofloxacin: insomnia, myalgias and arthralgia. These later subsided [78]. Both patients were cured after months of treatment, but due to the regimens containing several antibiotics, it is unclear whether this is attributable to the intraventricular administration of levofloxacin.
2.5.8. Chloramphenicol
Chloramphenicol is an antibiotic that is little used in industrialised countries, but it is included in the WHO list of essential medicines to be used for several indications, such as meningitis [120]. Three cases were reported in the literature on IT use of chloramphenicol [76,77]. After IT administration, chloramphenicol sodium succinate will be hydrolysed to the microbiologically active chloramphenicol in the ventricular fluid. There is a considerable difference in the doses used in the first two cases in 1951 (up to 750 µg) [76] and the more recent case in 2005 (25 mg/day) [77]. The accompanying systemic doses were also different (12 g oral vs. 3 g IV), and in the recent case, no concentrations in the CSF were measured. Furthermore, besides the two cases Anderson et al. described in detail (Table 2), they also mentioned that several patients were treated with 3 mg administered into the lumbar theca for at least one week [76]. But, no further details on accompanying systemic therapy or CSF concentrations were given on these patients. In the cases reported in 1951, chloramphenicol was administered in pure crystalline form as an IT solution at a concentration of 100 µg/mL [76]. In the recent case, the formulation used was not mentioned. Due to the differences and incomplete information, an overall conclusion with regard to the IT doses therefore cannot be drawn.
In all three cases that were described in detail, no side effects were reported (Table 2). But, in the group of patients receiving 3 mg into the lumber theca, several side effects were noted: all patients reported depression with tearfulness, and patients with pre-existing cerebral tremor experienced marked accentuation of the tremor [76]. Both side effects disappeared with cessation of IT therapy.
2.5.9. Daptomycin
Daptomycin is a cyclic lipopeptide that is used to treat infections due to Gram-positive micro-organisms. After systemic administration, daptomycin penetrates poorly into the CSF compartment, due to its low lipophilicity, significant protein binding and large molecular weight, thus resulting in insufficient concentrations to treat intracerebral infections [52,121]. In total, eight cases have been described in the literature with IT administration of daptomycin in the treatment of intracerebral infections due to Enterococci or Staphylococci (see Table 4) [122,123,124,125,126,127,128,129]. The IT doses administered ranged from 2.5 mg to 10 mg per dose, with the dosing frequency ranging from once per day to once per 72 h. The dose of 5 mg was used most frequently. For seven out of the eight cases, a positive outcome was reported. The patient in the eighth reported case relapsed and needed a second (extended) period of treatment before he was cured [125]. No serious side effects were reported in these eight cases.

Table 4.
Cases with IT daptomycin.
3. Discussion, Overall Conclusions and Recommendations
Intrathecal administration of the antibiotics described in this review is off-label and is limited to those patients for whom clinicians run out of therapeutic options. When used without administration/preparation errors, and with the exception of benzylpenicillin, the reported side effects are generally mild and reversible. As side effects have been reported after both IV and IT administration, and due to the lack of clear correlation between CSF concentrations and toxicity, it could be questioned whether the administration of extremely high IV doses puts patients at lower risk of side effects compared to IT administration. It is possible that the CSF concentration that is aimed for causes a toxic effect regardless of the route of administration.
With the current limited knowledge of PK/PD in the CSF, it is not possible to choose an evidence-based efficacious dosing regimen, and also, routine TDM is not recommended in the literature. Generally, for lipophilic drugs with a molecular weight > 1000 g/mol and hydrophylic drugs with a molecular weight > 400 g/mol, once-daily IT dosing is usually performed [8]. For antibiotics for which there is experience with multiple-dosing regimen, the highest regimen can be used in case of a high drainage volume, a pathogen with a relatively high ECOFF and/or a large distribution of the CSF. All available data are summarised in Table 5. Clamping of the drain is necessary to make sure that the administered antibiotic is distributed over the CSF space. The duration of clamping depends on the intracranial pressure and the tolerance of the patient.

Table 5.
Summary of antibiotics with molecular weight, protein binding, hydrophylic or lipophilic nature and overview of IT doses used in clinical cases.
4. Methods
A search was conducted in PubMed using the name of the individual antibiotic in combination with ‘intrathecal’ or ‘intraventricular’. References from the papers included were also checked to find missing references. The antibiotics selected were based on those mentioned in the review paper of Nau et al. [8] as antibiotics are usually not administered intrathecally. Included in the final search were the following: benzylpenicillin, ampicillin, piperacillin, cefuroxime, cefotaxime, ceftriaxone, ceftazidime, imipenem, meropenem, ertapenem, ciprofloxacin, levofloxacin, moxifloxacin, co-trimoxazole, linezolid, metronidazole, chloramphenicol, rifampicin, fosfomycin, doxycycline and tetracycline.
Author Contributions
Conceptualization, A.E.M.; methodology, A.E.M.; literature searches, A.E.M.; data extraction, A.E.M. and P.v.V.; interpretation data A.E.M. and P.v.V.; writing—original draft preparation, A.E.M.; writing—review and editing, A.E.M. and P.v.V. and B.C.P.K.; visualization, A.E.M. and B.C.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neuberger, A.; Shofty, B.; Bishop, B.; Naffaa, M.E.; Binawi, T.; Babich, T.; Rappaport, Z.H.; Zaaroor, M.; Sviri, G.; Yahav, D.; et al. Risk factors associated with death or neurological deterioration among patients with Gram-negative postneurosurgical meningitis. Clin. Microbiol. Infect. 2016, 22, 573.e1–573.e4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Shi, Y.; Cao, Y.; Qian, L.; Lv, H.; Zhang, L.; Zhang, G. Clinical Feature, Therapy, Antimicrobial Resistance Gene Distribution, and Outcome of Nosocomial Meningitis Induced by Multidrug-Resistant Enterobacteriaceae-A Longitudinal Cohort Study From Two Neurosurgical Centers in Northern China. Front. Cell. Infect. Microbiol. 2022, 12, 839257. [Google Scholar] [CrossRef]
- de Champs, C.; Guelon, D.; Joyon, D.; Sirot, D.; Chanal, M.; Sirot, J. Treatment of a meningitis due to an Enterobacter aerogenes producing a derepressed cephalosporinase and a Klebsiella pneumoniae producing an extended-spectrum beta-lactamase. Infection 1991, 19, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Kerz, T.; von Loewenich, F.D.; Roberts, J.; Neulen, A.; Ringel, F. Cerebrospinal fluid penetration of very high-dose meropenem: A case report. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 47. [Google Scholar] [CrossRef]
- Viladrich, P.F.; Cabellos, C.; Pallares, R.; Tubau, F.; Martinez-Lacasa, J.; Linares, J.; Gudiol, F. High doses of cefotaxime in treatment of adult meningitis due to Streptococcus pneumoniae with decreased susceptibilities to broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 1996, 40, 218–220. [Google Scholar] [CrossRef] [PubMed]
- FDA. Polymyxin B for Injection USP 500,000 Units Rx Only; Reference ID: 3078059; FDA: Silver Spring, MD, USA, 2011.
- European Medicines Agency. European Medicines Agency Completes Review of Polymyxin-Based Medicines. EMA/643444/2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/10/WC500176334.pdf (accessed on 25 July 2023).
- Nau, R.; Blei, C.; Eiffert, H. Intrathecal Antibacterial and Antifungal Therapies. Clin. Microbiol. Rev. 2020, 33, e00190–e00119. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, W.; Yao, D.; Dai, H. Intrathecal or intraventricular antimicrobial therapy for post-neurosurgical intracranial infection due to multidrug-resistant and extensively drug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2019, 54, 556–561. [Google Scholar] [CrossRef]
- Ciummo, F.; Srinivas, P.; Biedny, J. Antimicrobial use in central nervous system infections. Curr. Opin. Infect. Dis. 2021, 34, 255–263. [Google Scholar] [CrossRef]
- Lewin, J.J., 3rd; Cook, A.M.; Gonzales, C.; Merola, D.; Neyens, R.; Peppard, W.J.; Brophy, G.M.; Kurczewski, L.; Giarratano, M.; Makii, J.; et al. Current Practices of Intraventricular Antibiotic Therapy in the Treatment of Meningitis and Ventriculitis: Results from a Multicenter Retrospective Cohort Study. Neurocrit. Care 2019, 30, 609–616. [Google Scholar] [CrossRef]
- Hasbun, R. Healthcare-associated ventriculitis: Current and emerging diagnostic and treatment strategies. Expert Rev. Anti Infect. Ther. 2021, 19, 993–999. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Baziaka, F.; Giamarellou, H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: A literature review. Int. J. Antimicrob. Agents 2013, 41, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Nau, R.; Sorgel, F.; Eiffert, H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Mader, M.M.; Czorlich, P.; Konig, C.; Fuhrmann, V.; Kluge, S.; Westphal, M.; Grensemann, J. Intrathecal penetration of meropenem and vancomycin administered by continuous infusion in patients suffering from ventriculitis—A retrospective analysis. Acta Neurochir. 2018, 160, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Blassmann, U.; Roehr, A.C.; Frey, O.R.; Vetter-Kerkhoff, C.; Thon, N.; Hope, W.; Briegel, J.; Huge, V. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: A prospective observational study. Crit. Care 2016, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Beach, J.E.; Perrott, J.; Turgeon, R.D.; Ensom, M.H.H. Penetration of Vancomycin into the Cerebrospinal Fluid: A Systematic Review. Clin. Pharmacokinet. 2017, 56, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Albanese, J.; Leone, M.; Bruguerolle, B.; Ayem, M.L.; Lacarelle, B.; Martin, C. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob. Agents Chemother. 2000, 44, 1356–1358. [Google Scholar] [CrossRef]
- Lutsar, I.; McCracken, G.H., Jr.; Friedland, I.R. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin. Infect. Dis. 1998, 27, 1117–1129. [Google Scholar] [CrossRef]
- Lin Wu, F.L.; Liu, S.S.; Yang, T.Y.; Win, M.F.; Lin, S.W.; Huang, C.F.; Wang, K.C.; Shen, L.J. A Larger Dose of Vancomycin Is Required in Adult Neurosurgical Intensive Care Unit Patients Due to Augmented Clearance. Ther. Drug. Monit. 2015, 37, 609–618. [Google Scholar] [CrossRef]
- Odio, C.; Thomas, M.L.; McCracken, G.H., Jr. Pharmacokinetics and bacteriological efficacy of mezlocillin in experimental Escherichia coli and Listeria monocytogenes meningitis. Antimicrob. Agents Chemother. 1984, 25, 427–432. [Google Scholar] [CrossRef]
- McCracken, G.H., Jr.; Nelson, J.D.; Grimm, L. Pharmacokinetics and bacteriological efficacy of cefoperazone, ceftriaxone, and moxalactam in experimental Streptococcus pneumoniae and Haemophilus influenzae meningitis. Antimicrob. Agents Chemother. 1982, 21, 262–267. [Google Scholar] [CrossRef]
- Courchesne, E.; Chisum, H.J.; Townsend, J.; Cowles, A.; Covington, J.; Egaas, B.; Harwood, M.; Hinds, S.; Press, G.A. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000, 216, 672–682. [Google Scholar] [CrossRef]
- Fleischhack, G.; Jaehde, U.; Bode, U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin. Pharmacokinet. 2005, 44, 1–31. [Google Scholar] [CrossRef]
- Lebret, A.; Hodel, J.; Rahmouni, A.; Decq, P.; Petit, E. Cerebrospinal fluid volume analysis for hydrocephalus diagnosis and clinical research. Comput. Med. Imaging Graph. 2013, 37, 224–233. [Google Scholar] [CrossRef]
- Gerber, J.; Tumani, H.; Kolenda, H.; Nau, R. Lumbar and ventricular CSF protein, leukocytes, and lactate in suspected bacterial CNS infections. Neurology 1998, 51, 1710–1714. [Google Scholar] [CrossRef]
- Shapiro, W.R.; Young, D.F.; Mehta, B.M. Methotrexate: Distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N. Engl. J. Med. 1975, 293, 161–166. [Google Scholar] [CrossRef]
- Kaiser, A.B.; McGee, Z.A. Aminoglycoside therapy of gram-negative bacillary meningitis. N. Engl. J. Med. 1975, 293, 1215–1220. [Google Scholar] [CrossRef]
- Ichie, T.; Urano, K.; Suzuki, D.; Okada, T.; Kobayashi, N.; Hayashi, H.; Sugiura, Y.; Yamamura, K.; Sugiyama, T. Influence of cerebral fluid drainage on the pharmacokinetics of vancomycin in neurosurgical patients. Pharmazie 2015, 70, 404–409. [Google Scholar]
- Li, X.; Wang, X.; Wu, Y.; Sun, S.; Chen, K.; Lu, Y.; Wang, Q.; Zhao, Z. Plasma and cerebrospinal fluid population pharmacokinetic modeling and simulation of meropenem after intravenous and intrathecal administration in postoperative neurosurgical patients. Diagn. Microbiol. Infect. Dis. 2019, 93, 386–392. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Wang, Q.; Zhao, Z. Population Pharmacokinetics of Combined Intravenous and Local Intrathecal Administration of Meropenem in Aneurysm Patients with Suspected Intracranial Infections After Craniotomy. Eur. J. Drug. Metab. Pharmacokinet. 2018, 43, 45–53. [Google Scholar] [CrossRef]
- Nilsson, C.; Stahlberg, F.; Thomsen, C.; Henriksen, O.; Herning, M.; Owman, C. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am. J. Physiol. 1992, 262, R20–R24. [Google Scholar] [CrossRef]
- Klein, O.; Demoulin, B.; Jean Auque, R.T.; Audibert, G.; Sainte-Rose, C.; Marchal, J.C.; Marchal, F. Cerebrospinal fluid outflow and intracranial pressure in hydrocephalic patients with external ventricular drainage. Acta Neurol. Scand. 2010, 122, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.F.; Kaiser, A.B.; Bowman, C.M.; McKee, K.T., Jr.; Trujillo, H.; McGee, Z.A. The pharmacokinetics and efficacy of an aminoglycoside administered into the cerebral ventricles in neonates: Implications for further evaluation of this route of therapy in meningitis. J. Infect. Dis. 1981, 143, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ooie, T.; Suzuki, H.; Terasaki, T.; Sugiyama, Y. Kinetics of quinolone antibiotics in rats: Efflux from cerebrospinal fluid to the circulation. Pharm. Res. 1996, 13, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Reesor, C.; Chow, A.W.; Kureishi, A.; Jewesson, P.J. Kinetics of intraventricular vancomycin in infections of cerebrospinal fluid shunts. J. Infect. Dis. 1988, 158, 1142–1143. [Google Scholar] [CrossRef]
- Imberti, R.; Cusato, M.; Accetta, G.; Marino, V.; Procaccio, F.; Del Gaudio, A.; Iotti, G.A.; Regazzi, M. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob. Agents Chemother. 2012, 56, 4416–4421. [Google Scholar] [CrossRef]
- Norrby, S.R. Role of cephalosporins in the treatment of bacterial meningitis in adults. Overview with special emphasis on ceftazidime. Am. J. Med. 1985, 79, 56–61. [Google Scholar] [CrossRef]
- Jongmans, C.; Muller, A.E.; Van Den Broek, P.; Cruz De Almeida, B.M.; Van Den Berg, C.; Van Oldenrijk, J.; Bos, P.K.; Koch, B.C.P. An Overview of the Protein Binding of Cephalosporins in Human Body Fluids: A Systematic Review. Front. Pharmacol. 2022, 13, 900551. [Google Scholar] [CrossRef]
- Nau, R.; Prange, H.W.; Muth, P.; Mahr, G.; Menck, S.; Kolenda, H.; Sorgel, F. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob. Agents Chemother. 1993, 37, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Ewoldt, T.M.J.; Bahmany, S.; Abdulla, A.; Muller, A.E.; Endeman, H.; Koch, B.C.P. Plasma protein binding of ceftriaxone in critically ill patients: Can we predict unbound fractions? J. Antimicrob. Chemother. 2023, 78, 1059–1065. [Google Scholar] [CrossRef]
- Farrington, N.; McEntee, L.; Johnson, A.; Unsworth, J.; Darlow, C.; Jimenez-Valverde, A.; Hornik, C.; Greenberg, R.; Schwartz, J.; Das, S.; et al. Pharmacodynamics of Meropenem and Tobramycin for Neonatal Meningoencephalitis: Novel Approaches to Facilitate the Development of New Agents to Address the Challenge of Antimicrobial Resistance. Antimicrob. Agents Chemother. 2022, 66, e0218121. [Google Scholar] [CrossRef]
- Bakken, J.S.; Bruun, J.N.; Gaustad, P.; Tasker, T.C. Penetration of amoxicillin and potassium clavulanate into the cerebrospinal fluid of patients with inflamed meninges. Antimicrob. Agents Chemother. 1986, 30, 481–484. [Google Scholar] [CrossRef]
- Knoop, M.; Schutze, M.; Piek, J.; Drewelow, B.; Mundkowski, R. Antibiotic prophylaxis in cerebrospinal fluid shunting: Reassessment of Cefotiam penetration into human CSF. Zentralbl. Neurochir. 2007, 68, 14–18. [Google Scholar] [CrossRef]
- Nau, R.; Prange, H.W.; Kinzig, M.; Frank, A.; Dressel, A.; Scholz, P.; Kolenda, H.; Sorgel, F. Cerebrospinal fluid ceftazidime kinetics in patients with external ventriculostomies. Antimicrob. Agents Chemother. 1996, 40, 763–766. [Google Scholar] [CrossRef]
- Nau, R.; Lassek, C.; Kinzig-Schippers, M.; Thiel, A.; Prange, H.W.; Sorgel, F. Disposition and elimination of meropenem in cerebrospinal fluid of hydrocephalic patients with external ventriculostomy. Antimicrob. Agents Chemother. 1998, 42, 2012–2016. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Dong, H.; Zhu, Y.; Cao, P.; Meng, L.; Wang, Y. Population Pharmacokinetics and Dosing Regimen Optimization of Linezolid in Cerebrospinal Fluid and Plasma of Post-operative Neurosurgical Patients. J. Pharm. Sci. 2023, 112, 884–892. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Gotfried, M.H.; Cwik, M.; Korth-Bradley, J.M.; Dukart, G.; Ellis-Grosse, E.J. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 2006, 58, 1221–1229. [Google Scholar] [CrossRef]
- Nau, R.; Prange, H.W.; Menck, S.; Kolenda, H.; Visser, K.; Seydel, J.K. Penetration of rifampicin into the cerebrospinal fluid of adults with uninflamed meninges. J. Antimicrob. Chemother. 1992, 29, 719–724. [Google Scholar] [CrossRef]
- Scotton, P.G.; Pea, F.; Giobbia, M.; Baraldo, M.; Vaglia, A.; Furlanut, M. Cerebrospinal fluid penetration of levofloxacin in patients with spontaneous acute bacterial meningitis. Clin. Infect. Dis. 2001, 33, e109–e111. [Google Scholar] [CrossRef][Green Version]
- Yogev, R.; Kolling, W.M.; Williams, T. Pharmacokinetic comparison of intravenous and oral chloramphenicol in patients with Haemophilus influenzae meningitis. Pediatrics 1981, 67, 656–660. [Google Scholar] [CrossRef]
- Piva, S.; Di Paolo, A.; Galeotti, L.; Ceccherini, F.; Cordoni, F.; Signorini, L.; Togni, T.; De Nicolo, A.; Rasulo, F.A.; Fagoni, N.; et al. Daptomycin Plasma and CSF Levels in Patients with Healthcare-Associated Meningitis. Neurocrit. Care 2019, 31, 116–124. [Google Scholar] [CrossRef]
- Kullar, R.; Chin, J.N.; Edwards, D.J.; Parker, D.; Coplin, W.M.; Rybak, M.J. Pharmacokinetics of single-dose daptomycin in patients with suspected or confirmed neurological infections. Antimicrob. Agents Chemother. 2011, 55, 3505–3509. [Google Scholar] [CrossRef]
- Tauber, M.G.; Doroshow, C.A.; Hackbarth, C.J.; Rusnak, M.G.; Drake, T.A.; Sande, M.A. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J. Infect. Dis. 1984, 149, 568–574. [Google Scholar] [CrossRef]
- Nau, R.; Sorgel, F.; Prange, H.W. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin. Pharmacokinet. 1998, 35, 223–246. [Google Scholar] [CrossRef]
- Nau, R.; Schmidt, T.; Kaye, K.; Froula, J.L.; Tauber, M.G. Quinolone antibiotics in therapy of experimental pneumococcal meningitis in rabbits. Antimicrob. Agents Chemother. 1995, 39, 593–597. [Google Scholar] [CrossRef]
- Ahmed, A.; Jafri, H.; Lutsar, I.; McCoig, C.C.; Trujillo, M.; Wubbel, L.; Shelton, S.; McCracken, G.H., Jr. Pharmacodynamics of vancomycin for the treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob. Agents Chemother. 1999, 43, 876–881. [Google Scholar] [CrossRef][Green Version]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Remes, F.; Tomas, R.; Jindrak, V.; Vanis, V.; Setlik, M. Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J. Neurosurg. 2013, 119, 1596–1602. [Google Scholar] [CrossRef]
- Khan, S.A.; Waqas, M.; Siddiqui, U.T.; Shamim, M.S.; Nathani, K.R.; Jooma, R.; Mehmood, F. Intrathecal and intraventricular antibiotics for postoperative Gram-negative meningitis and ventriculitis. Surg. Neurol. Int. 2017, 8, 226. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef]
- EUCAST Steering Committee. MIC and Zone Diameter Distributions and ECOFFs. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 1 June 2023).
- Ng, K.; Mabasa, V.H.; Chow, I.; Ensom, M.H. Systematic review of efficacy, pharmacokinetics, and administration of intraventricular vancomycin in adults. Neurocrit. Care 2014, 20, 158–171. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, H.; Zhu, C.; Chen, F.; Sun, S.; Liang, N.; Zheng, W. Efficacy and safety of intrathecal meropenem and vancomycin in the treatment of postoperative intracranial infection in patients with severe traumatic brain injury. Exp. Ther. Med. 2019, 17, 4605–4609. [Google Scholar] [CrossRef]
- Brossner, G.; Engelhardt, K.; Beer, R.; Pfausler, B.; Georgopoulos, A.; Schmutzhard, E. Accidental intrathecal infusion of cefotiam: Clinical presentation and management. Eur. J. Clin. Pharmacol. 2004, 60, 373–375. [Google Scholar] [CrossRef]
- Mohammed, N.; Savardekar, A.R.; Patra, D.P.; Narayan, V.; Nanda, A. The 21st-century challenge to neurocritical care: The rise of the superbug Acinetobacter baumannii. A meta-analysis of the role of intrathecal or intraventricular antimicrobial therapy in reduction of mortality. Neurosurg. Focus 2017, 43, E8. [Google Scholar] [CrossRef]
- Gower, D.J.; Barrows, A.A., 3rd; Kelly, D.L., Jr.; Pegram, S., Jr. Gram-negative bacillary meningitis in the adult: Review of 39 cases. South. Med. J. 1986, 79, 1499–1502. [Google Scholar] [CrossRef]
- McCracken, G.H., Jr.; Mize, S.G.; Threlkeld, N. Intraventricular gentamicin therapy in gram-negative bacillary meningitis of infancy. Report of the Second Neonatal Meningitis Cooperative Study Group. Lancet 1980, 1, 787–791. [Google Scholar]
- Karvouniaris, M.; Brotis, A.G.; Tsiamalou, P.; Fountas, K.N. The Role of Intraventricular Antibiotics in the Treatment of Nosocomial Ventriculitis/Meningitis from Gram-Negative Pathogens: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 120, e637–e650. [Google Scholar] [CrossRef]
- Muller, A.E.; Huttner, B.; Huttner, A. Therapeutic Drug Monitoring of Beta-Lactams and Other Antibiotics in the Intensive Care Unit: Which Agents, Which Patients and Which Infections? Drugs 2018, 78, 439–451. [Google Scholar] [CrossRef]
- Brotis, A.G.; Churis, I.; Karvouniaris, M. Local complications of adjunct intrathecal antibiotics for nosocomial meningitis associated with gram-negative pathogens: A meta-analysis. Neurosurg. Rev. 2021, 44, 139–152. [Google Scholar] [CrossRef]
- Chusri, S.; Sakarunchai, I.; Kositpantawong, N.; Panthuwong, S.; Santimaleeworagun, W.; Pattharachayakul, S.; Singkhamanan, K.; Doi, Y. Outcomes of adjunctive therapy with intrathecal or intraventricular administration of colistin for post-neurosurgical meningitis and ventriculitis due to carbapenem-resistant acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 646–650. [Google Scholar] [CrossRef]
- Deshayes, S.; Coquerel, A.; Verdon, R. Neurological Adverse Effects Attributable to beta-Lactam Antibiotics: A Literature Review. Drug Saf. 2017, 40, 1171–1198. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Mayhall, C.G.; Archer, N.H.; Lamb, V.A.; Spadora, A.C.; Baggett, J.W.; Ward, J.D.; Narayan, R.K. Ventriculostomy-related infections. A prospective epidemiologic study. N. Engl. J. Med. 1984, 310, 553–559. [Google Scholar] [CrossRef]
- Anderson, K.F.; Ellis, F.G. Intrathecal chloramphenicol in staphylococcal meningitis resistant to penicillin and streptomycin. Br. Med. J. 1951, 2, 1067–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scapellato, P.G.; Ormazabal, C.; Scapellato, J.L.; Bottaro, E.G. Meningitis due to vancomycin-resistant Enterococcus faecium successfully treated with combined intravenous and intraventricular chloramphenicol. J. Clin. Microbiol. 2005, 43, 3578–3579. [Google Scholar] [CrossRef]
- Berning, S.E.; Cherry, T.A.; Iseman, M.D. Novel treatment of meningitis caused by multidrug-resistant Mycobacterium tuberculosis with intrathecal levofloxacin and amikacin: Case report. Clin. Infect. Dis. 2001, 32, 643–646. [Google Scholar] [CrossRef]
- Upton, A.; Woodhouse, A.; Vaughan, R.; Newton, S.; Ellis-Pegler, R. Evolution of central nervous system multidrug-resistant Mycobacterium tuberculosis and late relapse of cryptic prosthetic hip joint tuberculosis: Complications during treatment of disseminated isoniazid-resistant tuberculosis in an immunocompromised host. J. Clin. Microbiol. 2009, 47, 507–510. [Google Scholar] [CrossRef]
- Lich, B.F.; Conner, A.K.; Burks, J.D.; Glenn, C.A.; Sughrue, M.E. Intrathecal/Intraventricular Linezolid in Multidrug-Resistant Enterococcus faecalis Ventriculitis. J. Neurol. Surg. Rep. 2016, 77, e160–e161. [Google Scholar] [CrossRef]
- Dajez, P.; Vincken, W.; Lambelin, D.; Noterman, J.; Yourassowski, E.; Telerman-Toppet, N. Intraventricular administration of rifampin for tuberculous meningitis. J. Neurol. 1981, 225, 153–156. [Google Scholar] [CrossRef]
- Vincken, W.; Meysman, M.; Verbeelen, D.; Lauwers, S.; D’Haens, J. Intraventricular rifampicin in severe tuberculous meningo-encephalitis. Eur. Respir. J. 1992, 5, 891–893. [Google Scholar] [CrossRef]
- Senbaga, N.; Davies, E.M. Inadvertent intrathecal administration of rifampicin. Br. J. Clin. Pharmacol. 2005, 60, 116. [Google Scholar] [CrossRef]
- Spittle, C.R.; Phillips, B.M. A case of E. coli meningitis treated with systemic and intrathecal ampicillin. Postgrad. Med. J. 1962, 38, 168–171. [Google Scholar] [CrossRef][Green Version]
- Thilmann, A.F.; Mobius, E.; Podoll, K. Intraventricular antibiotic therapy. Nervenarzt 1992, 63, 108–112. [Google Scholar]
- Abraham, E.P.; Chain, E.; Fletcher, C.M.; Gardner, A.D.; Heatley, N.G.; Jennings, M.A. Further observations on penicillin. Lancet 1941, 238, 177–189. [Google Scholar] [CrossRef]
- Wood, F.C.; Dash, C. Intrathecal penicillin. Br. Med. J. 1978, 2, 1090. [Google Scholar] [CrossRef][Green Version]
- Jepson, R.P.; Whitty, C.W. Pheumococcal meningitis after head injury, treated with intrathecal penicillin. Lancet 1946, 1, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Erickson, T.C.; Masten, M.G.; Suckle, H.M. Complications of intrathecal use of penicillin. J. Am. Med. Assoc. 1946, 132, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Siegal, S. Transverse myelopathy following recovery from pneumococcic meningitis. J. Am. Med. Assoc. 1945, 129, 547. [Google Scholar] [CrossRef]
- Sweet, L.K. The treatment of pneumococcic meningitis with penicillin. J. Am. Med. Assoc. 1945, 127, 263. [Google Scholar] [CrossRef]
- Cohen, M.M. Fatality following the use of intrathecal penicillin; Case report. J. Neuropathol. Exp. Neurol. 1952, 11, 335–339. [Google Scholar] [CrossRef]
- Clara, N. CSF exchange after the erroneous intrathecal injection of 800 mg ceftriaxone for pneumococcal meningitis. J. Antimicrob. Chemother. 1986, 17, 263–265. [Google Scholar] [CrossRef]
- Fisher, L.S.; Chow, A.W.; Yoshikawa, T.T.; Guze, L.B. Cephalothin and cephaloridine therapy for bacterial meningitis. Ann. Intern. Med. 1975, 82, 689–693. [Google Scholar] [CrossRef]
- Konig, C.; Grensemann, J.; Czorlich, P.; Schlemm, E.; Kluge, S.; Wicha, S.G. A dosing nomograph for cerebrospinal fluid penetration of meropenem applied by continuous infusion in patients with nosocomial ventriculitis. Clin. Microbiol. Infect. 2022, 28, 1022.e9–1022.e16. [Google Scholar] [CrossRef] [PubMed]
- EUCAST Steering Committee. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 12.0. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 1 June 2023).
- Hosmann, A.; Ritscher, L.; Burgmann, H.; Al Jalali, V.; Wulkersdorfer, B.; Wolfl-Duchek, M.; Sanz Codina, M.; Jager, W.; Poschner, S.; Plochl, W.; et al. Meropenem concentrations in brain tissue of neurointensive care patients exceed CSF levels. J. Antimicrob. Chemother. 2021, 76, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Bain, K.T.; Wittbrodt, E.T. Linezolid for the treatment of resistant gram-positive cocci. Ann. Pharmacother. 2001, 35, 566–575. [Google Scholar] [CrossRef]
- Gill, C.J.; Murphy, M.A.; Hamer, D.H. Treatment of Staphylococcus epidermidis ventriculo-peritoneal shunt infection with linezolid. J. Infect. 2002, 45, 129–132. [Google Scholar] [CrossRef]
- FDA. Highlights of Prescribing Information-Tigecycline. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208744 (accessed on 2 December 2022).
- Ray, L.; Levasseur, K.; Nicolau, D.P.; Scheetz, M.H. Cerebral spinal fluid penetration of tigecycline in a patient with Acinetobacter baumannii cerebritis. Ann. Pharmacother. 2010, 44, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vardakas, K.Z.; Tsiveriotis, K.P.; Triarides, N.A.; Tansarli, G.S. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int. J. Antimicrob. Agents 2014, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Luo, X.; Li, X.; Li, Q.; Huo, J.; Yang, L.; Zhu, L.; Feng, W.; Zhou, J.; Shi, G.; et al. Development and validation of an LC-MS/MS method for the determination of tigecycline in human plasma and cerebrospinal fluid and its application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Q.; Zhan, R.C.; Jia, W.; Zhang, B.Q.; Wang, J.J. A case report of intraventricular tigecycline therapy for intracranial infection with extremely drug resistant Acinetobacter baumannii. Medicine 2017, 96, e7703. [Google Scholar] [CrossRef]
- Lauretti, L.; D’Alessandris, Q.G.; Fantoni, M.; D’Inzeo, T.; Fernandez, E.; Pallini, R.; Scoppettuolo, G. First reported case of intraventricular tigecycline for meningitis from extremely drug-resistant Acinetobacter baumannii. J. Neurosurg. 2017, 127, 370–373. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, Z.; Zhao, M. Case Report of Successful Treatment of Extensively Drug-Resistant Acinetobacter baumannii Ventriculitis with Intravenous plus Intraventricular Tigecycline. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Tsolaki, V.; Karvouniaris, M.; Manoulakas, E.; Kotlia, P.; Karadontas, V.; Fotakopoulos, G.; Zakynthinos, E.; Makris, D. Intraventricular CNS treatment with Colistin-Tigecycline combination: A case series. J. Crit. Care 2018, 47, 338–341. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.; Zhao, J.; Wang, Q.; Zhou, J. Intraventricular administration of tigecycline for the treatment of multidrug-resistant bacterial meningitis after craniotomy: A case report. J. Chemother. 2018, 30, 49–52. [Google Scholar] [CrossRef]
- Deng, Z.W.; Wang, J.; Qiu, C.F.; Yang, Y.; Shi, Z.H.; Zhou, J.L. A case report of intraventricular and intrathecal tigecycline infusions for an extensively drug-resistant intracranial Acinetobacter baumannii infection. Medicine 2019, 98, e15139. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Alsaleh, H.; Baradwan, A.; Alfawares, R.; Alobaid, A.; Rasheed, A.; Soliman, I. Intraventricular Tigecycline as a Last Resort Therapy in a Patient with Difficult-to-Treat Healthcare-Associated Acinetobacter baumannii Ventriculitis: A Case Report. SN Compr. Clin. Med. 2020, 2, 1683–1687. [Google Scholar] [CrossRef]
- Li, L.M.; Zheng, W.J.; Shi, S.W. Spinal arachnoiditis followed by intrathecal tigecycline therapy for central nervous system infection by extremely drug-resistant Acinetobacter baumannii. J. Int. Med. Res. 2020, 48, 300060520920405. [Google Scholar] [CrossRef] [PubMed]
- Soto-Hernandez, J.L.; Soto-Ramirez, A.; Perez-Neri, I.; Angeles-Morales, V.; Cardenas, G.; Barradas, V.A. Multidrug-resistant Klebsiella oxytoca ventriculitis, successfully treated with intraventricular tigecycline: A case report. Clin. Neurol. Neurosurg. 2020, 188, 105592. [Google Scholar] [CrossRef]
- Zhong, L.; Shi, X.Z.; Su, L.; Liu, Z.F. Sequential intraventricular injection of tigecycline and polymyxin B in the treatment of intracranial Acinetobacter baumannii infection after trauma: A case report and review of the literature. Mil. Med. Res. 2020, 7, 23. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wu, G.; Wang, H.; Xu, X. Intravenous plus intraventricular tigecycline-amikacin therapy for the treatment of carbapenem-resistant Klebsiella pneumoniae ventriculitis: A case report. Medicine 2022, 101, e29635. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Yu, X.; Wang, J.; Cheng, L.; Hu, S.; Han, G. Intrathecal injection of tigecycline in treatment of multidrug-resistant Acinetobacter baumannii meningitis: A case report. Eur. J. Hosp. Pharm. 2017, 24, 182–184. [Google Scholar] [CrossRef]
- Nicasio, A.M.; Crandon, J.L.; Nicolau, D.P. In vivo pharmacodynamic profile of tigecycline against phenotypically diverse Escherichia coli and Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2009, 53, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kang, H.S.; Sohn, C.H.; Oh, B.M. Cauda equina syndrome misdiagnosed as aggravated hydrocephalus: Neurological complication of intrathecal colistin in post-surgical meningitis. Acta Neurochir. 2011, 153, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Christ, W. Central nervous system toxicity of quinolones: Human and animal findings. J. Antimicrob. Chemother. 1990, 26 (Suppl. B), 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Kizu, J.; Kawamura, M. Effects of anti-inflammatory drugs on convulsant activity of quinolones: A comparative study of drug interaction between quinolones and anti-inflammatory drugs. J. Infect. Chemother. 2003, 9, 314–320. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Model List of Essential Medicines, 22nd List (WHO/MHP/HPS/EML/2021.02); World Health Organization: Geneva, Switzerland, 2021.
- Lee, B.J.; Vu, B.N.; Seddon, A.N.; Hodgson, H.A.; Wang, S.K. Treatment Considerations for CNS Infections Caused by Vancomycin-Resistant Enterococcus faecium: A Focused Review of Linezolid and Daptomycin. Ann. Pharmacother. 2020, 54, 1243–1251. [Google Scholar] [CrossRef]
- Denetclaw, T.H.; Suehiro, I.; Wang, P.K.; Tolliver, G.L. Successful treatment of ventriculostomy-associated meningitis caused by multidrug resistant coagulase-negative Staphylococcus epidermidis using low-volume intrathecal daptomycin and loading strategy. Ann. Pharmacother. 2014, 48, 1376–1379. [Google Scholar] [CrossRef]
- Dhariwal, A.; Leff, R.; Allen, M.; Cherian, B. Intrathecal daptomycin use in a challenging case of Enterococcus faecium ventriculitis. Access Microbiol. 2022, 4, 000230. [Google Scholar] [CrossRef]
- Dietz, N.; Barra, M.; Zhang, M.; Zacharaiah, M.; Coumans, J.V. Acute myeloid leukemia with central nervous system extension and subdural seeding of vancomycin-resistant Enterococcus faecium after bilateral subdural hematomas treated with subdural daptomycin administration. Surg. Neurol. Int. 2019, 10, 171. [Google Scholar] [CrossRef]
- Elvy, J.; Porter, D.; Brown, E. Treatment of external ventricular drain-associated ventriculitis caused by Enterococcus faecalis with intraventricular daptomycin. J. Antimicrob. Chemother. 2008, 61, 461–462. [Google Scholar] [CrossRef]
- Erritouni, M.; Ktaich, N.; Rahal, J.J.; Figueroa, D.; Nieto, J.; Urban, C.; Mariano, N.; Eisinger, F.; Abayev, J.; Nicolau, D.; et al. Use of daptomycin for the treatment of methicillin-resistant coagulase-negative staphylococcal ventriculitis. Case Rep. Med. 2012, 2012, 593578. [Google Scholar] [CrossRef]
- Kahler, R.; Holloway, K. Successful Use of Intrathecal Daptomycin to Treat Meningitis Due to Vancomycin-Resistant Enterococcus faecium. Infect. Dis. Clin. Pract. 2012, 20, 416–418. [Google Scholar] [CrossRef]
- Mueller, S.W.; Kiser, T.H.; Anderson, T.A.; Neumann, R.T. Intraventricular daptomycin and intravenous linezolid for the treatment of external ventricular-drain-associated ventriculitis due to vancomycin-resistant Enterococcus faecium. Ann. Pharmacother. 2012, 46, e35. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Muzevich, K.; Edmond, M.B.; Bearman, G.; Stevens, M.P. Central nervous system infections due to vancomycin-resistant enterococci: Case series and review of the literature. Int. J. Infect. Dis. 2014, 25, 26–31. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).