Impact of Mt. Olympus Honeys on Virulence Factors Implicated in Pathogenesis Exerted by Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results and Discussion
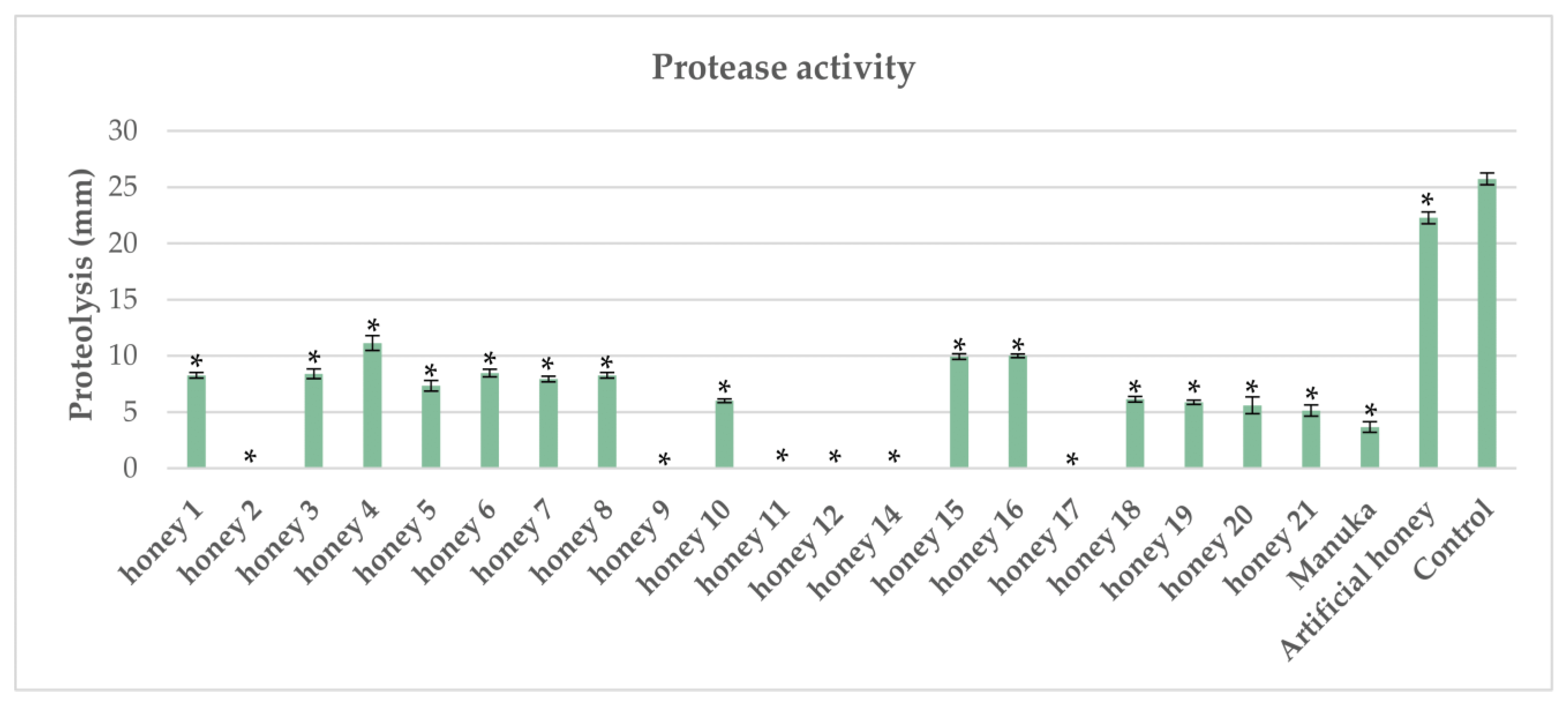
2.1. Impact of Honeys on Protease Activity in Pseudomonas aeruginosa
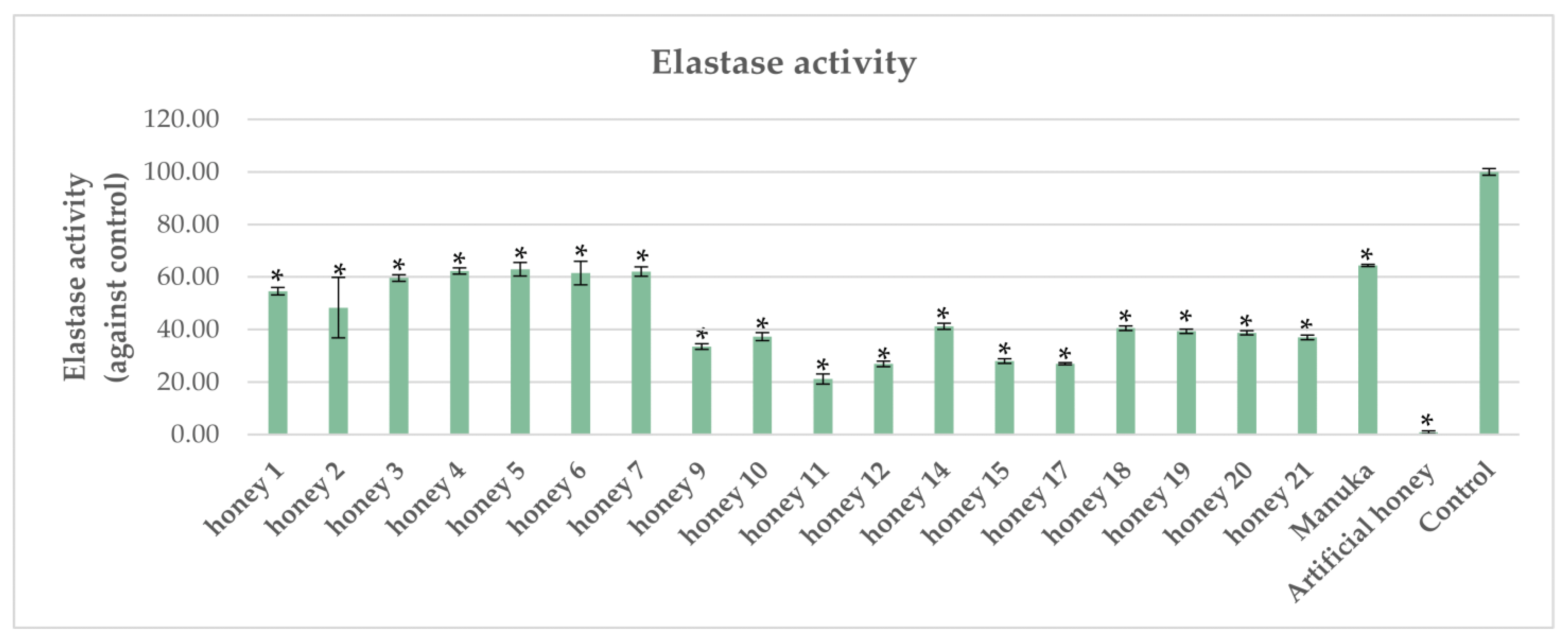
2.2. Impact of Honeys on Elastase Activity in P. aeruginosa
2.3. Impact of Honeys on Pyoverdine Production in P. aeruginosa
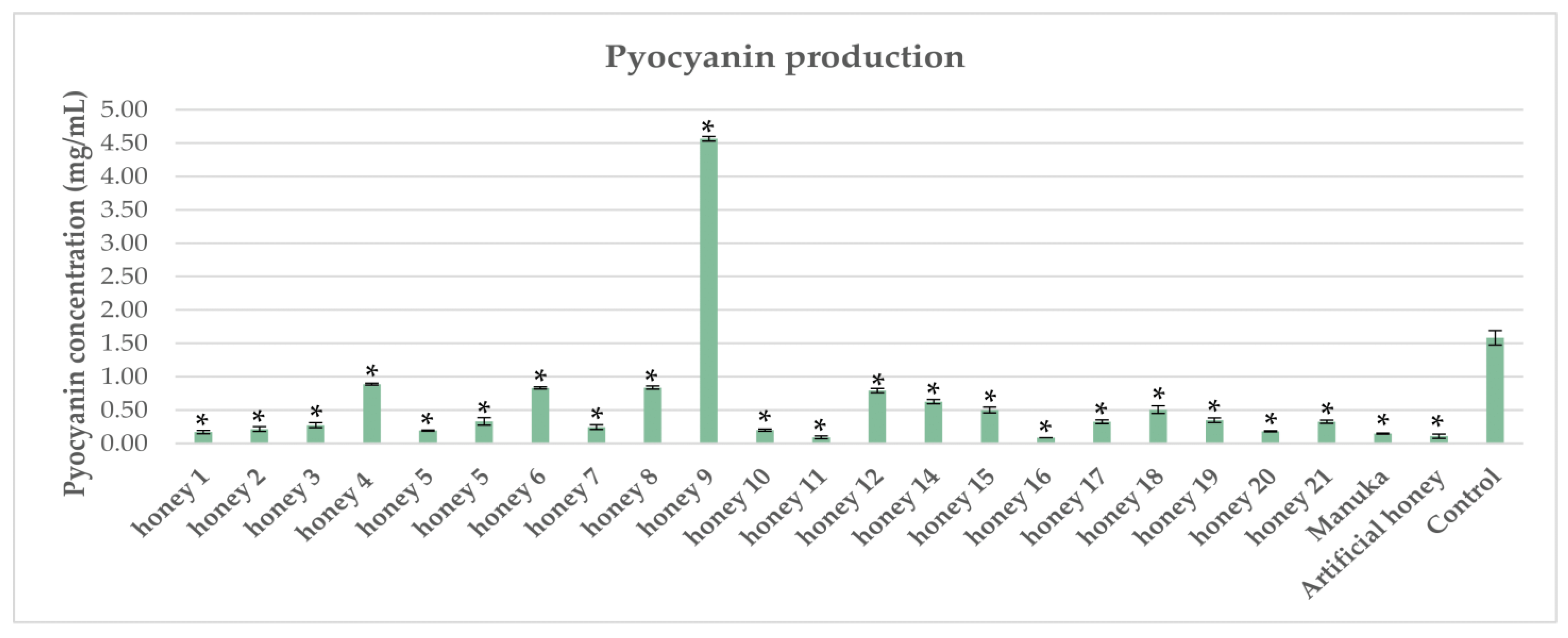
2.4. Impact of Honeys on Pyocyanin Production in P. aeruginosa
2.5. Impact of Honeys on Swimming Motility of P. aeruginosa
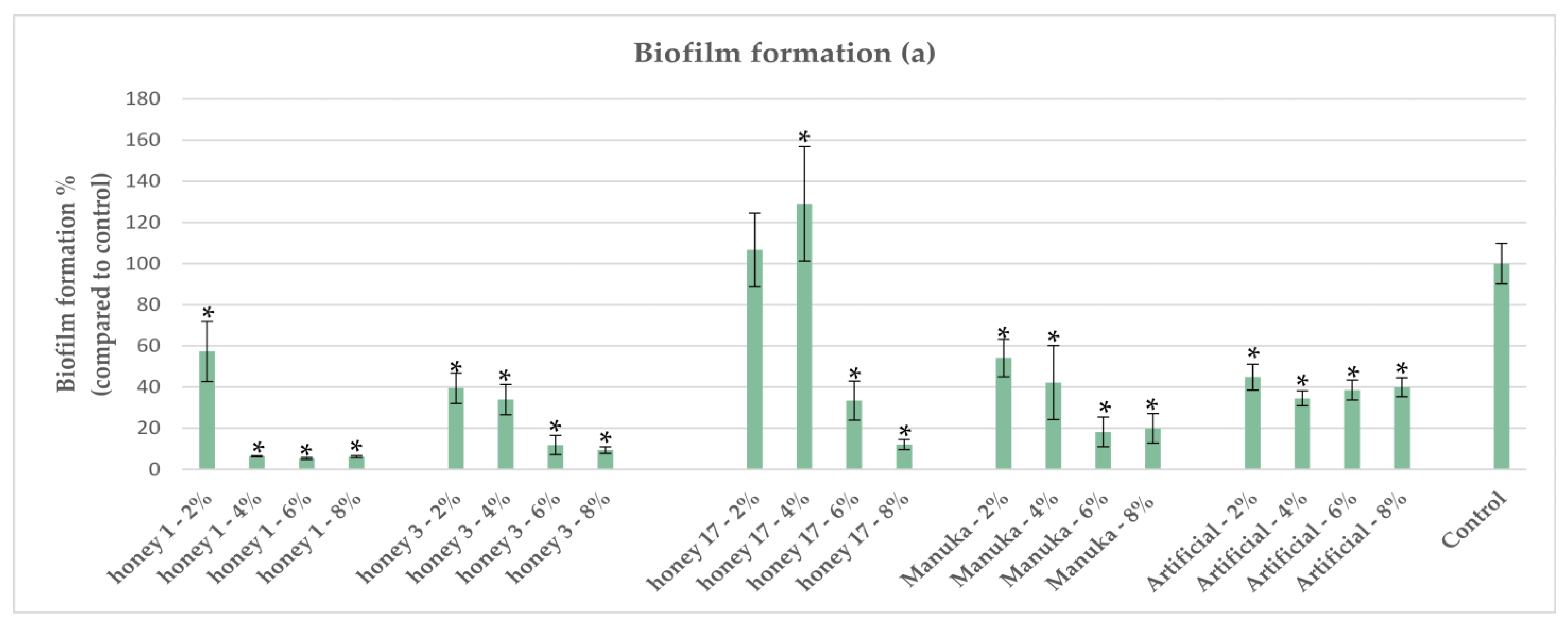
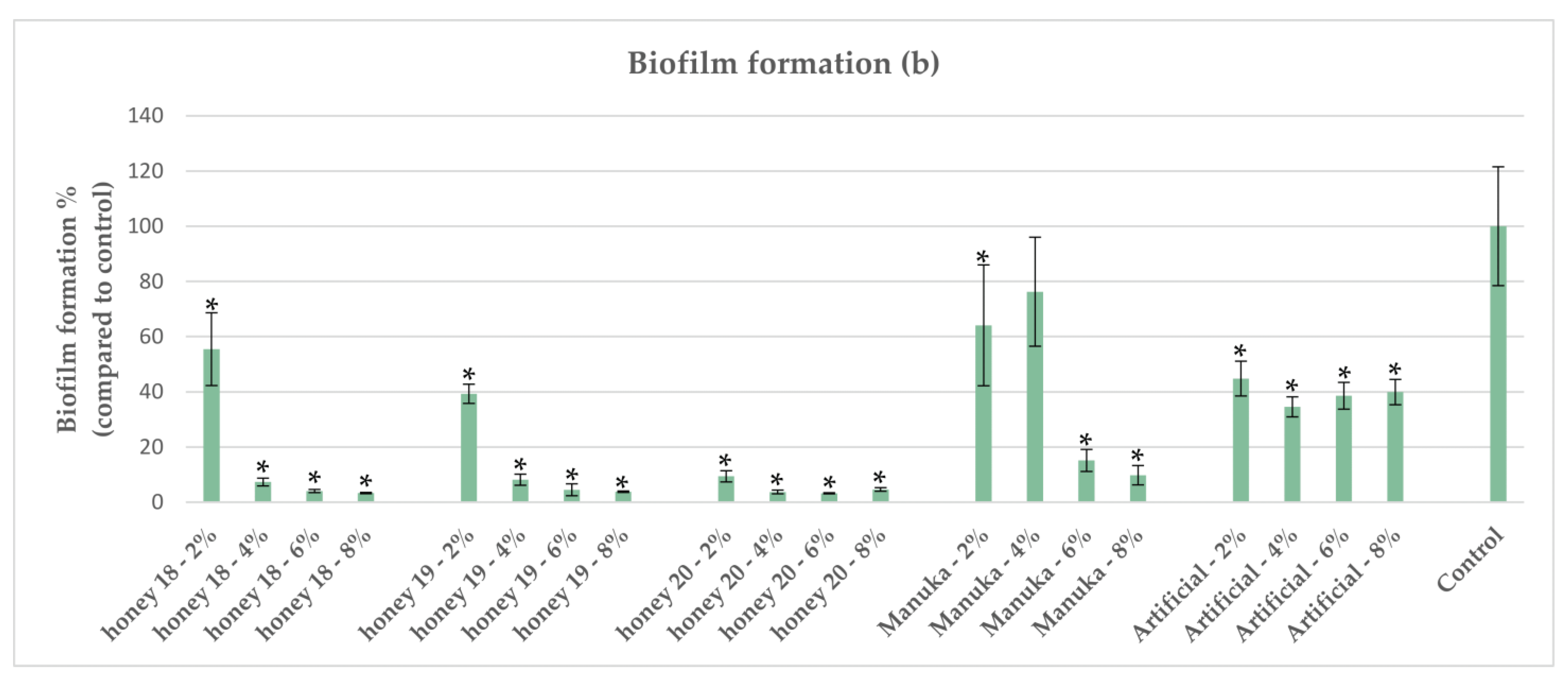
2.6. Impact of Honeys on Biofilm Formation of P. aeruginosa
3. Materials and Methods
3.1. Honey Samples
3.2. Bacterial Strain and Growth Conditions
3.3. Protease Activity Assay
3.4. Elastase Activity Assay
3.5. Pyoverdine Assay
3.6. Pyocyanin Assay
3.7. Swimming Motility Assay
3.8. Inhibition of Biofilm Formation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alamu, J.; Kakithakara Vajravelu, L.; Venkatesan, B.; Thulukanam, J. Correlation of Phenotypic and Genotypic Virulence Markers, Antimicrobial Susceptibility Pattern, and Outcome of Pseudomonas aeruginosa Sepsis Infection. Microb. Pathog. 2022, 170, 105716. [Google Scholar] [CrossRef]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka Honey Reduces the Motility of Pseudomonas aeruginosa by Suppression of Flagella-Associated Genes. J. Antimicrob. Chemother. 2015, 70, 716–725. [Google Scholar] [CrossRef]
- Nikolaidis, M.; Mossialos, D.; Oliver, S.G.; Amoutzias, G.D. Comparative Analysis of the Core Proteomes among the Pseudomonas Major Evolutionary Groups Reveals Species-Specific Adaptations for Pseudomonas aeruginosa and Pseudomonas chlororaphis. Diversity 2020, 12, 289. [Google Scholar] [CrossRef]
- El-Mahdy, R.; El-Kannishy, G. Virulence Factors of Carbapenem-Resistant Pseudomonas aeruginosa in Hospital-Acquired Infections in Mansoura, Egypt. Infect. Drug Resist. 2019, 12, 3455–3461. [Google Scholar] [CrossRef]
- Başkan, C.; Sırıken, B.; Tüfekci, E.F.; Kılınç, Ç.; Ertürk, Ö.; Erol, İ. Presence of Quorum Sensing System, Virulence Genes, Biofilm Formation and Relationship among Them and Class 1 Integron in Carbapenem-Resistant Clinical Pseudomonas aeruginosa Isolates. Arch. Microbiol. 2022, 204, 464. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ye, L.; Li, D.; Lin, S.; Deng, W.; Zhang, L.; Liang, J.; Li, J.; Wei, Q.; Wang, K. Effects of Daphnetin on Biofilm Formation and Motility of Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2022, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.; Kumari, S.; Dixit, M.; Sharma, N.K. Cu-Catalyzed Synthesis of Alkylaminotroponyl Sulfones as Pseudomonas aeruginosa Quorum Sensing Inhibitors Targeting LasI/R QS Circuitry. Chem. Asian J. 2022, 17, e202200866. [Google Scholar] [CrossRef]
- Mossialos, D.; Amoutzias, G.D. Role of Siderophores in Cystic Fibrosis Pathogenesis: Foes or Friends? Int. J. Med. Microbiol. 2009, 299, 87–98. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Déziel, E.; Comeau, Y.; Villemur, R. Initiation of Biofilm Formation by Pseudomonas aeruginosa 57RP Correlates with Emergence of Hyperpiliated and Highly Adherent Phenotypic Variants Deficient in Swimming, Swarming, and Twitching Motilities. J. Bacteriol. 2001, 183, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.; Safrin, M.; Olson, J.C.; Ohman, D.E. Secreted LasA of Pseudomonas aeruginosa Is a Staphylolytic Protease. J. Biol. Chem. 1993, 268, 7503–7508. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Wu, J.; Han, B.; Zhao, S.; Zhong, Y.; Han, W.; Gao, J.; Wang, S. Bioactive Characterization of Multifloral Honeys from Apis cerana cerana, Apis dorsata, and Lepidotrigona flavibasis. Food Res. Int. 2022, 161, 111808. [Google Scholar] [CrossRef] [PubMed]
- Missio, P.; Gauche, C.; Gonzaga, L.V.; Carolina, A.; Costa, O. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Wultańska, D.; Paterczyk, B.; Nowakowska, J.; Pituch, H. The Effect of Selected Bee Products on Adhesion and Biofilm of Clostridioides difficile Strains Belonging to Different Ribotypes. Molecules 2022, 27, 7385. [Google Scholar] [CrossRef] [PubMed]
- Chhawchharia, A.; Haines, R.R.; Green, K.J.; Barnett, T.C.; Bowen, A.C.; Hammer, K.A. In Vitro Antibacterial Activity of Western Australian Honeys, and Manuka Honey, against Bacteria Implicated in Impetigo. Complement. Ther. Clin. Pract. 2022, 49, 101640. [Google Scholar] [CrossRef]
- Kačániová, M.; Borotová, P.; Galovičová, L.; Kunová, S.; Štefániková, J.; Kowalczewski, P.Ł.; Šedík, P. Antimicrobial and Antioxidant Activity of Different Honey Samples from Beekeepers and Commercial Producers. Antibiotics 2022, 11, 1163. [Google Scholar] [CrossRef]
- Al-Sayaghi, A.M.; Al-Kabsi, A.M.; Abduh, M.S.; Saghir, S.A.M.; Alshawsh, M.A. Antibacterial Mechanism of Action of Two Types of Honey against Escherichia coli through Interfering with Bacterial Membrane Permeability, Inhibiting Proteins, and Inducing Bacterial DNA Damage. Antibiotics 2022, 11, 1182. [Google Scholar] [CrossRef]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening Bioactivity and Bioactive Constituents of Nordic Unifloral Honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef]
- Liaqat, I.; Gulab, B.; Aftab, N. Honey Potential As Antibacterial, Antibiofilm, Antiquorum Sensing and Biofilm Dispersal Agent Against Pathogenic Bacteria. Biofilms Adv. Res. Appl. 2021, 434, 189–205. [Google Scholar]
- Kafantaris, I.; Tsadila, C.; Nikolaidis, M.; Tsavea, E.; Dimitriou, T.G.; Iliopoulos, I.; Amoutzias, G.D.; Mossialos, D. Transcriptomic Analysis of Pseudomonas aeruginosa Response to Pine Honey via Rna Sequencing Indicates Multiple Mechanisms of Antibacterial Activity. Foods 2021, 10, 936. [Google Scholar] [CrossRef]
- Tsavea, E.; Vardaka, F.P.; Savvidaki, E.; Kellil, A.; Kanelis, D.; Bucekova, M.; Grigorakis, S.; Godocikova, J.; Gotsiou, P.; Dimou, M.; et al. Physicochemical Characterization and Biological Properties of Pine Honey Produced across Greece. Foods 2022, 11, 943. [Google Scholar] [CrossRef]
- Papanikolaou, G.E.; Gousios, G.; Cremers, N.A.J. Use of Medical-Grade Honey to Treat Clinically Infected Heel Pressure Ulcers in High-Risk Patients: A Prospective Case Series. Antibiotics 2023, 12, 605. [Google Scholar] [CrossRef]
- Pleeging, C.C.F.; Coenye, T.; Mossialos, D.; de Rooster, H.; Chrysostomou, D.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 2020, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial Activity of Honey. Honey Anal. 2017, 1, 215–232. [Google Scholar] [CrossRef]
- Tsavea, E.; Mossialos, D. Antibacterial Activity of Honeys Produced in Mount Olympus Area against Nosocomial and Foodborne Pathogens Is Mainly Attributed to Hydrogen Peroxide and Proteinaceous Compounds. J. Apic. Res. 2019, 58, 756–763. [Google Scholar] [CrossRef]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and Antioxidant Activity of Different Types of Honey Derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Vokou, D.; Petanidou, T.; Bellos, D. Pollination Ecology and Reproductive Potential of Jankaea Heldreichii (Gesneriaceae); a Tertiary Relict on Mt Olympus, Greece. Biol. Conserv. 1990, 52, 125–133. [Google Scholar] [CrossRef]
- Güneş, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of Phenolic Compounds Profile in Chestnut and Floral Honeys and Their Antioxidant and Antimicrobial Activities. J. Food Biochem. 2017, 41, e12345. [Google Scholar] [CrossRef]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix tetrasperma Impair Virulence and Inhibit Quorumsensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef]
- Yin, H.; Deng, Y.; Wang, H.; Liu, W.; Zhuang, X.; Chu, W. Tea Polyphenols as an Antivirulence Compound Disrupt Quorum-Sensing Regulated Pathogenicity of Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 16158. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.C.M.; Viganor, L.; De Castro, A.A.; Da Cunha, E.F.F.; Mello, T.P.; Mattos, L.M.; Pereira, M.D.; Hunt, M.C.; O’Shaughnessy, M.; Howe, O.; et al. Disarming Pseudomonas aeruginosa Virulence by the Inhibitory Action of 1,10-Phenanthroline-5,6-Dione-Based Compounds: Elastase B (LasB) as a Chemotherapeutic Target. Front. Microbiol. 2019, 10, 1701. [Google Scholar] [CrossRef]
- Cigana, C.; Castandet, J.; Sprynski, N.; Melessike, M.; Beyria, L.; Ranucci, S.; Alcalá-Franco, B.; Rossi, A.; Bragonzi, A.; Zalacain, M.; et al. Pseudomonas aeruginosa Elastase Contributes to the Establishment of Chronic Lung Colonization and Modulates the Immune Response in a Murine Model. Front. Microbiol. 2021, 11, 620819. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Zhou, S.; Jiang, Y.; Zhu, W.; Zhuang, X.; Fu, J. Effect of Traditional Chinese Herbal Medicine with Antiquorum Sensing Activity on Pseudomonas aeruginosa. Evid. Based Complement. Altern. Med. 2013, 2013, 648257. [Google Scholar] [CrossRef] [PubMed]
- Shirlaw, O.; Billah, Z.; Attar, B.; Hughes, L.; Qasaymeh, R.M.; Seidel, V.; Efthimiou, G. Antibiofilm Activity of Heather and Manuka Honeys and Antivirulence Potential of Some of Their Constituents on the DsbA1 Enzyme of Pseudomonas aeruginosa. Antibiotics 2020, 9, 911. [Google Scholar] [CrossRef]
- Łasica, A.M.; Jagusztyn-Krynicka, E.K. The Role of Dsb Proteins of Gram-Negative Bacteria in the Process of Pathogenesis. FEMS Microbiol. Rev. 2007, 31, 626–636. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria—Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Wang, R.; Starkey, M.; Hazan, R.; Rahme, L.G. Honey’s Ability to Counter Bacterial Infections Arises from Both Bactericidal Compounds and QS Inhibition. Front. Microbiol. 2012, 3, 144. [Google Scholar] [CrossRef]
- Ankley, L.M.; Monteiro, M.P.; Camp, K.M.; O’Quinn, R.; Castillo, A.R. Manuka Honey Chelates Iron and Impacts Iron Regulation in Key Bacterial Pathogens. J. Appl. Microbiol. 2020, 128, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Aldarhami, A.; Patel, M.; Adnan, M.; Hamdi, A.; Snoussi, M.; Qanash, H.; Imam, M.; Monjed, M.K.; Khateb, A.M. The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities. Pharmaceuticals 2022, 15, 1212. [Google Scholar] [CrossRef] [PubMed]
- Vijendra Kumar, N.; Murthy, P.S.; Manjunatha, J.R.; Bettadaiah, B.K. Synthesis and Quorum Sensing Inhibitory Activity of Key Phenolic Compounds of Ginger and Their Derivatives. Food Chem. 2014, 159, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The Hierarchy Quorum Sensing Network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef]
- Pandit, S.; Ravikumar, V.; Abdel-Haleem, A.M.; Derouiche, A.; Mokkapati, V.R.S.S.; Sihlbom, C.; Mineta, K.; Gojobori, T.; Gao, X.; Westerlund, F.; et al. Low Concentrations of Vitamin C Reduce the Synthesis of Extracellular Polymers and Destabilize Bacterial Biofilms. Front. Microbiol. 2017, 8, 2599. [Google Scholar] [CrossRef]
- Farkas, Á.; Balázs, V.L.; Kõszegi, T.; Csepregi, R.; Kerekes, E.; Horváth, G.; Szabó, P.; Gaál, K.; Kocsis, M. Antibacterial and Biofilm Degradation Effects of Hungarian Honeys Linked with Botanical Origin, Antioxidant Capacity and Mineral Content. Front. Nutr. 2022, 9, 953470. [Google Scholar] [CrossRef]
- Proaño, A.; Coello, D.; Villacrés-Granda, I.; Ballesteros, I.; Debut, A.; Vizuete, K.; Brenciani, A.; Álvarez-Suarez, J.M. The Osmotic Action of Sugar Combined with Hydrogen Peroxide and Bee-Derived Antibacterial Peptide Defensin-1 Is Crucial for the Antibiofilm Activity of Eucalyptus Honey. LWT 2021, 136, 110379. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing Phytochemicals as Anti-Virulent Agents to Attenuate Quorum Sensing-Regulated Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2022, 15, 1695–1718. [Google Scholar] [CrossRef]
- El Zowalaty, M.E.; Al Thani, A.A.; Webster, T.J.; El Zowalaty, A.E.; Schweizer, H.P.; Nasrallah, G.K.; Marei, H.E.; Ashour, H.M. Pseudomonas aeruginosa: Arsenal of Resistance Mechanisms, Decades of Changing Resistance Profiles, and Future Antimicrobial Therapies. Future Microbiol. 2015, 10, 1683–1706. [Google Scholar] [CrossRef]
- Jadaun, V.; Prateeksha; Singh, B.R.; Paliya, B.S.; Upreti, D.K.; Rao, C.V.; Rawat, A.K.S.; Singh, B.N. Honey Enhances the Anti-Quorum Sensing Activity and Anti-Biofilm Potential of Curcumin. RSC Adv. 2015, 5, 71060–71070. [Google Scholar] [CrossRef]
- Prithiviraj, B.; Bais, H.P.; Weir, T.; Suresh, B.; Najarro, E.H.; Dayakar, B.V.; Schweizer, H.P.; Vivanco, J.M. Down Regulation of Virulence Factors of Pseudomonas aeruginosa by Salicylic Acid Attenuates Its Virulence on Arabidopsis Thaliana and Caenorhabditis Elegans. Infect. Immun. 2005, 73, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.M.; Miguel, M.G.; Boas, M.V.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on Their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional Analysis of Scottish Honeys with Antimicrobial Activity against Antibiotic-Resistant Bacteria Reveals Novel Antimicrobial Components. LWT 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Anthimidou, E.; Mossialos, D. Antibacterial Activity of Greek and Cypriot Honeys against Staphylococcus aureus and Pseudomonas aeruginosa in Comparison to Manuka Honey. J. Med. Food 2013, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Gambello, M.J.; Iglewski, B.H. Cloning and Characterization of the Pseudomonas aeruginosa LasR Gene, a Transcriptional Activator of Elastase Expression. J. Bacteriol. 1991, 173, 3000–3009. [Google Scholar] [CrossRef]
- McMorran, B.J.; Shantha Kumara, H.M.C.; Sullivan, K.; Lamont, I.L. Involvement of a Transformylase Enzyme in Siderophore Synthesis in Pseudomonas aeruginosa. Microbiology 2001, 147, 1517–1524. [Google Scholar] [CrossRef]
- James, H.E.; Beare, P.A.; Martin, L.W.; Lamont, I.L. Mutational Analysis of a Bifunctional Ferrisiderophore Receptor and Signal-Transducing Protein from Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 4514–4520. [Google Scholar] [CrossRef]
- Hazan, R.; He, J.; Xiao, G.; Dekimpe, V.; Apidianakis, Y.; Lesic, B.; Astrakas, C.; Déziel, E.; Lépine, F.; Rahme, L.G. Homeostatic Interplay between Bacterial Cell-Cell Signaling and Iron in Virulence. PLoS Pathog. 2010, 6, e1000810. [Google Scholar] [CrossRef]
- O’May, C.; Tufenkji, N. The Swarming Motility of Pseudomonas aeruginosa Is Blocked by Cranberry Proanthocyanidins and Other Tannin-Containing Materials. Appl. Environ. Microbiol. 2011, 77, 3061–3067. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, June 28–July 3 2010. [Google Scholar]


| Harvest Date | Geographical Location | Botanical Source | Honey Number |
|---|---|---|---|
| July, 2013 | Karia | Polyfloral | 1 |
| July, 2013 | Elassona | Alfalfa and herbs | 2 |
| July, 2013 | Sikea | Polyfloral | 3 |
| July, 2013 | Paliampela | Oregano and herbs | 4 |
| July, 2013 | Domeniko | Polyfloral | 5 |
| July, 2013 | Samina | Polyfloral | 6 |
| July, 2013 | Sarantaporo | Polyfloral | 7 |
| July, 2013 | Krania | Mint, herbs, and acacia | 8 |
| July, 2013 | Azoro | Polyfloral | 9 |
| July, 2013 | Verdikoussia | Polyfloral | 10 |
| July, 2013 | Kalithea | Polyfloral | 11 |
| June, 2012 | Karia | Polyfloral | 12 |
| 2012 | Domeniko | Polyfloral and conifers | 14 |
| July, 2014 | Karia | Polyfloral | 15 |
| 2012 | Elassona | Polyfloral | 16 |
| 2014 | Drimos | Herbs | 17 |
| August, 2014 | Karia | Acacia, Abies, and Sideritis | 18 |
| August, 2014 | Azoros | Polyfloral and conifers | 19 |
| 2014 | Galanovrissi | Polyfloral and conifers | 20 |
| July, 2014 | Karia | Polyfloral and conifers | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsavea, E.; Tzika, P.; Katsivelou, E.; Adamopoulou, A.; Nikolaidis, M.; Amoutzias, G.D.; Mossialos, D. Impact of Mt. Olympus Honeys on Virulence Factors Implicated in Pathogenesis Exerted by Pseudomonas aeruginosa. Antibiotics 2023, 12, 998. https://doi.org/10.3390/antibiotics12060998
Tsavea E, Tzika P, Katsivelou E, Adamopoulou A, Nikolaidis M, Amoutzias GD, Mossialos D. Impact of Mt. Olympus Honeys on Virulence Factors Implicated in Pathogenesis Exerted by Pseudomonas aeruginosa. Antibiotics. 2023; 12(6):998. https://doi.org/10.3390/antibiotics12060998
Chicago/Turabian StyleTsavea, Eleni, Paraskevi Tzika, Eleni Katsivelou, Anna Adamopoulou, Marios Nikolaidis, Grigorios D. Amoutzias, and Dimitris Mossialos. 2023. "Impact of Mt. Olympus Honeys on Virulence Factors Implicated in Pathogenesis Exerted by Pseudomonas aeruginosa" Antibiotics 12, no. 6: 998. https://doi.org/10.3390/antibiotics12060998
APA StyleTsavea, E., Tzika, P., Katsivelou, E., Adamopoulou, A., Nikolaidis, M., Amoutzias, G. D., & Mossialos, D. (2023). Impact of Mt. Olympus Honeys on Virulence Factors Implicated in Pathogenesis Exerted by Pseudomonas aeruginosa. Antibiotics, 12(6), 998. https://doi.org/10.3390/antibiotics12060998








