Carbapenem Use in the Last Days of Life: A Nationwide Korean Study
Abstract
1. Introduction
2. Results
2.1. Characteristics of Carbapenem Use in Patients in the Last Two Weeks of Their Life
2.2. Comparison of Characteristics between “Optimal” and “Not Optimal” Carbapenem Prescriptions in Patients without an ID Specialist Consultation
3. Discussion
4. Materials and Methods
4.1. Study Setting and Population
4.2. Data Collection and Definitions
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breilh, D.; Texier-Maugein, J.; Allaouchiche, B.; Saux, M.C.; Boselli, E. Carbapenems. J. Chemother. 2013, 25, 1–17. [Google Scholar] [CrossRef]
- Armand-Lefèvre, L.; Angebault, C.; Barbier, F.; Hamelet, E.; Defrance, G.; Ruppé, E.; Bronchard, R.; Lepeule, R.; Lucet, J.-C.; El Mniai, A.; et al. Emergence of Imipenem-Resistant Gram-Negative Bacilli in Intestinal Flora of Intensive Care Patients. Antimicrob. Agents Chemother. 2013, 57, 1488–1495. [Google Scholar] [CrossRef]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Kwak, Y.G.; Moon, C.; Kim, E.S.; Kim, B.-N. Frequent Prescription of Antibiotics and High Burden of Antibiotic Resistance among Deceased Patients in General Medical Wards of Acute Care Hospitals in Korea. PLoS ONE 2016, 11, e0146852. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Yang, K.S.; Lee, S.E.; Kim, H.J.; Sohn, J.W.; Kim, M.J. Effects of Group 1 versus Group 2 Carbapenems on the Susceptibility of Acinetobacter baumannii to Carbapenems: A Before and After Intervention Study of Carbapenem-Use Stewardship. PLoS ONE 2014, 9, e99101. [Google Scholar] [CrossRef]
- Horikoshi, Y.; Suwa, J.; Higuchi, H.; Kaneko, T.; Furuichi, M.; Aizawa, Y.; Fukuoka, K.; Okazaki, K.; Ito, K.; Shoji, T. Sustained pediatric antimicrobial stewardship program with consultation to infectious diseases reduced carbapenem resistance and infection-related mortality. Int. J. Infect. Dis. 2017, 64, 69–73. [Google Scholar] [CrossRef]
- McLaughlin, M.; Advincula, M.R.; Malczynski, M.; Qi, C.; Bolon, M.; Scheetz, M.H. Correlations of Antibiotic Use and Carbapenem Resistance in Enterobacteriaceae. Antimicrob. Agents Chemother. 2013, 57, 5131–5133. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 5 May 2023).
- Gauzit, R.; Pean, Y.; Alfandari, S.; Bru, J.-P.; Bedos, J.-P.; Rabaud, C.; Robert, J. Carbapenem use in French hospitals: A nationwide survey at the patient level. Int. J. Antimicrob. Agents 2015, 46, 707–712. [Google Scholar] [CrossRef]
- Wi, Y.M.; Kwon, K.T.; Hwang, S.; Bae, S.; Kim, Y.; Chang, H.-H.; Kim, S.-W.; Cheong, H.S.; Lee, S.; Jung, D.S.; et al. Use of Antibiotics Within the Last 14 Days of Life in Korean Patients: A Nationwide Study. J. Korean Med. Sci. 2023, 38, e66. [Google Scholar] [CrossRef]
- Coppry, M.; Jeanne-Leroyer, C.; Noize, P.; Dumartin, C.; Boyer, A.; Bertrand, X.; Rogues, A.M. Antibiotics associated with acquisition of carbapenem-resistant Pseudomonas aeruginosa in ICUs: A multicentre nested case-case-control study. J. Antimicrob. Chemother. 2019, 74, 503–510. [Google Scholar] [CrossRef]
- Woerther, P.L.; Lepeule, R.; Burdet, C.; Decousser, J.W.; Ruppé, É.; Barbier, F. Carbapenems and alternative β-lactams for the treatment of infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae: What impact on intestinal colonisation re-sistance? Int. J. Antimicrob. Agents 2018, 52, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Avendano, E.E.; Chan, J.; Merchant, S.; Puzniak, L. Risk factors for hospitalized patients with resistant or multi-drug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob Resist Infect Control 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.F.V.I.; Severin, J.A.; Lesaffre, E.M.E.H.; Vos, M.C. A Systematic Review and Meta-Analyses Show that Carbapenem Use and Medical Devices Are the Leading Risk Factors for Carbapenem-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 2626–2637. [Google Scholar] [CrossRef]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Frasquet, J.; Sánchez, M.; Martín, M.; Ramirez, P. Influence of antibiotic pressure on multi-drug resistant Klebsiella pneumoniae colonisation in critically ill patients. Antimicrob. Resist. Infect. Control 2019, 8, 38. [Google Scholar] [CrossRef]
- Gaw, C.E.; Hamilton, K.W.; Gerber, J.S.; Szymczak, J.E. Physician Perceptions Regarding Antimicrobial Use in End-of-Life Care. Infect. Control. Hosp. Epidemiol. 2018, 39, 383–390. [Google Scholar] [CrossRef]
- Stocker, H.; Mehlhorn, C.; Jordan, K.; Eckholt, L.; Jefferys, L.; Arastéh, K. Clinical and economic effects of an antimicrobial stewardship intervention in a surgical intensive care unit. Infection 2020, 48, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Nilholm, H.; Holmstrand, L.; Ahl, J.; Månsson, F.; Odenholt, I.; Tham, J.; Resman, F. An Audit-Based, Infectious Disease Specialist-Guided Antimicrobial Stewardship Pro-gram Profoundly Reduced Antibiotic Use Without Negatively Affecting Patient Outcomes. Open Forum Infect. Dis. 2015, 2, ofv042. [Google Scholar] [CrossRef]
- Paulsen, J.; Solligård, E.; Damås, J.K.; DeWan, A.; Åsvold, B.O.; Bracken, M.B. The Impact of Infectious Disease Specialist Consultation for Staphylococcus aureus Bloodstream Infections: A Systematic Review. Open Forum Infect. Dis. 2016, 3, ofw048. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Kritsotakis, E.I.; Mathioudaki, A.; Vouidaski, A.; Spanias, C.; Petrodaskalaki, M.; Ioannou, P.; Chamilos, G.; Kofteridis, D.P. A carbapenem-focused antimicrobial stewardship programme implemented during the COVID-19 pandemic in a setting of high endemicity for multidrug-resistant Gram-negative bacteria. J. Antimicrob. Chemother. 2023, 78, 1000–1008. [Google Scholar] [CrossRef]
- Butt, A.A.; Al Kaabi, N.; Khan, T.; Saifuddin, M.; Khan, M.; Krishnanreddy, K.M.; Jasim, W.H.; Sara, M.; Pitout, M.; Weber, S. Impact of Infectious Diseases Team Consultation on Antimicrobial Use, Length of Stay and Mortality. Am. J. Med. Sci. 2015, 350, 191–194. [Google Scholar] [CrossRef]
- Messacar, K.; Pearce, K.; Pyle, L.; Hurst, A.L.; Child, J.; Parker, S.K.; Campbell, K. A Handshake from Antimicrobial Stewardship Opens Doors for Infectious Disease Consultations. Clin. Infect. Dis. 2017, 64, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Lee, L.W.; Liew, Y.X.; Krishna, L.; Chlebicki, M.P.; Chung, S.J.; Kwa, A.L.-H. Antibiotic stewardship program (ASP) in palliative care: Antibiotics, to give or not to give. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 41, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ivers, N.; Jamtvedt, G.; Flottorp, S.; Young, J.M.; Odgaard-Jensen, J.; French, S.D.; O’Brien, M.A.; Johansen, M.; Grimshaw, J.; Oxman, A.D. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2012, 6, CD000259. [Google Scholar] [CrossRef] [PubMed]
| Carbapenem Use (n = 533) | Non-Carbapenem Use (n = 668) | p Value | |
|---|---|---|---|
| Age, years, median (IQR) | 71 (61.0–79.0) | 72 (63.0–80.0) | 0.158 |
| Gender, n (%) | 0.159 | ||
| Male | 338 (63.4) | 397 (59.8) | |
| Female | 195 (36.6) | 271 (40.8) | |
| Underlying disease, n (%) | |||
| Cancer | 224 (42.0) | 303 (45.4) | 0.247 |
| Cardiovascular disease | 30 (5.6) | 61 (9.1) | 0.023 |
| Renal disease | 17 (3.2) | 12 (1.8) | 0.118 |
| Chronic lung disease | 20 (3.8) | 25 (3.7) | 0.993 |
| Diabetes | 13 (2.4) | 9 (1.3) | 0.161 |
| Cerebrovascular disease | 44 (8.3) | 92 (13.8) | 0.003 |
| Liver disease | 17 (3.2) | 27 (4.0) | 0.435 |
| Gastrointestinal disorder | 14 (2.6) | 9 (1.3) | |
| Cause of death, n (%) | |||
| Any infectious disease | 341 (64.0) | 193 (30.6) | <0.001 |
| Cancer | 110 (20.6) | 210 (33.3) | <0.001 |
| Cerebrovascular disease | 17 (3.2) | 68 (10.8) | <0.001 |
| Cardiovascular disease | 20 (3.8) | 54 (8.6) | 0.001 |
| Lung disease | 9 (1.7) | 43 (6.8) | <0.001 |
| Liver disease | 10 (1.9) | 27 (4.3) | 0.023 |
| Renal disease | 9 (1.7) | 20 (3.2) | 0.116 |
| Gastrointestinal bleeding | 8 (1.5) | 16 (2.5) | 0.231 |
| The completion of LST document prior to death, n (%) | |||
| LST document completed ≤14 days prior to death | 359 (67.4) | 465 (69.6) | 0.403 |
| LST document completed >14 days prior to death | 46 (8.6) | 70 (10.5) | 0.281 |
| Microbiological study, n (%) | 510 (95.7) | 556 (83.2) | <0.001 |
| Multidrug-resistant pathogen | 205 (40.2) | 114 (20.5) | <0.001 |
| Number of antibiotic changes, median (IQR) | 4 (3–5) | 2 (1–3) | <0.001 |
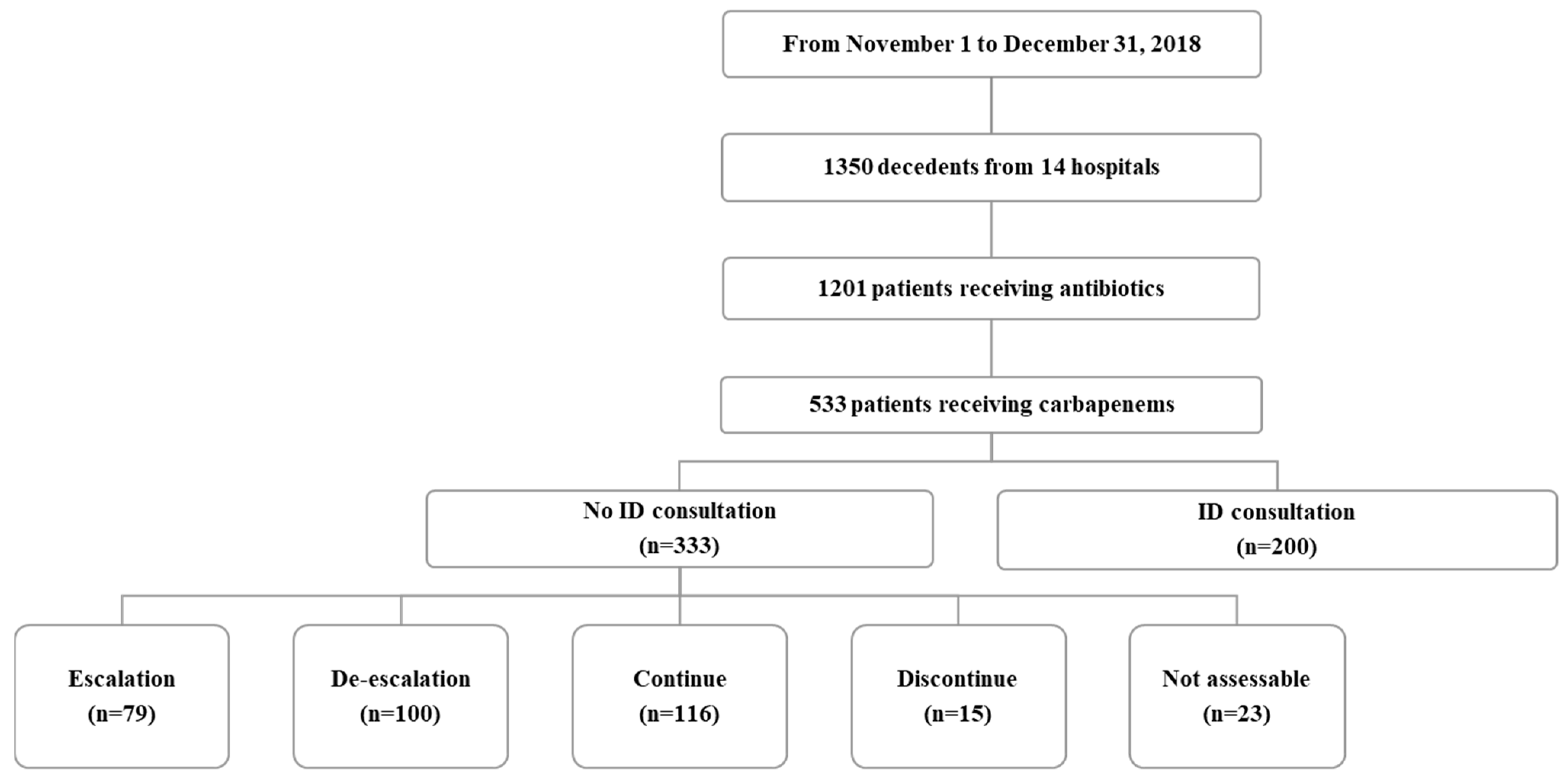
| ID specialist consultation, n (%) | 200 (37.5) | 127 (19.0) | <0.001 |
| No ID specialist consultation, n (%) | 333 (62.5) | 541 (81.0) | <0.001 |
| Escalation | 79 (23.7) | 74 (13.7) | |
| De-escalation | 100 (30.3) | 69 (12.8) | |
| Continue | 116 (34.8) | 205 (37.9) | |
| Stop | 15 (2.8) | 144 (26.6) | |
| Not assessable | 23 (4.3) | 49 (9.1) |
| Patients’ Characteristics | Not Optimal Use (n = 194) | Optimal Use (n = 116) | Unadjusted OR (95% CI) | p Value |
|---|---|---|---|---|
| Carbapenem treatment duration, days | 7 (3–12) | 6 (3–12) | 1.01 (0.96–1.04) | 0.882 |
| Age (years), median (IQR) | 74.0 (60.0–79.0) | 72.0 (62.8–80.0) | 1.01 (0.99–1.02) | 0.601 |
| Gender, n (%) | ||||
| Male | 128 (66.0) | 69 (59.5) | 1.32 (0.82–2.12) | 0.251 |
| Female | 66 (34.0) | 47 (40.5) | 0.76 (0.47–1.22) | 0.757 |
| Underlying co-morbidities, n (%) | ||||
| Cancer | 97 (50.0) | 54 (46.6) | 1.15 (0.72–1.82) | 0.557 |
| Cardiovascular diseases | 15 (7.7) | 4 (3.4) | 2.35 (0.76–7.25) | 0.138 |
| Renal diseases | 10 (5.2) | 2 (1.7) | 3.10 (0.67–14.39) | 0.149 |
| Lung diseases | 8 (4.1) | 4 (3.4) | 1.20 (0.36–4.09) | 0.766 |
| Diabetes | 3 (1.5) | 2 (1.7) | 0.90 (0.15–5.44) | 0.904 |
| Cerebrovascular diseases | 10 (5.2) | 4 (3.4) | 1.52 (0.47–4.97) | 0.487 |
| Liver diseases | 4 (2.1) | 5 (4.3) | 0.47 (0.12–1.78) | 0.264 |
| Gastrointestinal disorders | 3 (1.5) | 2 (1.7) | 0.90 (0.15–5.44) | 0.904 |
| Cause of death, n (%) (n = 304) | ||||
| Any infectious disease | 118 (62.1) | 69 (60.5) | 1.07 (0.66–1.72) | 0.784 |
| Cancer | 44 (23.2) | 31 (27.2) | 0.81 (0.47–1.38) | 0.430 |
| Cerebrovascular disease | 4 (2.1) | 0 | 0.999 | |
| Cardiovascular disease | 10 (5.3) | 5 (4.4) | 1.21 (0.40–3.64) | 0.733 |
| Lung disease | 3 (1.6) | 3 (2.6) | 0.59 (0.12–2.99) | 0.527 |
| Liver disease | 5 (2.6) | 2 (1.8) | 1.51 (0.29–7.93) | 0.624 |
| Renal disease | 3 (1.6) | 2 (1.8) | 0.90 (0.15–5.46) | 0.907 |
| Gastrointestinal bleeding | 3 (1.6) | 2 (1.8) | 0.90 (0.15–5.46) | 0.907 |
| LST document completion prior to death, n (%) | ||||
| Completed ≤14 days prior to death | 142 (73.2) | 83 (71.6) | 1.09 (0.65–1.81) | 0.754 |
| Completed >14 days prior to death | 15 (7.7) | 6 (5.2) | 1.54 (0.58–4.08) | 0.389 |
| Not completed | 37 (19.1) | 27 (23.3) | 0.78 (0.44–1.36) | 0.777 |
| Number of antibiotic changes, median (IQR) | 3.0 (2.0–4.0) | 3.5 (3.0–5.0) | 0.83 (0.71–0.97) | 0.023 |
| Microbiological testing, n (%) | 183 (94.3) | 110 (94.8) | 0.91 (0.33–2.52) | 0.852 |
| Multidrug-resistant pathogen (n = 293) | 57 (31.1) | 40 (36.4) | 0.79 (0.48–1.30) | 0.359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wi, Y.M.; Kwon, K.T.; Jeon, C.-H.; Kim, S.-H.; Hwang, S.; Bae, S.; Kim, Y.; Chang, H.-H.; Kim, S.-W.; Cheong, H.S.; et al. Carbapenem Use in the Last Days of Life: A Nationwide Korean Study. Antibiotics 2023, 12, 964. https://doi.org/10.3390/antibiotics12060964
Wi YM, Kwon KT, Jeon C-H, Kim S-H, Hwang S, Bae S, Kim Y, Chang H-H, Kim S-W, Cheong HS, et al. Carbapenem Use in the Last Days of Life: A Nationwide Korean Study. Antibiotics. 2023; 12(6):964. https://doi.org/10.3390/antibiotics12060964
Chicago/Turabian StyleWi, Yu Mi, Ki Tae Kwon, Cheon-Hoo Jeon, Si-Ho Kim, Soyoon Hwang, Sohyun Bae, Yoonjung Kim, Hyun-Ha Chang, Shin-Woo Kim, Hae Suk Cheong, and et al. 2023. "Carbapenem Use in the Last Days of Life: A Nationwide Korean Study" Antibiotics 12, no. 6: 964. https://doi.org/10.3390/antibiotics12060964
APA StyleWi, Y. M., Kwon, K. T., Jeon, C.-H., Kim, S.-H., Hwang, S., Bae, S., Kim, Y., Chang, H.-H., Kim, S.-W., Cheong, H. S., Lee, S., Jung, D. S., Sohn, K. M., Moon, C., Heo, S. T., Kim, B., Lee, M. S., Hur, J., Kim, J., ... Antimicrobial Stewardship Research Committee of Korean Society for Antimicrobial Therapy. (2023). Carbapenem Use in the Last Days of Life: A Nationwide Korean Study. Antibiotics, 12(6), 964. https://doi.org/10.3390/antibiotics12060964






