The Impact of Low-Level Benzalkonium Chloride Exposure on Staphylococcus spp. Strains and Control by Photoinactivation
Abstract
1. Introduction
2. Results
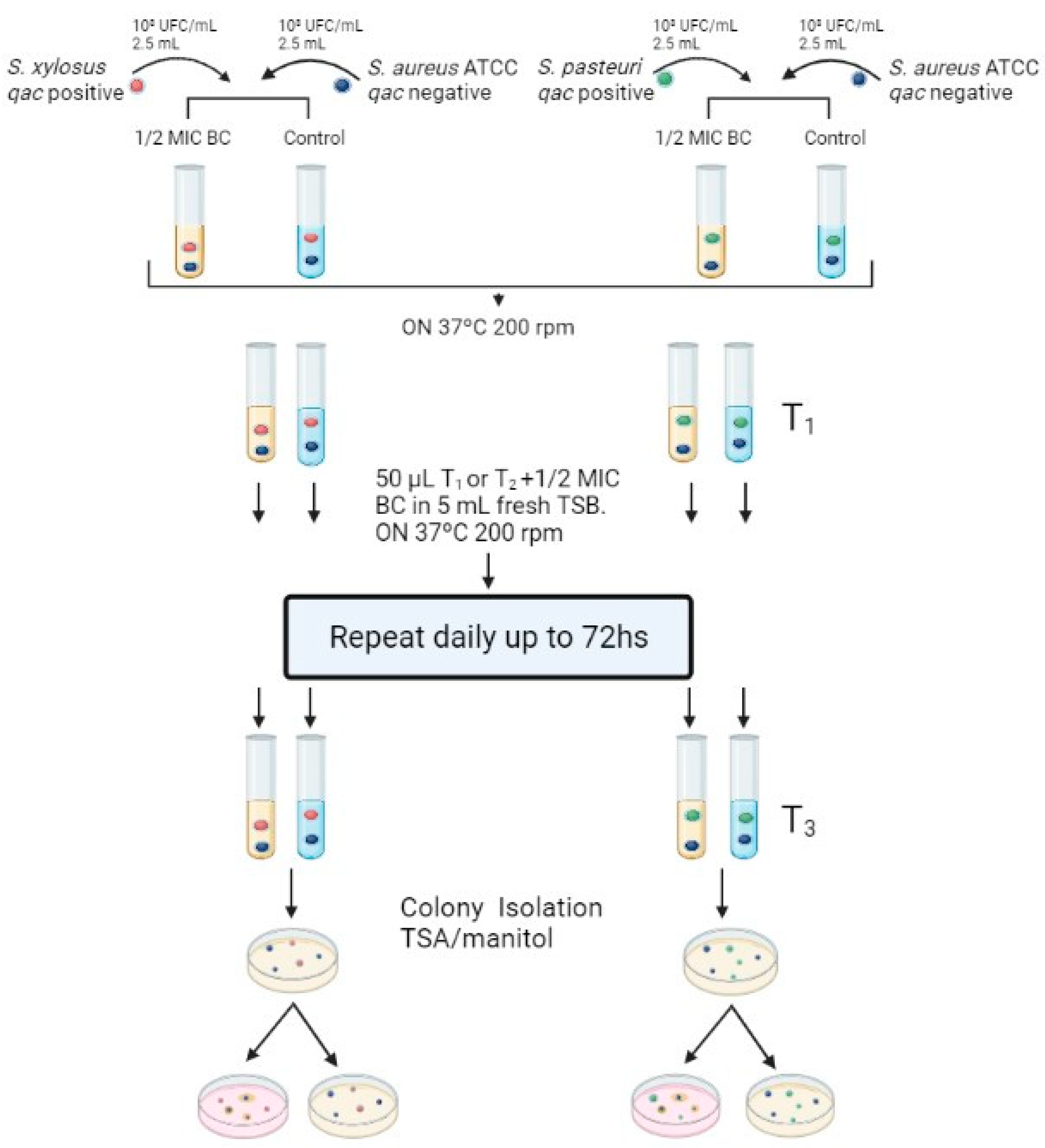
2.1. Exposure to Subinhibitory Concentration of Benzalkonium Chloride
2.2. Agar Diffusion and MIC of Benzalkonium Chloride
2.3. Photodynamic Inactivation (PDI) of Planktonic Cultures
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Determination of BC Minimum Inhibitory Concentration
4.3. Benzalkonium Chloride Exposure Assays
4.4. Antimicrobial Susceptibility Testing by Agar Disk Diffusion
4.5. Light Source
4.6. Photosensitizer
4.7. Photoinactivation of Planktonic Cultures
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Buck, J.; Ha, V.; Naushad, S.; Nobrega, D.B.; Luby, C.; Middleton, J.R.; De Vliegher, S.; Barkema, H.W. Non-Aureus Staphylococci and Bovine Udder Health: Current Understanding and Knowledge Gaps. Front. Vet. Sci. 2021, 8, 658031. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Buzón-Durán, L.; Riesco-Peláez, F.; Alonso-Calleja, C. Effect of Sub-Lethal Concentrations of Biocides on the Structural Parameters and Viability of the Biofilms Formed by Salmonella Typhimurium. Foodborne Pathog. Dis. 2017, 14, 350–356. [Google Scholar] [CrossRef]
- Klibi, A.; Maaroufi, A.; Torres, C.; Jouini, A. Detection and Characterization of Methicillin-Resistant and Susceptible Coagulase-Negative Staphylococci in Milk from Cows with Clinical Mastitis in Tunisia. Int. J. Antimicrob. Agents 2018, 52, 930–935. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Heir, E.; Sørum, H.; Holck, A. Genetic Linkage Between Resistance to Quaternary Ammonium Compounds and β -Lactam Antibiotics in Food-Related Staphylococcus spp. Microb. Drug Resist. 2001, 7, 363–371. [Google Scholar] [CrossRef]
- Paul, D.; Chakraborty, R.; Mandal, S.M. Biocides and Health-Care Agents Are More than Just Antibiotics: Inducing Cross to Co-Resistance in Microbes. Ecotoxicol. Environ. Saf. 2019, 174, 601–610. [Google Scholar] [CrossRef]
- Sellera, F.P.; Sabino, C.P.; Ribeiro, M.S.; Gargano, R.G.; Benites, N.R.; Melville, P.A.; Pogliani, F.C. In Vitro Photoinactivation of Bovine Mastitis Related Pathogens. Photodiagn. Photodyn. Ther. 2016, 13, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Ajose, D.J.; Oluwarinde, B.O.; Abolarinwa, T.O.; Fri, J.; Montso, K.P.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Combating Bovine Mastitis in the Dairy Sector in an Era of Antimicrobial Resistance: Ethno-Veterinary Medicinal Option as a Viable Alternative Approach. Front. Vet. Sci. 2022, 9, 800322. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G. A Critical Appraisal of Probiotics for Mastitis Control. Front. Vet. Sci. 2018, 5, 251. [Google Scholar] [CrossRef]
- Chan, M.K.L.; Koo, S.H.; Quek, Q.; Pang, W.S.; Jiang, B.; Ng, L.S.Y.; Tan, S.H.; Tan, T.Y. Development of a Real-Time Assay to Determine the Frequency of Qac Genes in Methicillin Resistant Staphylococcus Aureus. J. Microbiol. Methods 2018, 153, 133–138. [Google Scholar] [CrossRef]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the Wide Use of Quaternary Ammonium Compounds Enhance the Selection and Spread of Antimicrobial Resistance and Thus Threaten Our Health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E. Outbreaks Associated with Contaminated Antiseptics and Disinfectants. Antimicrob. Agents Chemother. 2007, 51, 4217–4224. [Google Scholar] [CrossRef]
- Dionisio, F.; Matic, I.; Radman, M.; Rodrigues, O.R.; Taddei, F. Plasmids Spread Very Fast in Heterogeneous Bacterial Communities. Genetics 2002, 162, 1525–1532. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic Tolerance Facilitates the Evolution of Resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Ning, X.; He, G.; Zeng, W.; Xia, Y. The Photosensitizer-Based Therapies Enhance the Repairing of Skin Wounds. Front. Med. 2022, 9, 915548. [Google Scholar] [CrossRef] [PubMed]
- Hoorijani, M.N.; Rostami, H.; Pourhajibagher, M.; Chiniforush, N.; Heidari, M.; Pourakbari, B.; Kazemian, H.; Davari, K.; Amini, V.; Raoofian, R.; et al. The Effect of Antimicrobial Photodynamic Therapy on the Expression of Novel Methicillin Resistance Markers Determined Using CDNA-AFLP Approach in Staphylococcus Aureus. Photodiagn. Photodyn. Ther. 2017, 19, 249–255. [Google Scholar] [CrossRef]
- Silva, L.O.; Da Silva Souza, K.L.; De Jesus Beloti, L.; Neto, W.M.R.; Núñez, S.C.; Frias, D.F.R. Use of Photodynamic Therapy and Photobiomodulation as Alternatives for Microbial Control on Clinical and Subclinical Mastitis in Sheep. Lasers Med. Sci. 2022, 37, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Schug, A.R.; Bartel, A.; Scholtzek, A.D.; Meurer, M.; Brombach, J.; Hensel, V.; Fanning, S.; Schwarz, S.; Feßler, A.T. Biocide Susceptibility Testing of Bacteria: Development of a Broth Microdilution Method. Vet. Microbiol. 2020, 248, 108791. [Google Scholar] [CrossRef] [PubMed]
- Soni, I.; Chakrapani, H.; Chopra, S. Draft Genome Sequence of Methicillin-Sensitive Staphylococcus Aureus ATCC 29213. Genome Announc. 2015, 3, e01095-15. [Google Scholar] [CrossRef]
- Abreu, A.C.D.S.; Crippa, B.L.; Souza, V.V.M.A.D.; Nuñez, K.V.M.; Almeida, J.M.D.; Rodrigues, M.X.; Silva, N.C.C. Assessment of Sanitiser Efficacy against Staphylococcus Spp. Isolated from Minas Frescal Cheese Producers in São Paulo, Brazil. Int. Dairy J. 2021, 123, 105171. [Google Scholar] [CrossRef]
- CLSI. 2019 Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI Standard VET01; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Karatzas, K.A.G.; Webber, M.A.; Jorgensen, F.; Woodward, M.J.; Piddock, L.J.V.; Humphrey, T.J. Prolonged Treatment of Salmonella Enterica Serovar Typhimurium with Commercial Disinfectants Selects for Multiple Antibiotic Resistance, Increased Efflux and Reduced Invasiveness. J. Antimicrob. Chemother. 2007, 60, 947–955. [Google Scholar] [CrossRef] [PubMed]
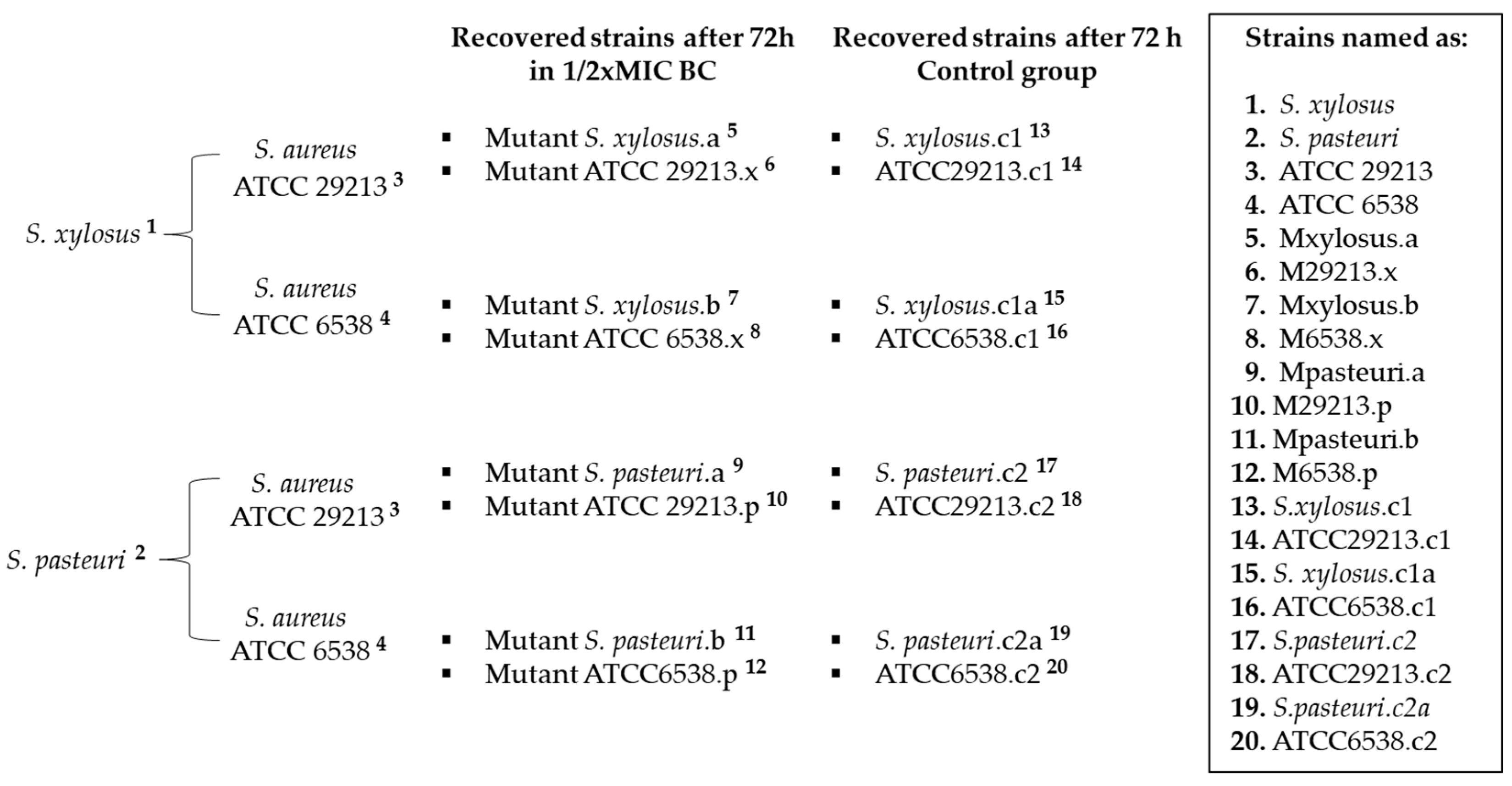

| Strains | MIC BC µg/mL | MIC Oxacilin µg/mL | Clindamicin | Cefalotin | Cefazolin | Penicillin |
|---|---|---|---|---|---|---|
| 1. S. xylosus | 7.8 | 6 | R | S | S | S |
| 5. Mxylosus.a | 7.8 | 6 | R | S | S | S |
| 7. Mxylosus.b | 7.8 | 6 | R | S | S | S |
| 2. S. pasteuri | 7.8 | 6 | R | S | S | S |
| 9. Mpasteuri.a | 15.7 | 6 | R | S | S | S |
| 11. Mpasteuri.b | 15.7 | 6 | R | S | S | S |
| 3. ATCC 29213 | 7.8 | 0.125 | S | S | S | S |
| 6. M29213.x | 7.8 | 0.125 | S | S | S | S |
| 10. M29213.p | 15.7 | 0.125 | S | S | S | S |
| 4. ATCC 6538 | 3.9 | 0.094 | S | S | S | S |
| 8. M6538.x | 7.8 | 0.094 | S | S | S | S |
| 12. M6538.p | 15.7 | 0.094 | R | R | R | R |
| Strain | CFU/mL | % Death | |
|---|---|---|---|
| No Light | Light | ||
| 1 | 5.5 × 107 | 5 × 101 | 99.99 |
| 7 | 5 × 107 | 0 | 100 |
| 2 | 4.55 × 107 | 0 | 100 |
| 11 | 3.8 × 107 | 0 | 100 |
| 3 | 1.55 × 107 | 1.1 × 103 | 99.99 |
| 10 | 2.5 × 107 | 4.8 × 103 | 99.98 |
| 4 | 1.25 × 107 | 3 × 104 | 99.76 |
| 12 | 6.25 × 106 | 1.75 × 102 | 99.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonsaglia, E.C.R.; Calvo, G.H.; Sordelli, D.O.; Silva, N.C.C.; Rall, V.L.M.; Casas, A.; Buzzola, F. The Impact of Low-Level Benzalkonium Chloride Exposure on Staphylococcus spp. Strains and Control by Photoinactivation. Antibiotics 2023, 12, 1244. https://doi.org/10.3390/antibiotics12081244
Bonsaglia ECR, Calvo GH, Sordelli DO, Silva NCC, Rall VLM, Casas A, Buzzola F. The Impact of Low-Level Benzalkonium Chloride Exposure on Staphylococcus spp. Strains and Control by Photoinactivation. Antibiotics. 2023; 12(8):1244. https://doi.org/10.3390/antibiotics12081244
Chicago/Turabian StyleBonsaglia, Erika C. R., Gustavo H. Calvo, Daniel O. Sordelli, Nathalia C. C. Silva, Vera L. M. Rall, Adriana Casas, and Fernanda Buzzola. 2023. "The Impact of Low-Level Benzalkonium Chloride Exposure on Staphylococcus spp. Strains and Control by Photoinactivation" Antibiotics 12, no. 8: 1244. https://doi.org/10.3390/antibiotics12081244
APA StyleBonsaglia, E. C. R., Calvo, G. H., Sordelli, D. O., Silva, N. C. C., Rall, V. L. M., Casas, A., & Buzzola, F. (2023). The Impact of Low-Level Benzalkonium Chloride Exposure on Staphylococcus spp. Strains and Control by Photoinactivation. Antibiotics, 12(8), 1244. https://doi.org/10.3390/antibiotics12081244





