Simulation of Vancomycin Exposure Using Trough and Peak Levels Achieves the Target Area under the Steady-State Concentration–Time Curve in ICU Patients
Abstract
1. Introduction
2. Methods
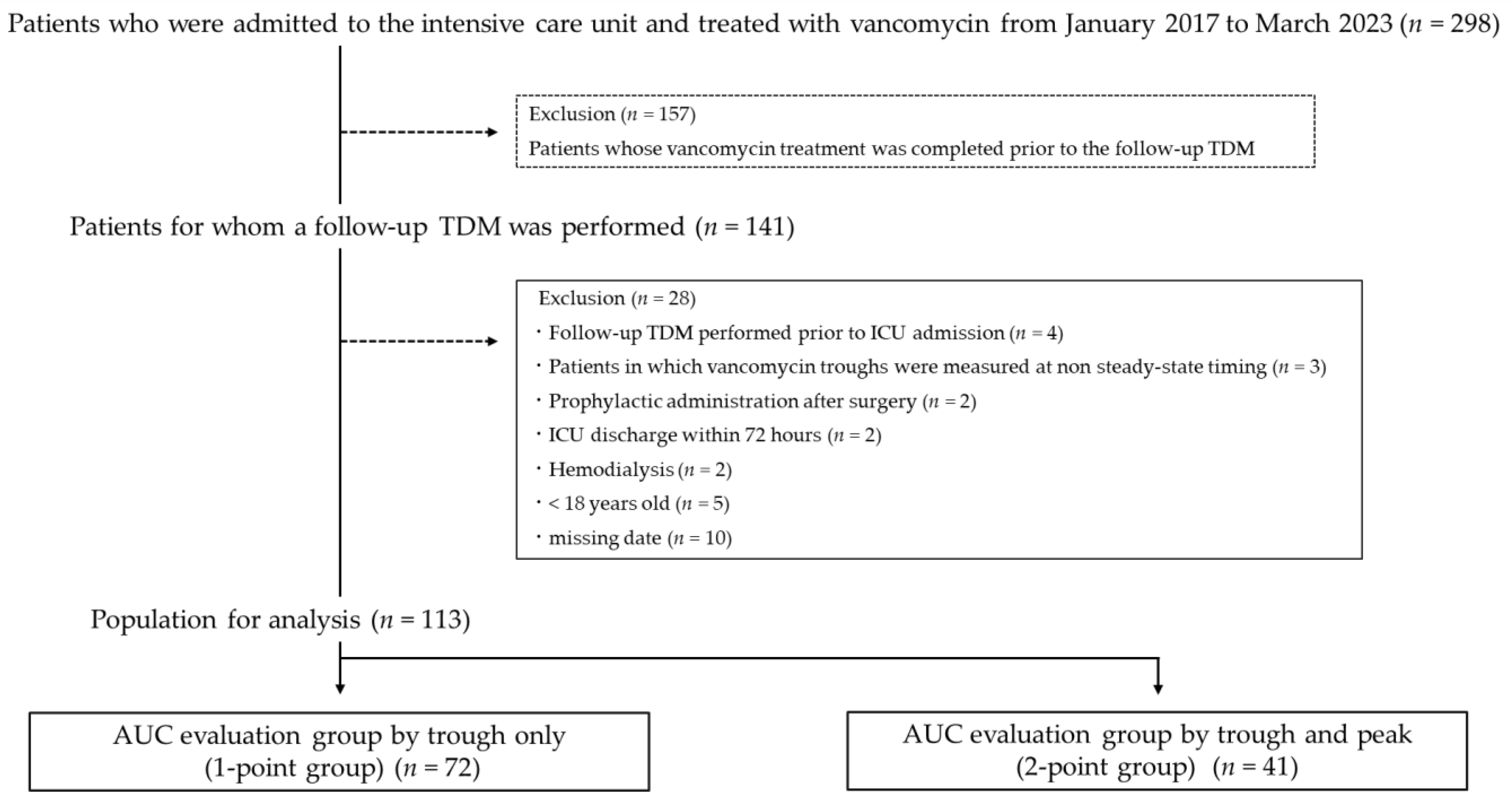
2.1. Study Design and Participants
2.2. VCM Dosing and Pharmacodynamics Data
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Participant Comparison
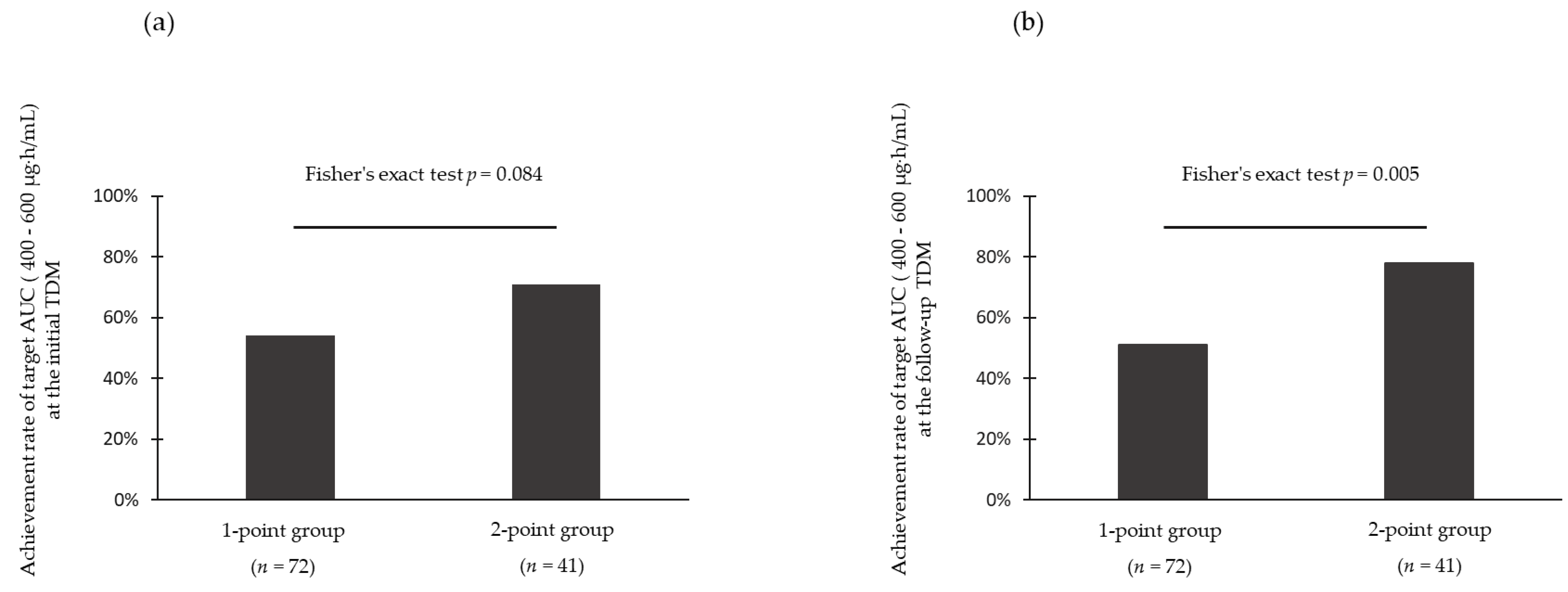
3.2. Achievement Rate of Target Area under the Curve
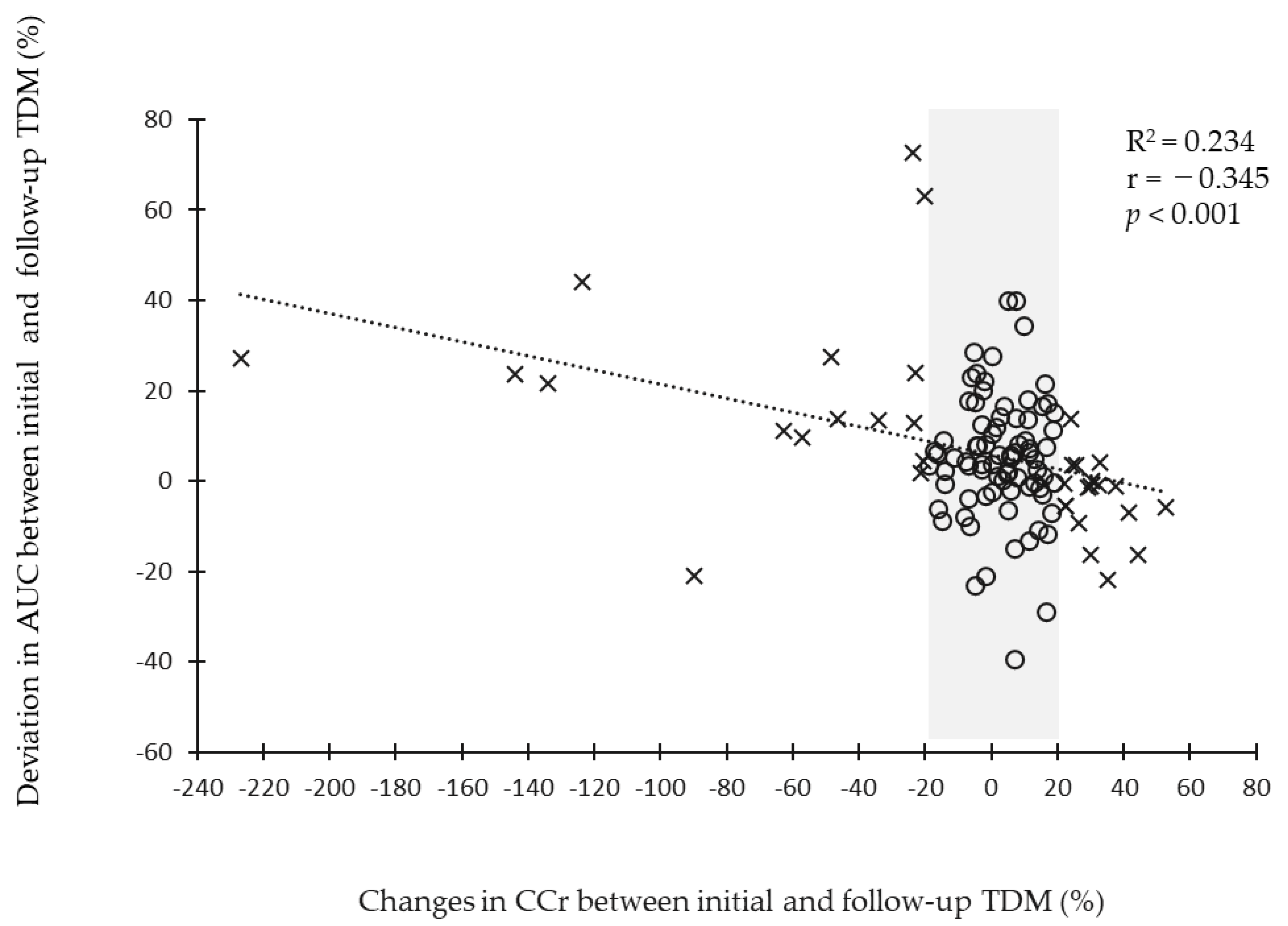
3.3. Relationship between Changes in Renal Function and Area under the Curve Deviation
3.4. Logistic Regression Analysis of Factors Associated with Achievement Rate of Target Area under the Curve at the Follow-Up Therapeutic Drug Monitoring
3.5. Characteristics of Patients with or without Changes in Creatinine Clearance
4. Discussion
5. Conclusions
6. Clinical Recommendations
- The use of two points for the AUC estimation of vancomycin, trough and peak concentrations, increases the accuracy of the estimation;
- For patients admitted to the ICU in particular, where inter-individual variability is high, AUC estimation using a two-point blood collection is necessary;
- Changes in renal function are predictors of AUC deviation, and caution should be exercised for changes in CCr with a 20% or more increase (or decrease).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Oda, K.; Shoji, K.; Hanai, Y.; Takahashi, Y.; Fujii, S.; Hamada, Y.; Kimura, T.; Mayumi, T.; Ueda, T.; et al. Clinical Practice Guidelines for Therapeutic Drug Monitoring of Vancomycin in the Framework of Model-Informed Precision Dosing: A Consensus Review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Pharmaceutics 2022, 14, 489. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Putt, M.T.; Boots, R.J.; Lipman, J. ARC—Augmented Renal Clearance. Curr. Pharm. Biotechnol. 2011, 12, 2020–2029. [Google Scholar] [CrossRef]
- Shimamoto, Y.; Fukuda, T.; Tanaka, K.; Komori, K.; Sadamitsu, D. Systemic inflammatory response syndrome criteria and vancomycin dose requirement in patients with sepsis. Intensive Care Med. 2013, 39, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Sousa, E.; Martins, P.J.; Pimentel, J.M. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int. J. Antimicrob. Agents 2012, 39, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Nunn, M.O.; Corallo, C.E.; Aubron, C.; Poole, S.; Dooley, M.J.; Cheng, A.C. Vancomycin Dosing: Assessment of Time to Therapeutic Concentration and Predictive Accuracy of Pharmacokinetic Modeling Software. Ann. Pharmacother. 2011, 45, 757–763. [Google Scholar] [CrossRef]
- Carter, B.L.; Damer, K.M.; Walroth, T.A.; Buening, N.R.; Foster, D.R.; Sood, R. A Systematic Review of Vancomycin Dosing and Monitoring in Burn Patients. J. Burn Care Res. 2015, 36, 641–650. [Google Scholar] [CrossRef]
- Monteiro, J.F.; Hahn, S.R.; Gonçalves, J.; Fresco, P. Vancomycin therapeutic drug monitoring and population pharmacokinetic models in special patient subpopulations. Pharmacol. Res. Perspect. 2018, 6, e00420. [Google Scholar] [CrossRef]
- Uchino, S. Acute Renal Failure in Critically Ill PatientsA Multinational, Multicenter Study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Hashimoto, N.; Kimura, T.; Hamada, Y.; Niwa, T.; Hanai, Y.; Chuma, M.; Fujii, S.; Matsumoto, K.; Shigemi, A.; Kawamura, H.; et al. Candidates for area under the concentration–time curve (AUC)-guided dosing and risk reduction based on analyses of risk factors associated with nephrotoxicity in vancomycin-treated patients. J. Glob. Antimicrob. Resist. 2021, 27, 12–19. [Google Scholar] [CrossRef]
- Lodise, T.P.; Patel, N.; Lomaestro, B.M.; Rodvold, K.A.; Drusano, G.L. Relationship between Initial Vancomycin Concentration-Time Profile and Nephrotoxicity among Hospitalized Patients. Clin. Infect. Dis. 2009, 49, 507–514. [Google Scholar] [CrossRef]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are Vancomycin Trough Concentrations Adequate for Optimal Dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef]
- Oda, K.; Hashiguchi, Y.; Kimura, T.; Tsuji, Y.; Shoji, K.; Takahashi, Y.; Matsumoto, K.; Kawamura, H.; Saito, H.; Takesue, Y. Performance of Area under the Concentration-Time Curve Estimations of Vancomycin with Limited Sampling by a Newly Developed Web Application. Pharm. Res. 2021, 38, 637–646. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; Deutschman, C.S.; Escobar, G.J.; Angus, D.C.; Iwashyna, T.J.; Brunkhorst, F.M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotscahfer, J.C.; Moellering, J.R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin Therapeutic Guidelines: A Summary of Consensus Recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Vandecasteele, S.J.; De Vriese, A.S. Recent changes in vancomycin use in renal failure. Kidney Int. 2010, 77, 760–764. [Google Scholar] [CrossRef]
- Zamoner, W.; Eid, K.Z.C.; de Almeida, L.M.B.; Pierri, I.G.; dos Santos, A.; Balbi, A.L.; Ponce, D. The Serum Concentration of Vancomycin as a Diagnostic Predictor of Nephrotoxic Acute Kidney Injury in Critically Ill Patients. Antibiotics 2022, 11, 112. [Google Scholar] [CrossRef]
- Carter, A.W.; Engoren, M. Factors associated with occurrence and severity of acute kidney injury in patients with Sepsis—A retrospective database study. J. Crit. Care 2022, 72, 154150. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Crass, R.L.; Rodvold, K.A.; Mueller, B.A.; Pai, M.P. Renal Dosing of Antibiotics: Are We Jumping the Gun? Clin. Infect. Dis. 2018, 68, 1596–1602. [Google Scholar] [CrossRef]
- Albanèse, J.; Leone, M.; Delmas, A.; Martin, C. Terlipressin or norepinephrine in hyperdynamic septic shock: A prospective, randomized study. Crit. Care Med. 2005, 33, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Schortgen, F.; Schetz, M. Does this critically ill patient with oliguria need more fluids, a vasopressor, or neither? Intensive Care Med. 2017, 43, 907–910. [Google Scholar] [CrossRef]
- Kiers, H.; Griesdale, D.E.; Litchfield, A.; Reynolds, S.; Gibney, R.; Chittock, D.; Pickkers, P.; Sweet, D.D. Effect of early achievement of physiologic resuscitation goals in septic patients admitted from the ward on the kidneys. J. Crit. Care 2010, 25, 563–569. [Google Scholar] [CrossRef]
- Turner, R.B.; Kojiro, K.; Shephard, E.A.; Won, R.; Chang, E.; Chan, D.; Elbarbry, F. Review and Validation of Bayesian Dose-Optimizing Software and Equations for Calculation of the Vancomycin Area Under the Curve in Critically Ill Patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 1174–1183. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Xi, X.; Dong, W.; Zhao, Z.; Chen, S. Development and validation of AKI prediction model in postoperative critically ill patients: A multicenter cohort study. Am. J. Transl. Res. 2022, 14, 5883–5895. [Google Scholar]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V.; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef]
- Yasuhara, M.; Iga, T.; Zenda, H.; Okumura, K.; Oguma, T.; Yano, Y.; Hori, R. Population Pharmacokinetics of Vancomycin in Japanese Adult Patients. Ther. Drug Monit. 1998, 20, 139–148. [Google Scholar] [CrossRef] [PubMed]
| All (n = 113) | 1-Point Group (n = 72) | 2-Point Group (n = 41) | p Value | |
|---|---|---|---|---|
| Age, years | 64.8 ± 13.0 | 64.5 ± 14.6 | 65.1 ± 9.7 | 0.724 |
| Female, n (%) | 33 (29.2%) | 19 (26.3%) | 14 (34.1%) | 0.383 |
| Height, cm | 162.7 ± 9.4 | 162.5 ± 9.8 | 163.0 ± 8.9 | 0.802 |
| Body weight, kg | 61.9 ± 13.2 | 62.3 ± 14.4 | 61.2 ± 11.0 | 0.749 |
| BMI, kg/m2 | 23.3 ± 4.1 | 23.4 ± 4.3 | 22.9 ± 3.8 | 0.772 |
| >25, kg/m2 | 30 (26.5%) | 18 (25.0%) | 12 (29.2%) | 0.621 |
| <18.5, kg/m2 | 10 (8.9%) | 5 (6.9%) | 5 (12.2%) | 0.345 |
| SOFA score, point | 7 (4, 11) | 7 (3, 9) | 9 (6, 11) | 0.037 |
| APACHE II score, point | 21 (15, 28) | 20 (12, 27) | 22 (17, 28) | 0.073 |
| Focus of infection, n (%) | 0.593 | |||
| Bacteremia | 37 (32.7%) | 26 (36.1%) | 11 (26.8%) | |
| Skin and soft tissue | 19 (16.8%) | 12 (16.6%) | 7 (17.1%) | |
| Abdomen | 19 (16.8%) | 9 (12.5%) | 10 (24.4%) | |
| Respiratory tract | 13 (11.5%) | 9 (12.5%) | 4 (9.8%) | |
| CRBSI | 8 (7.1%) | 4 (5.6%) | 4 (9.8%) | |
| Febrile neutropenia | 5 (4.4%) | 2 (2.8) | 3 (7.3) | |
| Device-related | 3 (2.7%) | 2 (2.8%) | 1 (2.4) | |
| Meningitis | 3 (2.7%) | 3 (4.2%) | 0 (0%) | |
| Bone | 2 (1.8%) | 2 (2.8%) | 0 (0%) | |
| Urinary tract | 1 (0.9%) | 1 (1.4%) | 0 (0%) | |
| Fever of unknown organ | 3 (2.7%) | 2 (2.8%) | 1 (2.4) | |
| Complications, n (%) | ||||
| Hypertension | 59 (52.2%) | 33 (45.8%) | 26 (63.4%) | 0.072 |
| Diabetes mellitus | 33 (29.2%) | 22 (30.6%) | 11 (26.8%) | 0.675 |
| Dyslipidemia | 24 (21.1%) | 16 (22.2%) | 8 (19.5%) | 0.735 |
| Laboratory data | ||||
| Albumin, g/dL | 2.2 (1.9, 2.6) | 2.1 (1.9, 2.6) | 2.2 (2.0, 2.5) | 0.788 |
| Creatinine, mg/dL | 0.8 (0.6, 1.2) | 0.7 (0.5, 1.1) | 0.8 (0.7, 1.2) | 0.068 |
| CCr at the vancomycin initiation, mL/min | 73.3 (44.8, 104.9) | 75.7 (53.7, 106.8) | 62.7 (41.9, 100.7) | 0.159 |
| CCr at the initial TDM, mL/min | 79.5 (51.1, 110.9) | 81.0 (56.6, 113.3) | 64.9 (40.5, 107.9) | 0.201 |
| CCr at the follow-up TDM, mL/min | 84.0 (45.3, 108.9) | 89.1 (51.8, 118.9) | 73.2 (42.8, 100.7) | 0.197 |
| % CCr, mL/min | 4.48 (−7.03, 15.46) | 4.62 (−6.42, 15.83) | 3.70 (−7.50, 15.46) | 0.905 |
| 20% or more increase, n (%) | 18 (16%) | 11 (15%) | 7 (17%) | 0.802 |
| 20% or more decrease, n (%) | 16 (14%) | 9 (13%) | 7 (17%) | 0.503 |
| eGFRcre, mL/min/1.73 m2 | 71.2 (43.9, 98.3) | 74.5 (47.5, 101.3) | 55.7 (40.5, 79.5) | 0.065 |
| eGFRcre < 60 mL/min/1.73 m2, n (%) | 44 (38.9%) | 28 (38.9%) | 16 (39.0%) | 0.989 |
| WBC, ×103/μL3 | 10.1 (6.4, 14.7) | 10.0 (6.4, 14.9) | 10.8 (6.3, 14.2) | 0.788 |
| CRP, mg/dL | 10.3 (5.1, 16.1) | 10.9 (5.3, 16.1) | 9.4 (4.3, 16.8) | 0.511 |
| BUN, mg/dL | 26 (17, 38) | 21 (16, 34) | 30 (24, 55) | 0.005 |
| BUN/Creatinine | 30 (21, 43) | 27 (21, 40) | 35 (26, 52) | 0.107 |
| Concomitant, n (%) | ||||
| Diuretics | 60 (53.1%) | 33 (45.8%) | 27 (65.9%) | 0.040 |
| Iodine-based contrast media | 57 (50.4%) | 37 (51.4%) | 20 (48.8%) | 0.789 |
| Catecholamine | 56 (49.6%) | 29 (40.3%) | 27 (65.9%) | 0.009 |
| NSAIDs | 40 (35.4%) | 33 (45.8%) | 7 (17.1%) | 0.002 |
| Tazobactam/Piperacillin | 21 (18.6%) | 15 (20.8%) | 6 (14.6%) | 0.415 |
| Trimethoprim/Sulfamethoxazole | 18 (15.9%) | 15 (20.8%) | 3 (7.3%) | 0.059 |
| Antibiotics | ||||
| Carbapenem | 37 (32.7%) | 28 (38.9%) | 9 (22.0%) | 0.065 |
| Cephalosporin | 17 (15.0%) | 12 (16.7) | 5 (12.1%) | 0.523 |
| Penicillin (excluding Tazobactam/Piperacillin) | 2 (1.8%) | 2 (2.8%) | 0 (0%) | 0.282 |
| Others | 1 (0.9%) | 1 (1.3%) | 0 (0%) | 0.449 |
| Vancomycin therapy | ||||
| Loading dose, n (%) | 30 (27%) | 11 (15.3%) | 19 (46.3%) | <0.001 |
| Maintenance dose to initial TDM, mg/kg/day | 13.9 (10.4, 16.5) | 14.2 (11.1, 16.7) | 12.6 (9.0, 16.2) | 0.215 |
| Maintenance dose after the initial TDM, mg/kg/day | 12.6 (9.5, 16.2) | 13.1 (9.9, 16.3) | 12.0 (8.4, 16.2) | 0.339 |
| Days to initial TDM, day | 2 (1, 2) | 2 (2, 3) | 1 (1, 2) | <0.001 |
| Days from initial TDM to follow-up TDM, day | 4 (4, 5) | 4 (4, 5) | 4 (3, 4) | <0.001 |
| Dosing interval for vancomycin to initial TDM, n (%) | 0.296 | |||
| 8 h | 4 (3.5%) | 2 (2.8%) | 2 (4.9%) | |
| 12 h | 84 (74.3%) | 57 (79.2%) | 27 (65.9%) | |
| 24 h | 25 (22.1%) | 13 (18.1%) | 12 (29.3%) | |
| Dosing interval for vancomycin after the initial TDM, n (%) | ||||
| 8 h | 8 (7.1%) | 8 (11.1%) | 0 (0%) | 0.072 |
| 12 h | 80 (70.8%) | 50 (69.4%) | 30 (7.1%) | |
| 24 h | 25 (22.1%) | 14 (19.4%) | 11 (26.9%) | |
| AUC parameter (μg·h/mL) | ||||
| AUC at the initial TDM | 486 (395, 544) | 497.1 (394.4, 578.9) | 452.0 (397.6, 510.9) | 0.031 |
| AUC at the follow-up TDM | 492 (426, 574) | 524.6 (428.1, 615.7) | 476.6 (420.2, 548.0) | 0.115 |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p Value | OR | (95% CI) | p Value | |
| Age; per 1-year increase | 1.00 | 0.97–1.03 | 0.848 | 1.00 | 0.97–1.04 | 0.901 |
| Sex; female | 1.23 | 0.54–2.80 | 0.626 | 0.99 | 0.35–2.83 | 0.984 |
| BMI; ≥25 kg/m2 | 0.54 | 0.23–1.25 | 0.150 | |||
| APACHE II score; per 1-point increase | 1.00 | 0.96–1.05 | 0.909 | |||
| SOFA score; per 1-point increase | 1.02 | 0.95–1.11 | 0.519 | |||
| Sepsis; yes | 0.71 | 0.33–1.53 | 0.385 | |||
| Burns; yes | 0.23 | 0.04–1.26 | 0.090 | |||
| Loading dose; yes | 2.11 | 0.84–5.28 | 0.112 | |||
| 2-point group; yes | 3.36 | 1.41–8.04 | 0.006 | 2.89 | 1.06–7.84 | 0.038 |
| 20% or more increase (or decrease) in CCr; yes | 0.37 | 0.16–0.84 | 0.017 | 0.25 | 0.09–0.67 | 0.006 |
| Days from initial TDM to follow-up TDM; >4 days | 0.33 | 0.15–0.75 | 0.008 | 0.44 | 0.18–1.09 | 0.075 |
| CRRT; yes | 0.55 | 0.23–1.29 | 0.169 | |||
| Catecholamine; yes | 1.13 | 0.53–2.40 | 0.756 | |||
| Diuretics; yes | 2.25 | 1.04–4.86 | 0.040 | 2.25 | 0.95–5.37 | 0.067 |
| Tazobactam/Piperacillin; yes | 1.78 | 0.63–4.95 | 0.284 | |||
| Without-Change Group (n = 79) | With-Change Group (n = 34) | p Value | |||
|---|---|---|---|---|---|
| Age, years | 64.6 ± 12.3 | 65.2 ± 14.6 | 0.932 | ||
| Female, n (%) | 17 (22%) | 16 (47%) | 0.006 | ||
| BMI, kg/m2 | 23.0 ± 3.9 | 23.9 ± 4.7 | 0.507 | ||
| SOFA score, point | 7 (3, 10) | 8 (6, 11) | 0.169 | ||
| APACHE II score, point | 20 (12, 25) | 22 (17, 28) | 0.144 | ||
| Sepsis, n (%) | 37 (47%) | 24 (71%) | 0.020 | ||
| Septic shock, n (%) | 28 (35%) | 19 (56%) | 0.043 | ||
| CRRT, n (%) | 15 (19%) | 13 (38%) | 0.035 | ||
| Complications, n (%) | |||||
| Hypertension | 42 (53%) | 17 (50%) | 0.757 | ||
| Diabetes mellitus | 23 (29%) | 10 (29%) | 0.975 | ||
| Dyslipidemia | 16 (20%) | 8 (24%) | 0.677 | ||
| Laboratory data | |||||
| Creatinine, mg/dL | 0.7 (0.6, 1.0) | 1.0 (0.6, 1.5) | 0.018 | ||
| CCr at the vancomycin initiation, mL/min | 76.4 (59.9, 108.3) | 49.6 (33.0, 87.5) | 0.015 | ||
| CCr at the initial TDM, mL/min | 82.3 (58.3, 122.5) | 58.7 (43.8, 92.4) | 0.024 | ||
| CCr at the follow-up TDM, mL/min | 87.8 (60.9, 122.8) | 61.2 (37.2, 98.6) | 0.032 | ||
| % CCr, mL/min | 3.70 (−4.76, 11.25) | 22.08 (−46.98, 30.24) | 0.621 | ||
| eGFRcre, mL/min/1.73 m2 | 76.8 (52.2, 101.4) | 53.3 (30.4, 72.9) | 0.004 | ||
| eGFRcre<60 mL/min/1.73 m2, n (%) | 27 (34%) | 17 (50%) | 0.114 | ||
| WBC, ×103/μL | 10.4 (6.8, 13.6) | 9.1 (5.8, 15.0) | 0.606 | ||
| CRP, mg/dL | 10.1 (5.0, 16.0) | 12.0 (5.9, 18.1) | 0.719 | ||
| BUN, mg/dL | 22 (16, 34) | 34 (22, 54) | 0.003 | ||
| BUN/Creatinine, mg/dL | 29 (20, 42) | 31 (23, 49) | 0.472 | ||
| Concomitant, n (%) | |||||
| Diuretics | 39 (49%) | 21 (62%) | 0.226 | ||
| Catecholamine | 34 (43%) | 22 (65%) | 0.035 | ||
| Vasopressin | 4 (5%) | 7 (21%) | 0.011 | ||
| Tazobactam/Piperacillin | 17 (22%) | 4 (12%) | 0.222 | ||
| Vancomycin therapy | |||||
| Loading dose, n (%) | 21 (27%) | 9 (26%) | 0.990 | ||
| Maintenance dose to initial TDM, mg/kg/day | 13.9 (10.2, 16.7) | 14.2 (10.6, 15.8) | 0.684 | ||
| Maintenance dose after initial TDM, mg/kg/day | 13.2 (9.1, 16.3) | 11.9 (10.3, 16.1) | 0.719 | ||
| Days to initial TDM, day | 2 (1, 2) | 2 (1, 3) | 0.906 | ||
| Days from initial TDM to follow-up TDM, day | 4 (4, 5) | 4 (4, 5) | 0.759 | ||
| AUC parameter (μg·h/mL) | |||||
| AUC at the initial TDM | 445.8 (378.1, 523.2) | 408.6 (330.3, 585.9) | 0.637 | ||
| AUC at the follow-up TDM | 495.8 (442.1, 561.9) | 464.5 (391.2, 616.3) | 0.712 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibe, Y.; Ishigo, T.; Fujii, S.; Takahashi, S.; Fukudo, M.; Sato, H. Simulation of Vancomycin Exposure Using Trough and Peak Levels Achieves the Target Area under the Steady-State Concentration–Time Curve in ICU Patients. Antibiotics 2023, 12, 1113. https://doi.org/10.3390/antibiotics12071113
Ibe Y, Ishigo T, Fujii S, Takahashi S, Fukudo M, Sato H. Simulation of Vancomycin Exposure Using Trough and Peak Levels Achieves the Target Area under the Steady-State Concentration–Time Curve in ICU Patients. Antibiotics. 2023; 12(7):1113. https://doi.org/10.3390/antibiotics12071113
Chicago/Turabian StyleIbe, Yuta, Tomoyuki Ishigo, Satoshi Fujii, Satoshi Takahashi, Masahide Fukudo, and Hideki Sato. 2023. "Simulation of Vancomycin Exposure Using Trough and Peak Levels Achieves the Target Area under the Steady-State Concentration–Time Curve in ICU Patients" Antibiotics 12, no. 7: 1113. https://doi.org/10.3390/antibiotics12071113
APA StyleIbe, Y., Ishigo, T., Fujii, S., Takahashi, S., Fukudo, M., & Sato, H. (2023). Simulation of Vancomycin Exposure Using Trough and Peak Levels Achieves the Target Area under the Steady-State Concentration–Time Curve in ICU Patients. Antibiotics, 12(7), 1113. https://doi.org/10.3390/antibiotics12071113






