Overcoming Drug Resistance in a Clinical C. albicans Strain Using Photoactivated Curcumin as an Adjuvant
Abstract
1. Introduction
2. Results
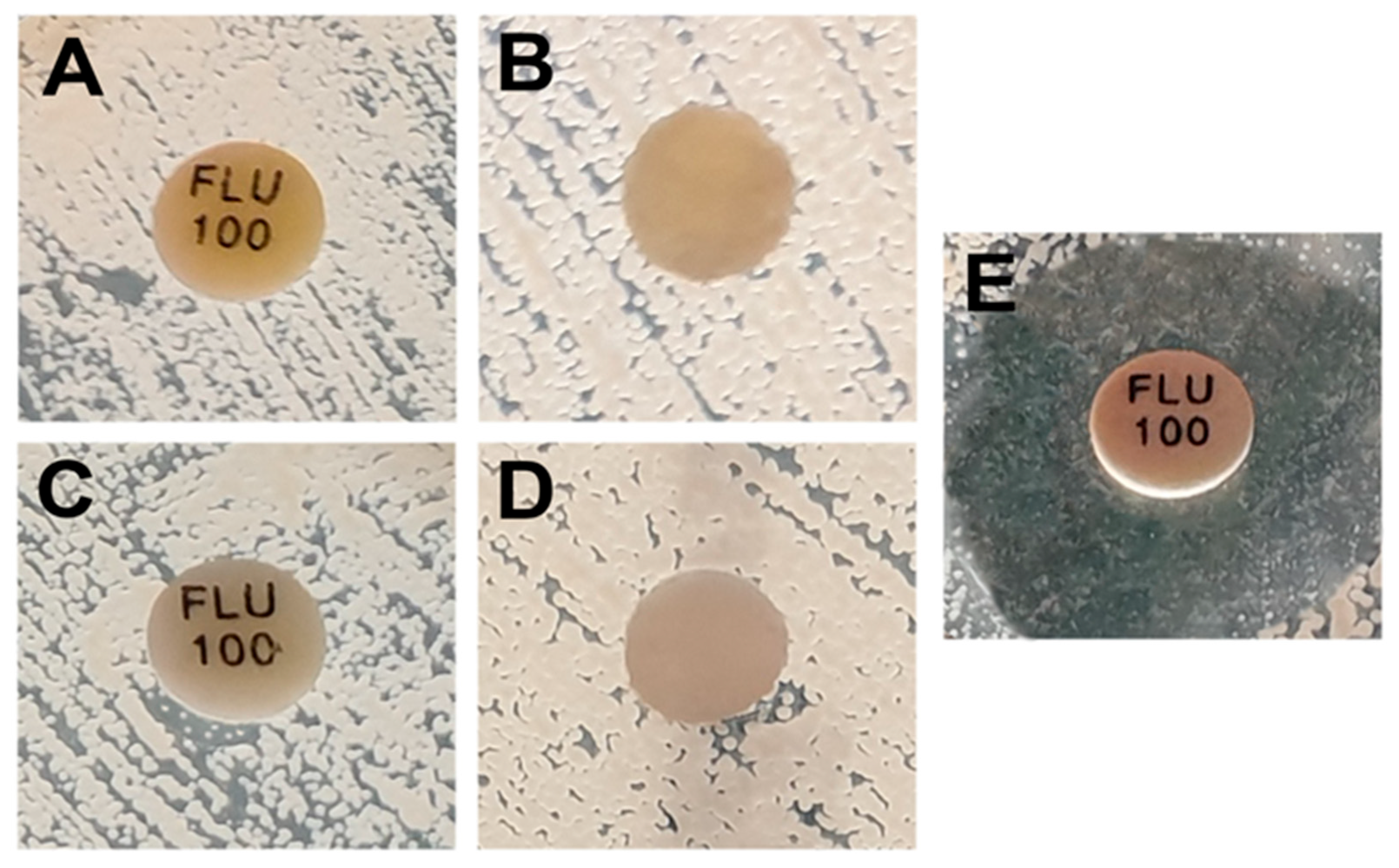
2.1. Determination of the Combined Effect of Fluconazole and Photo-Irradiated Curcumin Using the Difusimetric Method
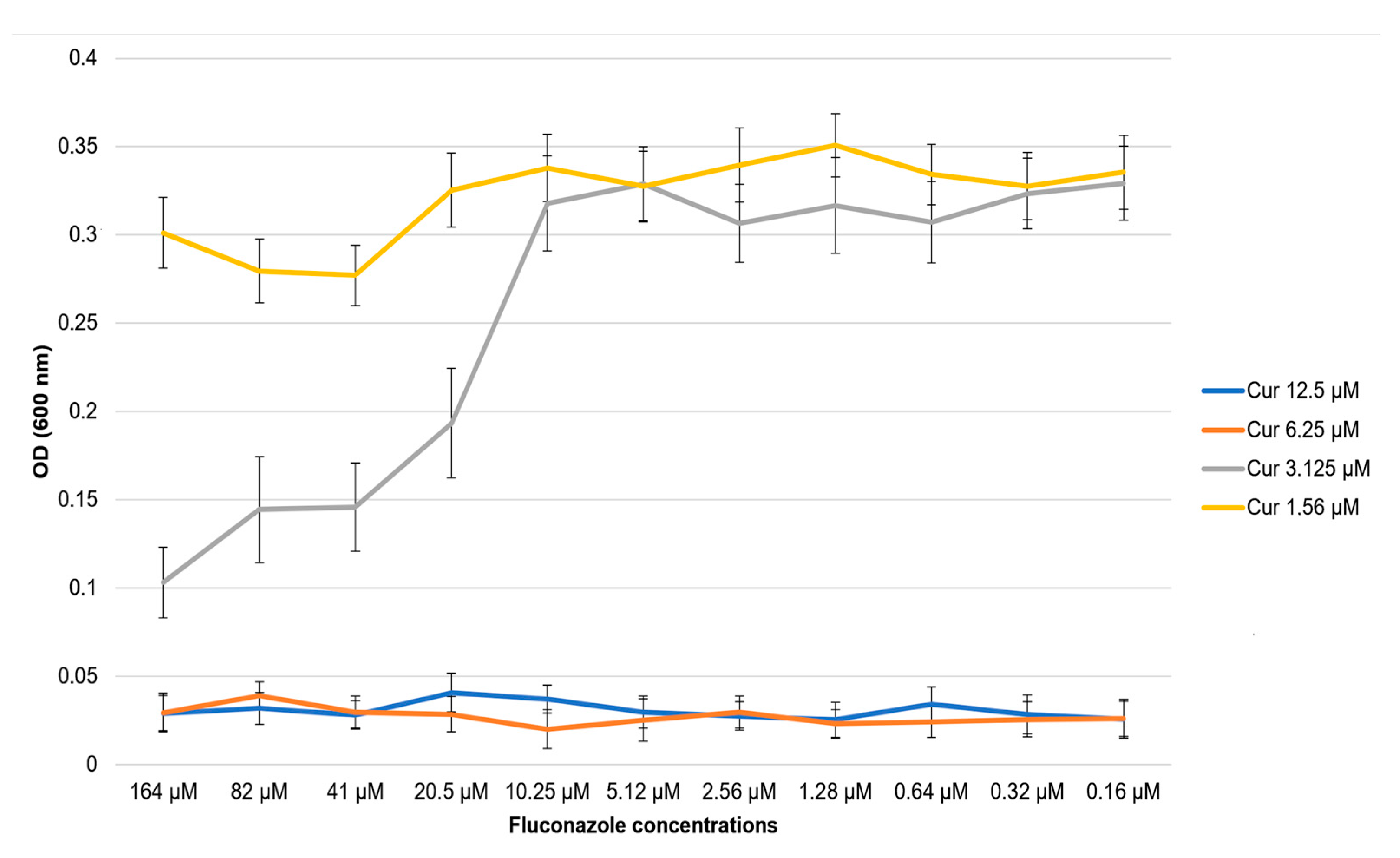
2.2. Determination of the Combined Effect of Fluconazole and Photo-Irradiated Curcumin Using the Microdilution Method
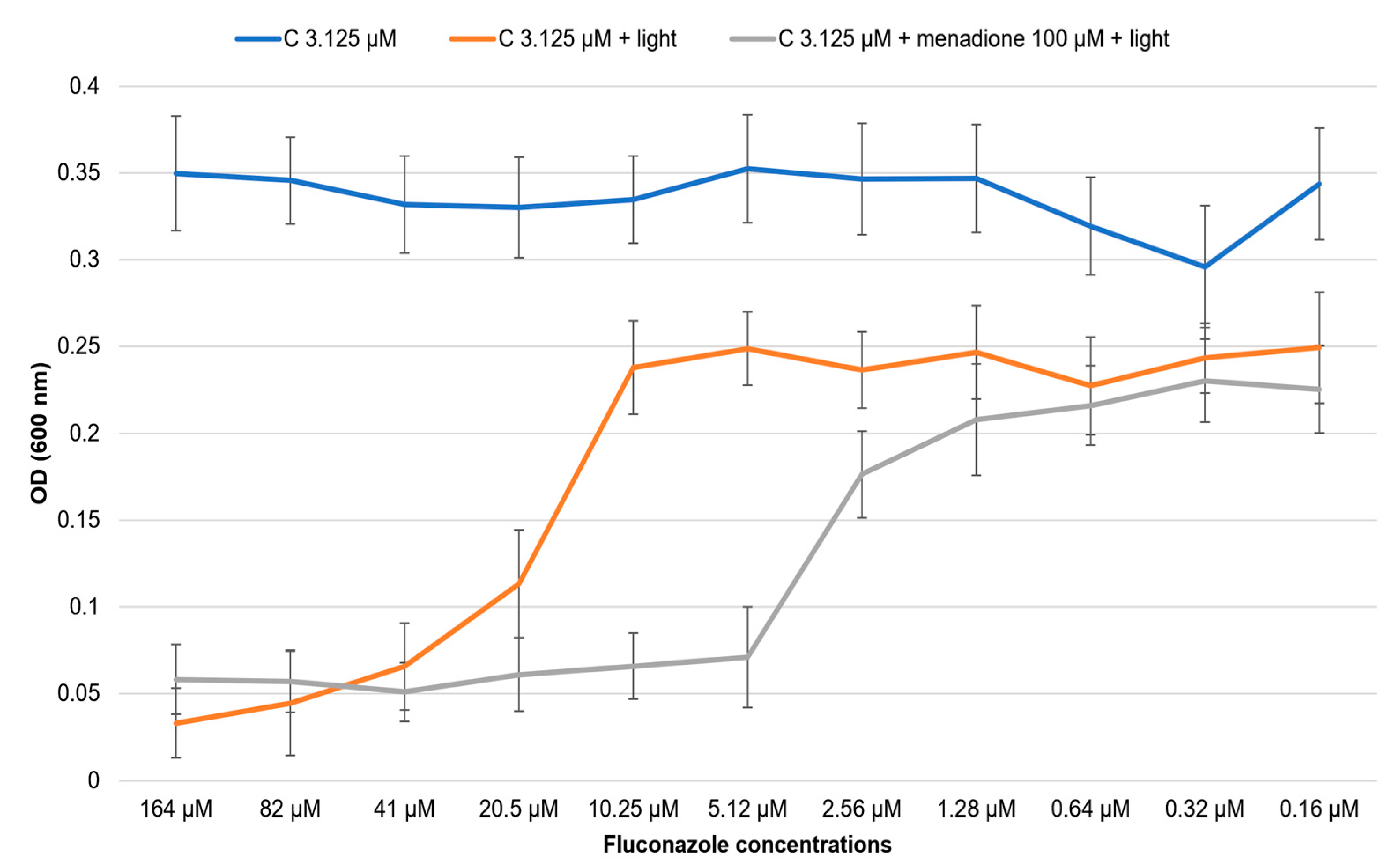
2.3. Influence of the Antioxidant/Prooxidant Addition on the Effect of the Co-Treatment of Fluconazole with Photo-Irradiate Curcumin
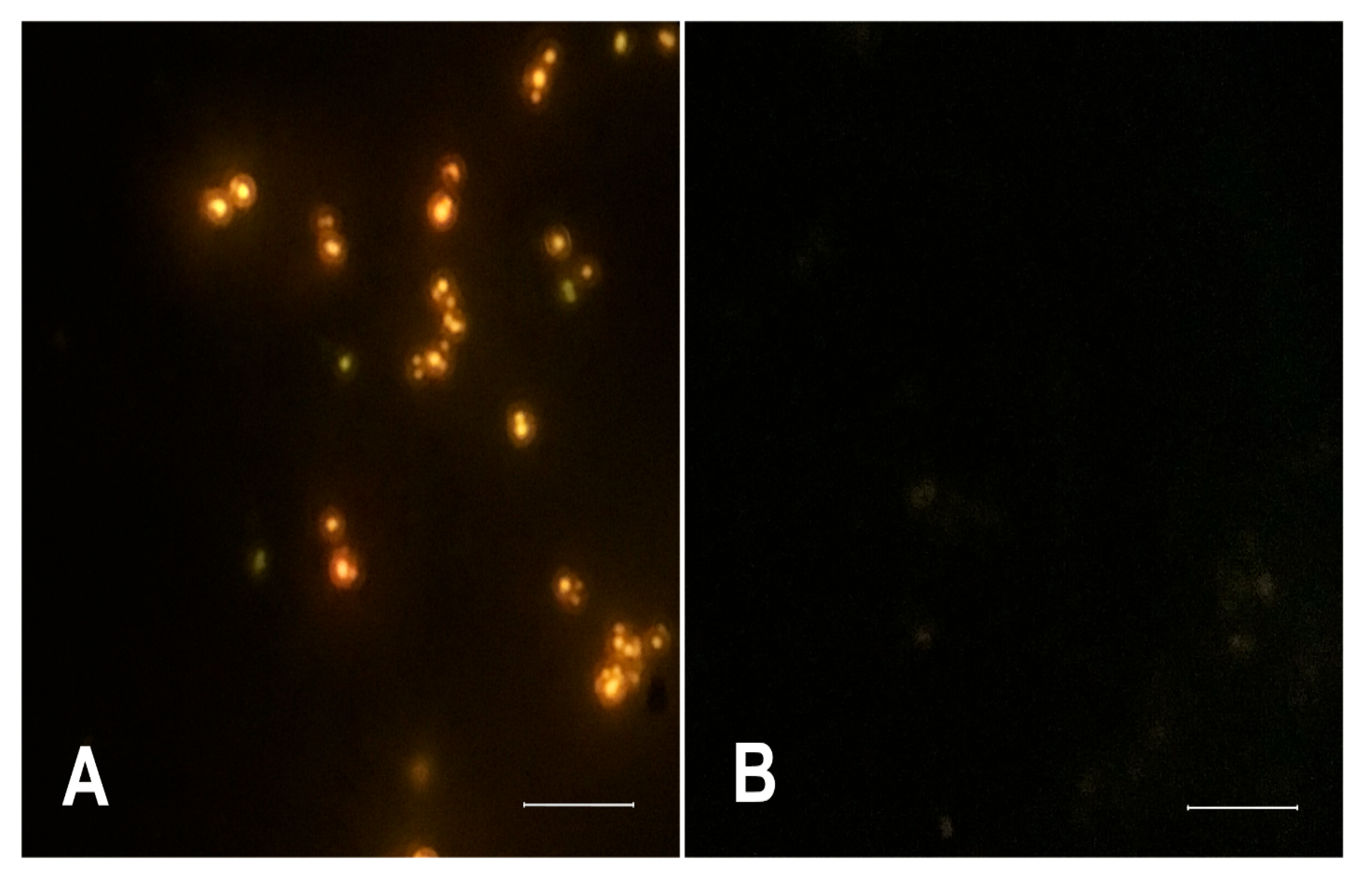
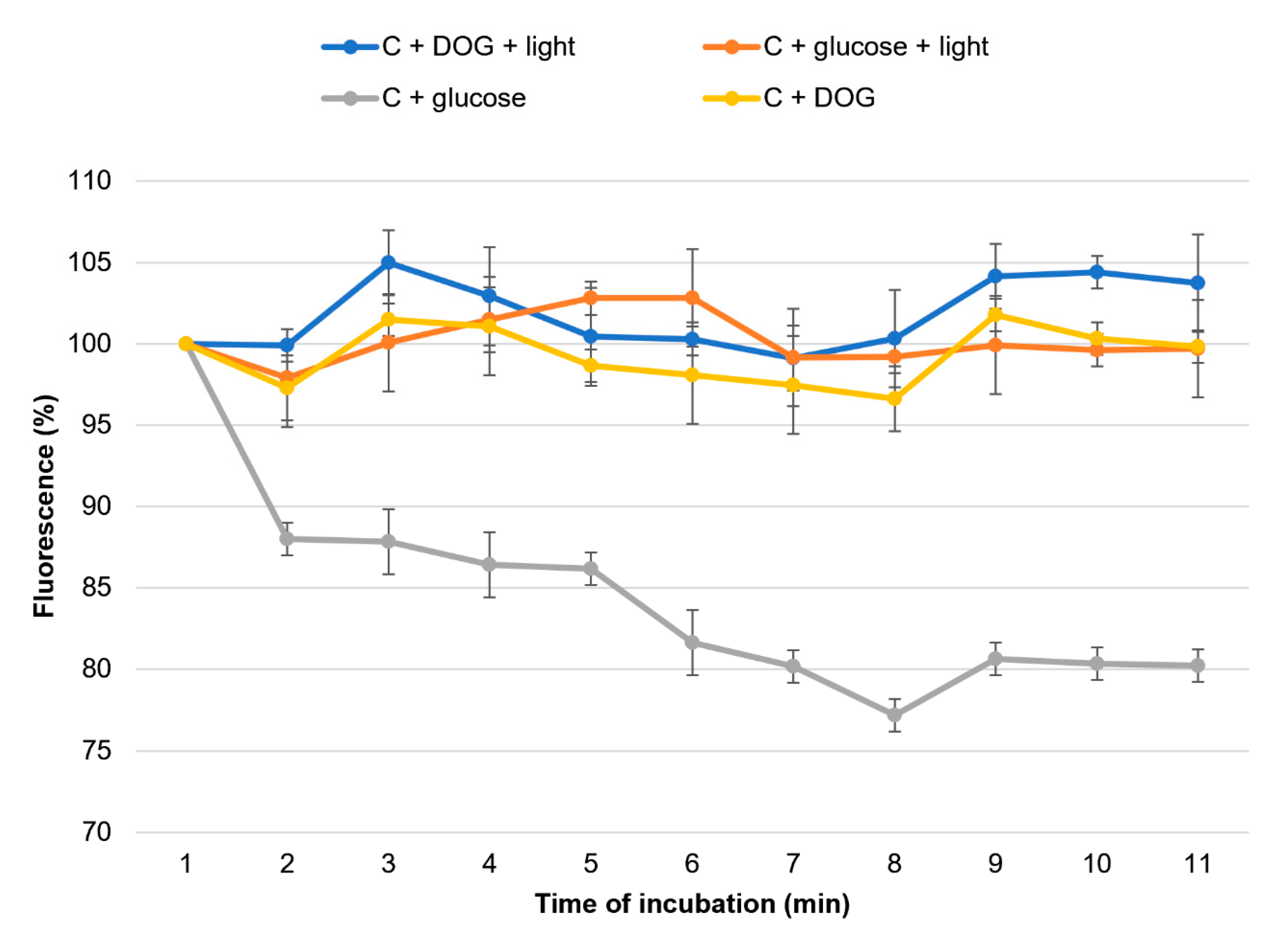
2.4. Fluorescence Microscopy Assay for Monitoring the Influence of Photo-Irradiated Curcumin on Nile Red Accumulation
2.5. Detection of Efflux Pumps Activity by Using Nile Red Efflux Assay
3. Discussion
4. Materials and Methods
4.1. Strain and Media
4.2. Reagents
4.3. Light Source for PDT
4.4. Determination of the Combined Effect of Fluconazole and Photo-Irradiated Curcumin Using the Difusimetric Method
4.5. Determination of the Combined Effect of Fluconazole and Photo-Irradiated Curcumin Using the Microdilution Method
4.6. Influence of the Antioxidant/Prooxidant Addition
4.7. Nile Red Accumulation Assay
4.8. Nile Red Efflux Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell 2020, 7, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Drummond, R.A.; Hohl, T.M. Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 2023, 23, 433–452. [Google Scholar] [CrossRef]
- Vanreppelen, G.; Wuyts, J.; Van Dijck, P.; Vandecruys, P. Sources of Antifungal Drugs. J. Fungi 2023, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Arathoon, E.G. Clinical efficacy of echinocandin antifungals. Curr. Opin. Infect. Dis. 2001, 14, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Preuss, C.V.; Amphotericin, B. StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482327/ (accessed on 24 June 2023).
- Matin, M.M.; Matin, P.; Rahman, R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Bio-Sci. 2022, 9, 864286. Available online: https://www.frontiersin.org/articles/10.3389/fmolb.2022.864286 (accessed on 24 June 2023).
- Chow, N.A.; Gade, L.; Tsay, S.V.; Forsberg, K.; Greenko, J.A.; Southwick, K.L.; Barrett, P.M.; Kerins, J.L.; Lockhart, S.R.; Chiller, T.M.; et al. Multiple introductions and subsequent transmis-sion of multidrug-resistant Candida auris in the USA: A molecular epidemiological survey. Lancet Infect. Dis. 2018, 18, 1377–1384. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Marichal, P.; Koymans, L.; Willemsens, S.; Bellens, D.; Verhasselt, P.; Luyten, W.; Borgers, M.; Ramaekers, F.C.S.; Odd, F.C.; Bossche, H.V. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 1999, 145 Pt 10, 2701–2713. [Google Scholar] [CrossRef]
- Sanglard, D.; Kuchler, K.; Ischer, F.; Pagani, J.L.; Monod, M.; Bille, J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 1995, 39, 2378–2386. [Google Scholar] [CrossRef]
- Perea, S.; López-Ribot, J.L.; Kirkpatrick, W.R.; McAtee, R.K.; Santillán, R.A.; Martínez, M.; Calabrese, D.; Sanglard, D.; Patterson, F. Prevalence of molecular mechanisms of resistance to azole antifungal agents in C. albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 2001, 45, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomes, A.S.; Curvelo, J.A.R.; Soares, R.A.; Ferreira-Pereira, A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of C. albicans showing a MDR phenotype. Med. Mycol. 2012, 50, 26–32. [Google Scholar] [CrossRef]
- Chen, X.; Ren, B.; Chen, M.; Liu, M.-X.; Ren, W.; Wang, Q.-X.; Zhang, L.-X.; Yan, G.-Y. ASDCD: Antifungal Synergistic Drug Combination Database. PLoS ONE 2014, 9, e86499. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.S. Turmeric—Chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Murugesh, J.; Annigeri, R.G.; Mangala, G.K.; Mythily, P.H.; Chandrakala, J. Evaluation of the antifungal efficacy of different con-centrations of Curcuma longa on Candida albicans: An in vitro study. J. Oral. Maxillofac. Pathol. 2019, 23, 305. [Google Scholar] [CrossRef]
- Shariati, A.; Didehdar, M.; Razavi, S.; Heidary, M.; Soroush, F.; Chegini, Z. Natural Compounds: A Hopeful Promise as an Antibi-ofilm Agent Against Candida Species. Front. Pharmacol. 2022, 13, 917787. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.d.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the Photodynamic Effects of Curcumin Against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Zhang, J.-H.; Chuang, W.-C.; Yu, K.-H.; Huang, X.-B.; Lee, Y.-C.; Lee, C.-I. An in Vitro Study on the Effect of Combined Treatment with Photodynamic and Chemical Therapies on Candida albicans. Int. J. Mol. Sci. 2018, 19, 337. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Estevam, M.L.; Nascimento, O.R.; Baptista, M.S.; Di Mascio, P.; Prado, F.M.; Faljoni-Alario, A.; Zucchi, M.D.R.; Nantes, I.L. Changes in the Spin State and Reactivity of Cytochrome c Induced by Photochemically Generated Singlet Oxygen and Free Radicals. J. Biol. Chem. 2004, 279, 39214–39222. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, V.; Kurachi, C.; Ferreira, J.; Marcassa, L.; Sibata, C.; Allison, R. PDT experience in Brazil: A regional profile. Photodiagnosis Photodyn. Ther. 2005, 2, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.; Venturini, M.; Sala, R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J. Photochem. Photobiol. B Biol. 2004, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Sharma, M.; Manoharlal, R.; Shukla, S.; Puri, N.; Prasad, T.; Ambudkar, S.V.; Prasad, R. Curcumin Modulates Efflux Mediated by Yeast ABC Multidrug Transporters and Is Synergistic with Antifungals. Antimicrob. Agents Chemother. 2009, 53, 3256–3265. [Google Scholar] [CrossRef]
- Solov’eva, M.E.; Solov’ev, V.V.; Faskhutdinova, A.A.; Kudryavtsev, A.A.; Akatov, V.S. Prooxidant and cytotoxic action of N-acetylcysteine and glutathione in combinations with vitamin B12b. Cell Tiss. Biol. 2007, 1, 40–49. [Google Scholar] [CrossRef]
- Miller, N.A.; Kaneshiro, A.K.; Konar, A.; Alonso-Mori, R.; Britz, A.; Deb, A.; Glownia, J.M.; Koralek, J.D.; Mallik, L.; Meadows, J.H.; et al. The Photoactive Excited State of the B12-Based Photoreceptor CarH. J. Phys. Chem. B 2020, 124, 10732–10738. [Google Scholar] [CrossRef]
- Ivnitski-Steele, I.; Holmes, A.R.; Lamping, E.; Monk, B.C.; Cannon, R.D.; Sklar, L.A. Identification of Nile Red as a fluorescent substrate of the C. albicans ABC transporters Cdr1p and Cdr2p and the MFS transporter Mdr1p. Anal. Biochem. 2009, 394, 87–91. [Google Scholar] [CrossRef]
- Toepfer, S.; Lackner, M.; Keniya, M.V.; Monk, B.C. Functional Expression of Recombinant Candida auris Proteins in Saccharomyces cerevisiae Enables Azole Susceptibility Evaluation and Drug Discovery. J. Fungi 2023, 9, 168. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Lyon, J.P.; Moreira, L.M.; de Moraes, P.C.G.; dos Santos, F.V.; de Resende, M.A. Photodynamic therapy for pathogenic fungi. Mycoses 2011, 54, e265–e271. [Google Scholar] [CrossRef] [PubMed]
- Munin, E.; Giroldo, L.M.; Alves, L.P.; Costa, M.S. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J. Photochem. Photobiol. B Biol. 2007, 88, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Giroldo, L.M.; Felipe, M.P.; de Oliveira, M.A.; Munin, E.; Alves, L.P.; Costa, M.S. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue in-creases membrane permeability in Candida albicans. Lasers Med. Sci. 2009, 24, 109–112. [Google Scholar] [CrossRef]
- Lyon, J.P.; Moreira, L.M.; Cardoso, M.A.G.; Saade, J.; Resende, M.A. Antifungal suscepitibility profile of candida spp. oral isolates obtained from denture wearers. Braz. J. Microbiol. 2008, 39, 668–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chabrier-Roselló, Y.; Foster, T.H.; Pérez-Nazario, N.; Mitra, S.; Haidaris, C.G. Sensitivity of C. albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob. Agents Chemother. 2005, 49, 4288–4295. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.M.; da Silva, D.L.; Sousa, G.R.; Amorim, J.C.F.; de Resende, M.A.; Pinotti, M.; Cisalpino, P.S. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J. Photochem. Photobiol. B Biol. 2009, 94, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; de Oliveira Mima, E.G.; Giampaolo, E.T.; Vergani, C.E.; Bagnato, V.S. Fungicidal effect of photodynamic therapy against fluconazole-resistant C. albicans and Candida glabrata. Mycoses 2011, 54, 123–130. [Google Scholar] [CrossRef]
- Martins, C.V.B.; da Silva, D.L.; Neres, A.T.M.; Magalhaes, T.F.F.; Watanabe, G.A.; Modolo, L.V.; Sabino, A.A.; de Fatima, A.; de Resende, M.A. Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 2008, 63, 337–339. [Google Scholar] [CrossRef]
- Sharma, M.; Manoharlal, R.; Puri, N.; Prasad, R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 2010, 30, 391–404. [Google Scholar] [CrossRef]
- Jianhua, W.; Hai, W. Antifungal susceptibility analysis of berberine, baicalin, eugenol and curcumin on Candida albicans. J. Med. Coll. PLA 2009, 24, 142–147. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; Machado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg. Med. 2011, 43, 927–934. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Miao, H.; Zhao, L.; Li, C.; Shang, Q.; Lu, H.; Fu, Z.; Cao, Y. Inhibitory effect of Shikonin on C. albicans growth. Biol. Pharm. Bull. 2012, 35, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Dbouk, N.H.; Covington, M.B.; Nguyen, K.; Chandrasekaran, S. Increase of reactive oxygen species contributes to growth inhibition by flucon-azole in Cryptococcus neoformans. BMC Microbiol. 2019, 19, 243. [Google Scholar] [CrossRef]
- Riggle, P.J.; Kumamoto, C.A. Transcriptional regulation of MDR1, encoding a drug efflux determinant, in fluconazole-resistant C. albicans strains through an Mcm1p binding site. Eukaryot. Cell 2006, 5, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.D.; Greenspan, P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: Comparison with oil red O. J. Histochem. Cytochem. 1985, 33, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Byrne, T.C.; Smith, K.L.; Hanson, K.E.; Anstrom, K.J.; Perfect, J.R.; Reller, L.B. Comparative Evaluation of Etest and Sensititre YeastOne Panels against the Clinical and Laboratory Standards Institute M27-A2 Reference Broth Microdilution Method for Testing Candida Susceptibility to Seven Antifungal Agents. J. Clin. Microbiol. 2007, 45, 698–706. [Google Scholar] [CrossRef]
- Keniya, M.V.; Fleischer, E.; Klinger, A.; Cannon, R.D.; Monk, B.C. Inhibitors of the C. albicans Major Facilitator Superfamily Transporter Mdr1p Re-sponsible for Fluconazole Resistance. PLoS ONE 2015, 10, e0126350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leferman, C.-E.; Stoica, L.; Tiglis, M.; Stoica, B.A.; Hancianu, M.; Ciubotaru, A.D.; Salaru, D.L.; Badescu, A.C.; Bogdanici, C.-M.; Ciureanu, I.-A.; et al. Overcoming Drug Resistance in a Clinical C. albicans Strain Using Photoactivated Curcumin as an Adjuvant. Antibiotics 2023, 12, 1230. https://doi.org/10.3390/antibiotics12081230
Leferman C-E, Stoica L, Tiglis M, Stoica BA, Hancianu M, Ciubotaru AD, Salaru DL, Badescu AC, Bogdanici C-M, Ciureanu I-A, et al. Overcoming Drug Resistance in a Clinical C. albicans Strain Using Photoactivated Curcumin as an Adjuvant. Antibiotics. 2023; 12(8):1230. https://doi.org/10.3390/antibiotics12081230
Chicago/Turabian StyleLeferman, Carmen-Ecaterina, Laura Stoica, Mirela Tiglis, Bogdan Alexandru Stoica, Monica Hancianu, Alin Dumitru Ciubotaru, Delia Lidia Salaru, Aida Corina Badescu, Camelia-Margareta Bogdanici, Ioan-Adrian Ciureanu, and et al. 2023. "Overcoming Drug Resistance in a Clinical C. albicans Strain Using Photoactivated Curcumin as an Adjuvant" Antibiotics 12, no. 8: 1230. https://doi.org/10.3390/antibiotics12081230
APA StyleLeferman, C.-E., Stoica, L., Tiglis, M., Stoica, B. A., Hancianu, M., Ciubotaru, A. D., Salaru, D. L., Badescu, A. C., Bogdanici, C.-M., Ciureanu, I.-A., & Ghiciuc, C.-M. (2023). Overcoming Drug Resistance in a Clinical C. albicans Strain Using Photoactivated Curcumin as an Adjuvant. Antibiotics, 12(8), 1230. https://doi.org/10.3390/antibiotics12081230






