Abstract
Urinary Tract Infections (UTIs) represent a common finding among females and an important basis for antibiotic treatment. Considering the significant increase in antibiotic resistance during the last decades, this study retrospectively follows the incidence of uropathogens and the evolution of resistance rates in the short and medium term. The current study was conducted at the “Prof. Dr. Th. Burghele” Clinical Hospital, including 1124 positive urine cultures, in three periods of four months between 2018 and 2022. Escherichia coli was the most frequent uropathogen (54.53%), followed by Klebsiella spp. (16.54%), and Enterococcus spp. (14.59%). The incidence of UTIs among the female population is directly proportional to age, with few exceptions. The highest overall resistance in Gram-negative uropathogens was observed for levofloxacin 30.69%, followed by ceftazidime 13.77% and amikacin 9.86%. The highest resistance in Gram-positive uropathogens was observed for levofloxacin 2018-R = 34.34%, 2020-R = 50.0%, and 2022-R = 44.92%, and penicillin 2018-R = 36.36%, 2020-R = 41.17%, and 2022-R = 37.68%. In Gram-negative uropathogens, a linear evolution was observed for ceftazidime 2018-R = 11.08%, 2020-R = 13.58%, and 2022-R = 17.33%, and levofloxacin 2018-R = 28.45%, 2020-R = 33.33%, and 2022-R = 35.0%. The current knowledge dictates the need to continuously assess antimicrobial resistance patterns, information that is necessary for treatment recommendations. The present study aims to determine the current situation and the evolution trends according to the current locoregional situation.
Keywords:
UTIs; females; AMR; uropathogens; antibiotic resistance; Escherichia coli; Klebsiella; COVID-19 1. Introduction
Urinary tract infections (UTIs) represent a common colonization followed by an inflammatory process of the urinary tract with various uropathogens. It is a widespread pathology, affecting over 150 million people around the globe annually, with over 10 million ambulatory visits and some 2 million emergency room visits estimated annually in the United States alone [1,2]. Hospitalizations for UTIs are expensive, costing almost USD 3 billion annually [3]. The growing resistance to routinely used antibiotics in both outpatient and inpatient settings adds to the burden of UTIs on global health systems [4]. These are considered the second-most common bacterial infections in humans, after respiratory infections, requiring special attention for a quick diagnosis and an optimal and effective treatment [5].
A UTI is one of the most common infections affecting women at different stages of life. According to estimates [6,7], every woman will have experienced at least one UTI over her lifetime, with more than 50% of all women experiencing a symptomatic UTI at least once [8]. Age raises the risk of infection [9]. Different types of conditions include lower UTIs (cystitis) and upper UTIs (pyelonephritis) [10]. Several risk factors are associated with acquiring UTIs and recurrent infections in female patients, such as facilitated ascent, which causes a higher incidence and a more significant public health problem than for the male population [11,12,13].
Antimicrobial resistance is a growing, global, and severe challenge to medical care. As a result, there are rising costs for patient care, an increase in hospital stays, and an increase in mortality. It has been found that almost all frequent infections in clinical practice exhibit high levels of resistance to conventional antibiotic treatments. It has also been reported that many organisms are multidrug-resistant [14].
The discovery of antibiotics represented a cornerstone in modern medicine, and they have served as a medication with obvious benefits on multiple infection sites. Still, misuse and abuse have also led to a severe hazard to public health because resistant uropathogenic bacteria are becoming more common. The European Association of Urology (EAU) Guidelines encourage the prudent use of all antimicrobials and propose Antibiotic Stewardship as a critical milestone in daily clinical practice. Furthermore, it suggests adapting antibiotic use policies to the local rates of resistance and sensitivity found, which will help both delay the increase in resistance and improve the efficacy of those indicated empirically [15]. Few recent data on local resistance rates are available for the Romanian territory [16,17,18], especially for more extended periods. The research that is currently available analyzes the bacterial prevalence and antibiotic resistance only for a determined period of a year [16,17,18]. The clinical practice requires reports that evaluate resistance rates at any particular time and indicate evolutionary trends across short and medium periods. The present study aims to assess the etiology and incidence of UTIs in Romania’s female population and evaluate the evolution of resistance and sensitivity rates to the common antibiotics over several periods for of years.
2. Results
The present study was developed in one of the largest urology hospitals in Bucharest, Romania—“Prof. Dr. Th. Burghele” Clinical Hospital—for 5 years. A total number of 1124 female patients met the study criteria. The evaluation was divided into three distinct periods of 4 months each with an interval of 2 years between them, as follows: 1 September 2018–31 December 2018—478 patients; 1 September 2020–31 December 2020—277 patients; 1 September 2022–31 December 2022—369 patients.
The most frequently encountered pathogen was Escherichia coli, representing 613 of the strains tested (54.53%), followed by Klebsiella spp., representing 186 of the strains tested (16.54%), Proteus spp., representing 81 strains (7.2%) and Pseudomonas spp., representing 42 strains (3.73%) in the group of Gram-negative bacteria. The most frequent Gram-positive bacteria tested were Enterococcus spp., with 164 strains tested (14.59%), followed by Staphylococcus, with 38 strains tested (3.38%). The same ratio between the tested uropathogens was maintained for all periods and age groups studied. We observed a linear increase in the incidence of each uropathogen that was directly proportional to the rise in the patient’s age. There are also minor exceptions to the rule. In the case of Staphylococcus spp., a higher incidence of 0.72% was observed in the age group 30–44 years, followed by the absence of detection in the immediately following age group, so that only in the > 60 years age group was it detected at a higher incidence of 2.16%. A detailed record of uropathogens’ incidence in all the periods studied and the division into age groups is presented in Table 1.

Table 1.
Uropathogens’ incidence and age stratifications.
Considering Escherichia coli as the most frequently encountered uropathogen, it shows the overall highest rate of resistance among the studied antibiotics to levofloxacin R = 188 (30.66%), followed by amoxicillin-clavulanic ac R = 172 (28.05%), and ceftazidime R = 65 (10.6%). Maintained sensitivity was observed to fosfomycin 1 (0.16%), carbapenems—imipenem 3 (0.48%), meropenem 1 (0.16%), and nitrofurantoin 26 (4.24%).
There is a general tendency to increase this bacterial resistance to the tested antibiotics. The most alarming situations are found in the case of amikacin: 2018, R = 3.55% and 2022, R = 22.43%; amoxicillin-clavulanic acid: 2018, R = 21.34% and 2022, R = 37.07%; and ceftazidime: 2018, R = 7.11% and 2022, R = 17.07%. A decrease in resistance is observed for nitrofurantoin: 2018, R = 6.71%; 2020, R = 5.8%; and 2022, no resistance in the tested strains. A detailed evaluation of the resistance and sensitivity of Escherichia coli to the tested antibiotics in the studied periods is presented in Table 2.

Table 2.
Escherichia coli susceptibility and resistance patterns.
Regarding Klebsiella spp., the second-most frequent pathogen studied, it shows the overall highest resistance to amoxicillin-clavulanic acid R = 68 (36.55%), ceftazidime R = 36 (19.35%), and levofloxacin R = 42 (22.58%). Relatively preserved sensitivity is observed for carbapenems—imipenem R = 7 (3.76%) and meropenem R = 9 (4.83%). The greatest increase in the evolution of resistance during the studied period was observed for levofloxacin 2018, R = 14.1% and 2022, R = 26.22%. A decrease in resistance was observed in the case of carbapenems imipenem 2018, R6.41% and 2022, R = 1.63%; meropenem: 2018, R = 6.41% and 2022, R = 4.91%; and ceftazidime: 2018, R = 20.51% and 2022, R = 18.03%. A detailed evaluation of the resistance and sensitivity of Klebsiella spp. to the tested antibiotics in the studied periods is presented in Table 3.

Table 3.
Klebsiella spp. susceptibility and resistance patterns.
Pseudomonas spp. showed the highest resistance rates to all studied antibiotics throughout all evaluated periods. Alarming rates of resistance were registered for levofloxacin R = 23 (54.76%), ceftazidime R = 19 (45.23%), carbapenem—imipenem R = 17 (40.47%), and meropenem R = 16 (38.09%). It shows an overall increase in antibiotic resistance in all the tested antibiotics throughout the studied periods. A detailed evaluation of the resistance and sensitivity of Pseudomonas spp. to the tested antibiotics over different periods is presented in Table 4.

Table 4.
Pseudomonas spp. susceptibility and resistance patterns.
Proteus spp. displayed preserved rates of resistance to carbapenems—imipenem R = 4 (4.93%), meropenem R = 2 (2.46%), and ceftazidime R = 7 (8.64%). The highest resistance rates were observed for levofloxacin R = 30 (37.03%), followed by amoxicillin-clavulanic acid R = 25 (30.86%). Proteus demonstrated a decrease in resistance to all antibiotics studied throughout the evaluated periods in the study, except for levofloxacin, which showed an increase in resistance, as follows 2018, R = 32.35%, 2020, R = 34.61%, and 2022, R = 47.61%. A detailed evaluation of the resistance and sensitivity of Proteus spp. to the tested antibiotics in the studied periods is presented in Table 5.

Table 5.
Proteus spp. susceptibility and resistance patterns.
The most common Gram-positive bacterium, Enterococcus spp., showed the highest overall resistance to levofloxacin, R = 75 (45.73%), followed by penicillin, R = 56 (34.14%) and ampicillin, R = 31 (18.9%). Reduced resistance rates are observed for vancomycin, R = 5 (3.04%), Fosfomycin, R = 5 (3.04%), and nitrofurantoin, R = 10 (6.09%). No strain studied in the evaluated periods demonstrated resistance to linezolid. Resistance increases are observed in the case of all antibiotics tested, except for penicillin: 2018, R = 34.52% vs. 2022, R = 30.9% and ampicillin: 2018, R = 20.23% vs. 2022, R = 10.9%. A detailed evaluation of the resistance and sensitivity of Enterococcus spp. to the tested antibiotics in the studied periods is represented in Table 6.

Table 6.
Enterococcus spp. susceptibility and resistance patterns.
The least frequent uropathogen, Staphylococcus spp., showed the highest rate of resistance to penicillin, R = 20 (52.63%) and trimethoprim-sulfamethoxazole, R = 8 (21.05%). Maintained resistance rates were observed for nitrofurantoin R = 1 (2.63%) and linezolid R = 2 (5.6%). A decrease in the resistance of this uropathogen was observed in the case of trimethoprim-sulfamethoxazole and linezolid. A detailed evaluation of the resistance and sensitivity of Staphylococcus spp. to the tested antibiotics in the studied periods is represented in Table 7.

Table 7.
Staphylococcus spp. sensitivity and resistance patterns.
Considering all the Gram-negative strains’ overall resistance and sensitivity patterns, the highest resistance was observed for levofloxacin, followed by ceftazidime and amikacin. The highest sensitivity was observed for carbapenems—imipenem and meropenem. Considering the overall resistance and sensitivity patterns in all the Gram-positive strains, levofloxacin had the highest resistance, followed by penicillin. The most heightened sensitivity was observed for linezolid and nitrofurantoin. A visual representation of the overall resistance and sensitivity patterns in all the tested Gram-negative and positive strains is presented in Figure 1 and Figure 2.
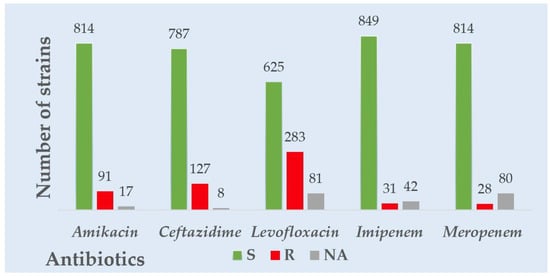
Figure 1.
Overall Gram-negative strains’ resistance to common antibiotics tested.
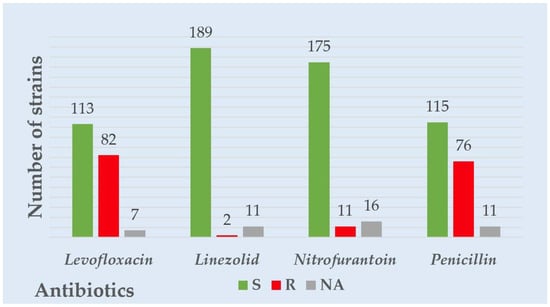
Figure 2.
Overall Gram-positive strains’ resistance to common antibiotics tested.
The evolution of the resistance profiles of Gram-negative pathogens over the 5 years studied displayed a tendency to increase for all the usual antibiotics used to treat UTIs. The numbers observed were as follows: amikacin (2018, R = 5.8%; 2020, R = 5.76%; 2022, R = 18.33%), ceftazidime (2018, R = 11.8%; 2020, R = 13.58%; 2022 R = 17.33%), and levofloxacin (2018, R = 25.59%; 2020, R = 33.33%; 2022 R = 35.0%). Some constant evolution of carbapenem resistance was observed. A graphic visualization of the evolution of the Gram-negative uropathogens’ resistance to the tested antibiotics during all the studied periods is presented in Figure 3.
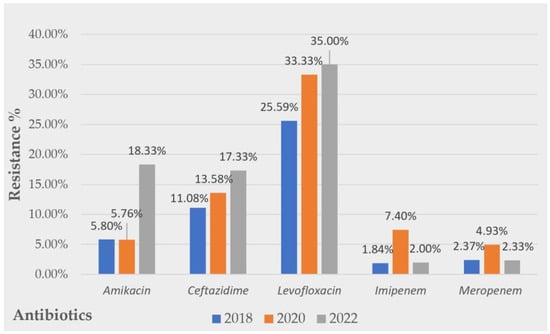
Figure 3.
Evolution of Gram-negative strains’ resistance over a 5 year period to common antibiotics tested.
Considering the evolution of Gram-positive uropathogens’ resistance patterns, inconstant rates of resistance were detected. No linear evolution tendency was observed for any of the tested antibiotics. However, alarming resistance rates were observed for levofloxacin and penicillin. A tripling in the nitrofurantoin resistance rate was observed from 2018, R = 3.03% to 2022, R = 10.14%. Favorable resistance rates were observed for linezolid. A graphic visualization of the evolution of the resistance Gram-positive uropathogens to the tested antibiotics along all the studied periods is presented in Figure 4.
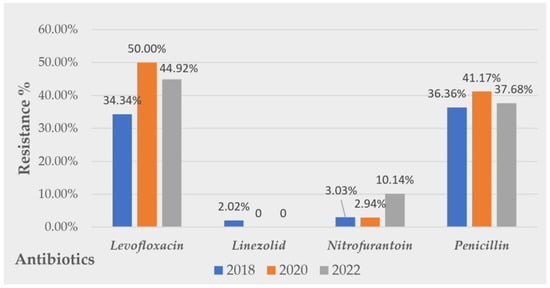
Figure 4.
Evolution of Gram-positive strains’ resistance over 5 years to common antibiotics tested.
3. Discussion
The resistance of uropathogens to the conventional classes of antibiotics is a public health problem that has become more and more pronounced recently, and is considered to be one of the top most important risk factors for the safety of humanity. The increasingly aggressive prescription of antibiotics globally by health services, the empiric administration of first-line antibiotics in uncomplicated urinary infections, and the over-the-counter sale of these classes of drugs in many developing countries have led, in recent decades, to an overwhelming increase in resistance rates to the most common classes of antibiotics [19].
3.1. Differences and Trends Regarding the Prevalence of Uropathogens in Relation to the Patient’s Age
In the entire group of patients and in all the studied periods, progressive increases were observed, which were directly proportional between the subjects’ age and the incidence of uropathogens. A review of the literature published in the Aging Health Journal showed a higher incidence of UTI among women compared to men and a proportional increase with the age of the subjects, reaching over 10% per year among women over 60 and over 30% per year among women over 85 years old [20,21]. A recently published review [22] showed that 1–5% of healthy premenopausal women, 4–19% of otherwise healthy older women and men, and 15–50% of institutionalized elderly people experience asymptomatic bacteriuria, showing a similar linear increase in the incidence rates to our results. In the Netherlands, research on UTIs in people over the age of 85 found that women had a 1.7-fold higher risk than men (incidence of 12.8 per 100 persons each year; incidence of 7.8 per 100 persons each year) [23]. Women’s incidence of UTIs increased from 9 to 11% in subjects aged 65 to 74, from 11.4 to 14.3%, and from 14.7 to 19.8% in subjects aged 75 to 84 and > 85 years, respectively, according to a large observational study of UTIs in older adults conducted in the United Kingdom [24].
Escherichia coli is the most common ubiquitous uropathogen in the entire population studied, in all age groups, representing a total of 54.33% of all studied pathogens, which is significantly lower than those between 60% and 90% reported in countries such as Morocco [25], Portugal [26], or Pakistan [27]. Similarly, high rates of Escherichia coli incidence were detected in Western European countries, such as France or Austria, reporting rates over 65% in the studied populations [28]. The studies show similar incidence rates between 45 and 55% to those detected in the present survey in countries close to Romania, such as Hungary or Italy [29,30]. Klebsiella spp. was the second-most common Gram-negative uropathogen isolated in the studied cohort, with the incidence increasing linearly with each evaluated age group; similar results to those presented were found in studies in Libya [31] and Iraq [32]. In the present study, the most frequent Gram-positive uropathogen was presented by Enterococcus spp. with an almost 15% incidence rate, with the prevalence increasing linearly with the age group. However, other studies have shown significantly lower incidence rates of only 2.8% (Pakistan) [33] and 4.8% (Nepal) [34].
3.2. Evolution of the Resistance Patterns of Gram-Negative Uropathogens
A linearly increasing evolution was observed in the case of Escherichia coli, the most frequent Gram-negative pathogen throughout the studied periods in the case of all tested antibiotics, with few exceptions. The highest resistance rates were observed for levofloxacin (R = 30.66%) and amoxicillin-clavulanic acid (R = 28.05%). Similar data were noticed in a recent systematic review and metanalysis published by Ballesteros-Monrreal et al. [35], which showed an alarming increase in recent years of multidrug-resistant uropathogenic Escherichia coli, with a high incidence of aminopenicillins resistant strains—extended spectrum beta-lactamase (ESBL) representing over 55%. Another recent paper from the United States [36] highlighted the increasing incidence of fluoroquinolone-resistant strains of both Escherichia coli and Klebsiella spp., with alarmingly high rates of recent resistance, and underlined the urgent need for necessary measures to change the empirical treatment of UTIs.
Extensive research from Spain published in 2021 [37] on Klebsiella spp. isolated strains from different specimens underlined similarities in resistance trends regarding multiple antibiotic classes, and it especially highlighted the alarming resistance profiles in urine strain isolates compared with other sites, such as blood or respiratory infections. The highest rates of resistance among Gram-negative pathogens were observed in the case of Pseudomonas spp. Our results share similarities with recent data published in 2021 in a large study [38] about the patterns of this bacteria in UTIs; it highlighted a 27.7% resistance to amikacin, which is similar to the 30.95% resistance rates found in our study; 50% resistance to cephalosporins, which is close to the 45.23% resistance found in our research. Still, it showed 38.7% resistance to levofloxacin, which is considerably lower than the 54.76% resistance in the present study. Proteus spp., the most important uropathogen correlating UTIs and urinary lithiasis, was encountered in 7.2% of cases. The highest resistance was observed to levofloxacin (R = 37.05%), followed by amoxicillin–clavulanic ac. (R = 30.86%); these results show a better sensitivity compared to a recent publication from 2021 [39], which underlines high resistance to trimethoprim-sulfamethoxazole (R = 97%), nalidixic ac. (R = 93%), and amoxicillin (R = 62%) in the case of Proteus spp. A study from Hungary [40] that followed the evolution of Gram-negative pathogens’ resistance over 10 years reported high rates of multidrug-resistant Proteus spp. in the analyzed samples.
The evolution of the resistance of Gram-negative bacteria throughout the evaluated period is significant. It showed linear increases in amikacin, ceftazidime, and levofloxacin. Similar results were obtained by a recent study published last year [41], following over 12 years of observations at Peking University Hospital in Beijing, China. It highlighted high resistance in Gram-negative pathogens for cephalosporins and fluoroquinolones and alarmingly increasing carbapenem rates, especially in the geriatric population. It also concluded that the latter category is more susceptible to multidrug-resistant strains. Research published in April 2022, observing 10 years of the uropathogens’ resistance at the University of Gondar, Ethiopia [42], found more resistant uropathogens strains with an alarming evolution, represented by an overall resistance to Escherichia coli of R = 74% (amoxicillin-clavulanic ac.), R = 55.6% (ciprofloxacin), R = 24% (amikacin), and R = 26.5% (meropenem); Klebsiella spp.—R = 86.3% (amoxicillin-clavulanic ac.), R = 53.3% (ciprofloxacin), R = 36.4% (amikacin), and R = 17.6% (meropenem). The study highlighted that more than 44% of the total strains were multidrug-resistant during the observed period, emphasizing the severe evolution of pathogen resistance in the short and medium term of the observation [42].
The evolution of Psudomonas spp. resistance to various classes of antibiotics is alarming, both in terms of the increased frequency of strains’ resistant to at least one antibiotic and multidrug-resistant bacteria. A review from last year [43] shows, according to ECDC data, that 33.9% of all strains of P. aeruginosa in Europe are resistant to at least one of the studied antibiotics (cephalosporins, fluoroquinolones, and aminoglycosides). This increased resistance is observed especially in southern and eastern European countries, such as Greece, Bulgaria, Serbia, Slovakia, and Romania, where over 50% of the strains tested are resistant to at least one antibiotic from these classes, which is similar to the current study [43].
3.3. Evolution of the Resistance Patterns of Gram-Positive Uropathogens
Enterococcus spp. was the most frequent Gram-positive uropathogen, presenting high heterogenicity of resistance to different classes of antibiotics. The most significant rise in the resistance was observed for levofloxacin, from R = 38.09% (2018) to R = 52.78% (2022), and nitrofurantoin, from R = 3.57% (2018) to R = 10.9% (2022). A large study from Poland [44] followed the antibiotic susceptibility of Enterococcus strains in urinary probes from urology and nephrology patients and highlighted exciting results in terms of resistance patterns. Similar to our study, it presented high resistance to aminopenicillins and fluoroquinolones, with a resistance to norfloxacin between 50 and 80%, depending on the isolated strain. Ferede ZT et al. [45] showed no resistance to linezolid for all tested Enterococci strains, which is similar to our results. They noted 93.3% sensitivity to vancomycin, which is identical to our findings of 93.29%. In contrast, they observed extremely high resistance to ampicillin (80%), whereas our study shows only 18% overall resistance, with the highest sensitivity in the most recent determination—R = 10.9% (2022). Another publication analyzing the antibiotic resistance of Enterococcus spp. and its relation with biofilm formation [46] shows alarming data about resistance to different antibiotics. High resistance to levofloxacin (over 77%) and tetracyclin (over 86%) was encountered. Meanwhile, it shows relatively preserved sensitivity to nitrofurantoin, which is similar to our study and can still be a good option for treatment in uncomplicated cases. The presence of multidrug Enterococcus strains emerging is an alarming signal in recent publications, highlighting the presence of up to seven different classes of antibiotic resistance in selected strains, which shows an unfavorable evolution of the current resistance of this pathogen.
The least common Gram-positive uropathogen, Staphylococcus spp., presents relatively low rates of resistance evolution, except for penicillin (R = 52.63%) and trimetoprim-sulfamethoxazole (R = 21.05%). A recent study from 2022, following 1327 patients with UTIs from Tanzania [47], underlined alarming rates of resistance of this pathogen, which were much higher than our results; it shows over 40% resistance to nitrofurantoin, compared to 2.63% in our study, and linezolid over 18% resistance, compared to 5.25% in the current research. Another paper from France [48] shows relatively similar resistance rates to penicillin (R = 59.0%) and fluoroquinolones (R = 36.3%). Meanwhile, data from Southern Ireland at University Hospital Waterford [49] shows a significantly increased incidence of super-resistant Staphylococcus aureus, resulting in almost 28% of this pathogen’s total strains being methicillin-resistant. However, this research shows similar resistance to our study of nitrofurantoin (R = 2.7%), underlining its positive benefit in the treatment of Gram-positive UTIs.
3.4. The Implications of the COVID-19 Pandemic on the AMR of Uropathogens
The high antibiotic use among COVID-19 patients has amplified the antimicrobial resistance (AMR) issue. Antibiotics do not treat COVID-19; nonetheless, they are frequently administered in patients with respiratory disease due to early diagnostic ambiguity and worry about bacterial co-infection or subsequent infection in those who have confirmed COVID-19. In earlier evaluations [50,51,52,53], it was discovered that COVID-19 patients received a high percentage of antibiotic prescriptions (about 75%), despite only a small percentage of them having bacterial infections, especially those outside of the intensive care unit setting. One way to optimize antibiotic prescription and, at least in part, stop the emergence of antibiotic resistance is to be aware of local and regional antimicrobial susceptibility differences (AMR); thus, knowing local resistance evolution is essential.
According to recent data from 45 public and private clinics in Ireland, 76% of research participants stated that COVID-19 had a negative impact on how well antibiotic stewardship programs were implemented [54]. A study from Egypt published this year [55] following the evolution of AMR during the pandemic shows a significant increase in resistance for the vast majority of the tested strains. Escherichia coli showed an important rise in resistance to carbapenems, ceftazidime, and amikacin; Klebsiella to nitrofurantoin, gentamycin, and amoxicillin-clavulanic ac; and Enterococcus to trimethoprim-sulfamethoxazole, levofloxacin, and amikacin. A survey conducted by our team last year [18] studied resistance evolution pre-pandemic and during the pandemic, and showed a significant increase in resistance to fluoroquinolones and carbapenems of both Klebsiella spp. and Pseudomonas. It also highlighted the increased resistance of Escherichia coli to amoxicillin-clavulanic ac., levofloxacin, ceftazidime, and nitrofurantoin. Comparative research [56] that evaluated uropathogens’ antibiotic resistance changes in Iran during 2020 and 2022 highlighted an important resistance increase for ampicillin, carbapenems, and ceftazidime for Escherichia coli. Resistance rates were increased for Klebsiella spp. to ampicillin, levofloxacin, and ceftazidime. Pseudomonas spp. presented the lowest sensitivity to cefepime and carbapenems [56]. Similar research, published in February 2023 and conducted in Morocco [57], studied uropathogenic bacterial resistance profiles before and after the COVID-19 outbreak. It shows a significant increase in resistance, close to our results, especially for Escherichia coli to amoxicillin and levofloxacin; for Klebsiella spp. to amoxicillin and ceftriaxone; and for Enterococcus to levofloxacin and ciprofloxacin. Surprisingly, it showed a decreased resistance for Klebsiella spp. to amikacin, carbapenems, and trimethoprim-sulfamethoxazole, which is similar to our study, and for Enterococcus spp. to ceftriaxone, carbapenems, and trimethoprim-sulfamethoxazole.
Although the pandemic has had a worldwide impact, the negative repercussions are likely to be severe, leading to a higher burden of AMR. As a result, active antibiotic stewardship in all hospitals, clinics, and communities is required to ensure a sustainable future. As a result, concentrated global efforts and leadership with a higher level of public involvement and international cooperation are urgently needed to mitigate the pandemic’s negative impact on AMR.
3.5. Limitations
A few limitations have to be considered. The lack of additional information about patients’ medical histories, such as the consumption of antibiotics, history of urinary tract surgeries, or history of indwelling catheters—all critical contributors to the emergence and spread of resistant uropathogen strains—represents a limitation of this study. “There is strength in numbers”; thus, another limitation is the reduced quantity of analyzed urine cultures. The conclusions would be improved when the estimation of the evaluated probes was higher. However, this study presents information from female patients with a range of different pathologies from a major teaching hospital in the country’s capital city. Another fact that might influence our conclusions is the short time between the examined intervals, before, during, and immediately after the COVID-19 pandemic; the more time passes after the viral outbreak, the more trustworthy conclusions can be drawn.
More study is necessary to understand the dynamics of the viral illness in the evolution of antibiotic resistance in uropathogens. Despite the limitations, this study has the potential to advance knowledge about the fundamental role that the pandemic is playing in the selection of resistant strains of bacteria involved in UTIs and the emerging of AMR.
4. Materials and Methods
4.1. Study Design and Setting
The current cross-sectional retrospective study was conducted during three different periods of four months each, before, during, and immediately after the COVID-19 outbreak, between September 2018 and December 2022, as follows: 1 September 2018–31 December 2018; 1 September 2020–31 December 2020; and 1 September 2022–31 December 2022 at a major Urology Clinic—“Prof. Dr. Th. Burghele”—from Bucharest, Romania.
4.2. Study Population
As mentioned earlier, a total of 12,845 urine probes were analyzed during the period, of which 1124 samples met the inclusion criteria for this study. It involved only female patients over 18 years old, with positive urine culture—more than 105 CFU/mL—and single bacterial strain on urine culture. The exclusion criteria of the current study were represented by male sex or patients under 18 years old, less than 105 CFU/mL, two or more bacterial strains on urine culture, and patients with indwelling catheters. A representative flowchart of the study population is presented in Figure 5.
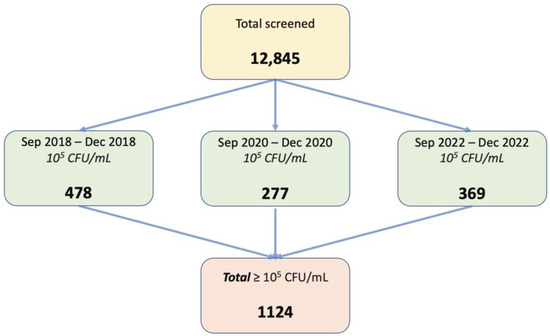
Figure 5.
Flow-chart on patients’ distribution in the study.
4.3. Data and Sample Collection
General information data were collected, such as age and sex for both hospitalized and non-hospitalized patients, while data regarding history, the behavioral, or clinical aspect of the selected study population were not able to be noted due to the retrospective character of the current research. After receiving adequate instructions, participants self-collected 5–10 mL of clean-catch, mid-stream urine (MSU) samples in a sterile urine container. In all cases, the collection of urine probes adhered to international safety guidelines [58]. Within 2 h after collection, samples were transferred to a designated box to the Clinic’s Microbiology Laboratories for further processing.
4.4. Quantitative Urine Culture, Bacterial Identification, and Antibiotic Susceptibility Test
After inoculating the urine samples with a sterile disposable loop on standard inoculation plates, they were incubated for 24 h. The bacteria were cultured on Columbia sheep agar and lactose agar using urine samples that had been obtained in a sterile container. In further instances, we cultured Staphylococcus spp. using the Chapman medium. On a culture media, significant microbial growth and colony morphology (e.g., color, size, and texture) and features were observed. Bacterial counts of more than 105 CFU/mL of no more than two microorganism species were considered significant. Colony morphology, Gram stain, and many standard biochemical tests (lactose fermentation, catalase, oxidase, indole, methyl red, etc.) were used to identify the bacteria. Zones of antibiotic inhibition were interpreted per the Clinical and Laboratory Standards Institute (CLSI) recommendations for Antimicrobials Susceptibility Testing (AST), which was carried out using the Kirby–Bauer disk diffusion method [59]. Discussions of bacterial culture, uropathogen identification, and the implemented antibiotic susceptibility tests have already been reported in previous publications [14,16,17,18,60].
5. Conclusions
From all the urine samples analyzed throughout the evaluated periods, Escherichia coli and Klebsiella spp. are the most frequent Gram-negative uropathogens, while Enterococcus spp. is the usual Gram-positive bacteria involved in UTIs. In the case of all the pathogens, important variations in the resistance patterns of the different antibiotics tested were observed, following increases in resistance in most cases in the short and medium term.
We discovered the highest overall rates of resistance for Pseudomonas spp. There are also situations where the sensitivity increased throughout the evaluated period, but these were the exceptions. Briefly, a negative impact of the COVID-19 pandemic outbreak was observed in the evolution of the resistance rate of the main classes of antibiotics used in UTIs in all studied uropathogens.
Further studies are required to determine if this evolution is a phenomenon encountered in more regions and to take firm and rapid measures to slow down the process of AMR spreading fueled by antibiotic overprescription.
Author Contributions
Conceptualization, C.M., R.-C.P. and A.P.; methodology, C.M. and R.-I.P.; validation, R.-C.P., B.F.G. and V.J.; formal analysis, C.M. and R.-I.P.; investigation, C.M., A.P., R.-I.P. and V.J.; resources, C.M. and A.P.; data curation, R.-C.P. and V.J.; writing—original draft preparation, C.M. and R.-I.P.; writing—review and editing, R.-C.P., A.P., B.F.G. and V.J.; visualization, A.P., B.F.G. and V.J.; supervision, R.-C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Prof. Dr. Th. Burghele” Clinical Hospital no. 2/2009.
Informed Consent Statement
The data collected retrospectively did not contain any personal information. For each patient, written informed consent was obtained.
Data Availability Statement
Data supporting the reported results are available from the authors.
Acknowledgments
The results regarding the pre-pandemic period are partially presented in the paper: “Spectrum and antibiotic resistance of uropathogens in Romanian females” published by Petca R.-C. et al. in Antibiotics, 2020, 9, 472, doi:10.3390/antibiotics9080472 [17]. The results regarding pre-pandemic and during the period are partially presented in the paper “Does the COVID Pandemic Modify the Antibiotic Resistance of Uropathogens in Female Patients? A New Storm?” published by Mareș C. et al. in Antibiotics, 2012, 11, 376, doi: 10.3390/antibiotics11030376 [18].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Schappert, S.M.; Rechtsteiner, E.A. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 13 2011, 169, 1–38. [Google Scholar] [PubMed]
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open Forum Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.V.; Babiker, A.; Master, R.N.; Luu, T.; Mathur, A.; Bordon, J. Antibiotic resistance among urinary isolates from female outpatients in the United States in 2003 and 2012. Antimicrob. Agents Chemother. 2016, 60, 2680–2683. [Google Scholar] [CrossRef]
- Mortazavi-Tabatabaei, S.A.R.; Ghaderkhani, J.; Nazari, A.; Sayehmiri, K.; Sayehmiri, F.; Pakzad, I. Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis. Int. J. Prev. Med. 2019, 10, 169. [Google Scholar]
- Fihn, S.D. Clinical practice. Acute uncomplicated urinary tract infection in women. N. Engl. J. Med. 2003, 349, 259–266. [Google Scholar] [CrossRef]
- Griebling, T.L. Urologic diseases in America project: Trends in resource use for urinary tract infections in women. J. Urol. 2005, 173, 1281–1287. [Google Scholar] [CrossRef]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Ikäheimo, R.; Siitonen, A.; Heiskanen, T.; Kärkkäinen, U.; Kuosmanen, P.; Lipponen, P.; Mäkelä, P.H. Recurrence of urinary tract infection in a primary care setting: Analysis of a 1-year follow-up of 179 women. Clin. Infect. Dis. 1996, 22, 91–99. [Google Scholar] [CrossRef]
- Hooton, T.M. Clinical practice. Uncomplicated urinary tract infection. N. Engl. J. Med. 2012, 366, 1028–1037. [Google Scholar] [CrossRef]
- Beerepoot, M.A.; ter Riet, G.; Nys, S.; van der Wal, W.M.; de Borgie, C.A.; de Reijke, T.M.; Prins, J.M.; Koeijers, J.; Verbon, A.; Stobberingh, E.; et al. Lactobacilli vs antibiotics to prevent urinary tract infections: A randomized, double-blind, noninferiority trial in postmenopausal women. Arch. Intern. Med. 2012, 172, 704–712. [Google Scholar] [CrossRef]
- Pfau, A.; Sacks, T. The bacterial flora of the vaginal vestibule, urethra and vagina in premenopausal women with recurrent urinary tract infections. J. Urol. 1981, 126, 630–634. [Google Scholar] [CrossRef]
- Raz, R. Urinary tract infection in postmenopausal women. Korean J. Urol. 2011, 52, 801–808. [Google Scholar] [CrossRef]
- Petca, R.-C.; Negoita, S.; Mares, C.; Petca, A.; Popescu, R.-I.; Chibelean, C.B. Heterogeneity of antibiotics multidrug-resistance profile of uropathogens in Romanian population. Antibiotics 2021, 10, 523. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyere, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F. EAU Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2022. [Google Scholar]
- Chibelean, C.B.; Petca, R.-C.; Mareș, C.; Popescu, R.-I.; Enikő, B.; Mehedințu, C.; Petca, A. A clinical perspective on the antimicrobial resistance spectrum of uropathogens in a Romanian male population. Microorganisms 2020, 8, 848. [Google Scholar] [CrossRef]
- Petca, R.-C.; Mareș, C.; Petca, A.; Negoiță, S.; Popescu, R.-I.; Boț, M.; Barabás, E.; Chibelean, C.B. Spectrum and antibiotic resistance of uropathogens in Romanian females. Antibiotics 2020, 9, 472. [Google Scholar] [CrossRef]
- Mareș, C.; Petca, R.-C.; Petca, A.; Popescu, R.-I.; Jinga, V. Does the COVID pandemic modify the antibiotic resistance of uropathogens in female patients? A new storm? Antibiotics 2022, 11, 376. [Google Scholar] [CrossRef]
- Lee Ventola, C. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Rowe, T.A.; Juthani-Mehta, M. Urinary tract infection in older adults. Aging Health 2013, 9, 519–528. [Google Scholar] [CrossRef]
- Eriksson, I.; Gustafson, Y.; Fagerström, L.; Olofsson, B. Prevalence and factors associated with urinary tract infections (UTIs) in very old women. Arch. Gerontol. Geriatr. 2010, 50, 132–135. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L. Urinary tract infections in the elderly: A review of disease characteristics and current treatment options. Drugs Context 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caljouw, M.A.; den Elzen, W.P.; Cools, H.J.; Gussekloo, J. Predictive factors of urinary tract infections among the oldest old in the general population. A population-based prospective follow-up study. BMC Med. 2011, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Farewell, D.; Jones, H.M.; Francis, N.A.; Paranjothy, S.; Butler, C.C. Incidence and antibiotic prescribing for clinically diagnosed urinary tract infection in older adults in UK primary care, 2004–2014. PLoS ONE 2018, 13, e0190521. [Google Scholar] [CrossRef] [PubMed]
- Arsalane, L.; Kamouni, Y.; Yahyaoui, H.; Bennouar, N.; Berraha, M.; Zouhair, S. Profil actuel de résistance aux antibiotiques des souches d’Escherichia coli uropathogènes et conséquences thérapeutiques. Prog. Urol. 2014, 24, 1058–1062. [Google Scholar]
- Silva, A.; Costa, E.; Freitas, A.; Almeida, A. Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics 2022, 11, 768. [Google Scholar] [CrossRef]
- Ramrakhia, S.; Raja, K.; Dev, K.; Kumar, A.; Kumar, V.; Kumar, B. Comparison of Incidence of Urinary Tract Infection in Diabetic vs Non-Diabetic and Associated Pathogens. Cureus 2020, 12, e10500. [Google Scholar] [CrossRef]
- Schito, G.C.; Naber, K.G.; Botto, H.; Palou, J.; Mazzei, T.; Gualco, L.; Marchese, A. The ARESC study: An international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 2009, 34, 407–413. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Urinary Tract Infections in Elderly Patients: A 10-Year Study on Their Epidemiology and Antibiotic Resistance Based on the WHO Access, Watch, Reserve (AWaRe) Classification. Antibiotics 2021, 10, 1098. [Google Scholar] [CrossRef]
- Folliero, V.; Caputo, P.; Della Rocca, M.T.; Chianese, A.; Galdiero, M.; Iovene, M.R.; Hay, C.; Franci, G.; Galdiero, M. Prevalence and antimicrobial susceptibility patterns of bacterial pathogens in urinary tract infections in University Hospital of Campania “Luigi Vanvitelli” between 2017 and 2018. Antibiotics 2020, 9, 215. [Google Scholar] [CrossRef]
- Ismail, F.; Haq, S.; Aiayad, M.; Abushiba, M.; Zorgani, A. Antibiotic resistance patterns of urinary pathogens in outpatients and inpatients: A report from Eastern Libya. Int. J. Urol. Nurs. 2022, 16, 55–61. [Google Scholar] [CrossRef]
- Polse, R.F.; Qarani, S.M.; Assafi, M.S.; Sabaly, N.; Ali, F. Incidence and Antibiotic Sensitivity of Klebsiella pneumonia isolated from urinary tract infection patients in Zakho emergency hospital/Iraq. J. Educ. Sci. 2020, 29, 257–268. [Google Scholar] [CrossRef]
- Muhammad, A.; Khan, S.N.; Ali, N.; Rehman, M.U.; Ali, I. Prevalence and antibiotic susceptibility pattern of uropathogens in outpatients at a tertiary care hospital. New Microbes New Infect. 2020, 36, 100716. [Google Scholar] [CrossRef]
- Pandey, B.; Pandit, M.; Jaiswal, S.; Sah, A.K.; Chand, R.S.; Shrestha, R. Antimicrobial susceptibility pattern of pathogenic bacteria causing urinary tract infection in tertiary care hospital in Kathmandu, Nepal. Int. J. Pharm. Sci. Res. 2020, 11, 6448–6455. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Mendez-Pfeiffer, P.; Barrios-Villa, E.; Arenas-Hernández, M.M.P.; Enciso-Martínez, Y.; Sepúlveda-Moreno, C.O.; Bolado-Martínez, E.; Valencia, D. Uropathogenic Escherichia coli in Mexico, an overview of virulence and resistance determinants: Systematic review and meta-analysis. Arch. Med. Res. 2023, 54, 247–260. [Google Scholar] [CrossRef]
- Faine, B.A.; Rech, M.A.; Vakkalanka, P.; Gross, A.; Brown, C.; Harding, S.J.; Slocum, G.; Zimmerman, D.; Zepeski, A.; Rewitzer, S.; et al. High prevalence of fluoroquinolone-resistant UTI among US emergency department patients diagnosed with urinary tract infection, 2018–2020. Acad. Emerg. Med. 2022, 29, 1096–1105. [Google Scholar] [CrossRef]
- Ballén, V.; Gabasa, Y.; Ratia, C.; Ortega, R.; Tejero, M.; Soto, S. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 2021, 11, 738223. [Google Scholar] [CrossRef]
- Al-Orphaly, M.; Hadi, H.A.; Eltayeb, F.K.; Al-Hail, H.; Samuel, B.G.; Sultan, A.A.; Skariah, S. Epidemiology of Multidrug-Resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere 2021, 6, e00202-21. [Google Scholar] [CrossRef]
- Tabatabaei, A.; Ahmadi, K.; Shabestari, A.N.; Khosravi, N.; Badamchi, A. Virulence genes and antimicrobial resistance pattern in Proteus mirabilis strains isolated from patients attended with urinary infections to Tertiary Hospitals, in Iran. Afr. Health Sci. 2021, 21, 1677–1684. [Google Scholar] [CrossRef]
- Gajdács, M.; Bátori, Z.; Ábrók, M.; Lázár, A.; Burián, K. Characterization of resistance in Gram-negative urinary isolates using existing and novel indicators of clinical relevance: A 10-year data analysis. Life 2020, 10, 16. [Google Scholar] [CrossRef]
- Huang, L.; Huang, C.; Yan, Y.; Sun, L.; Li, H. Urinary tract infection etiological profiles and antibiotic resistance patterns varied among different age categories: A retrospective study from a tertiary general hospital during a 12-year period. Front. Microbiol. 2022, 12, 813145. [Google Scholar] [CrossRef]
- Kasew, D.; Desalegn, B.; Aynalem, M.; Tila, S.; Diriba, D.; Afework, B.; Getie, M.; Biset, S.; Baynes, H.W. Antimicrobial resistance trend of bacterial uropathogens at the university of Gondar comprehensive specialized hospital, northwest Ethiopia: A 10 years retrospective study. PLoS ONE 2022, 17, e0266878. [Google Scholar] [CrossRef] [PubMed]
- AL-Khikani, F.H.; Ayit, A.S. Pseudomonas Aeruginosa a tenacious uropathogen: Increasing challenges and few solutions. Biomed. Biotechnol. Res. J. 2022, 6, 311–318. [Google Scholar] [CrossRef]
- Kraszewska, Z.; Skowron, K.; Kwiecinska-Piróg, J.; Grudlewska-Buda, K.; Przekwas, J.; Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Antibiotic resistance of Enterococcus spp. isolated from the urine of patients hospitalized in the university hospital in North-Central Poland, 2016–2021. Antibiotics 2022, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Ferede, Z.T.; Tullu, K.D.; Derese, S.G.; Yeshanew, A.G. Prevalence and antimicrobial susceptibility pattern of Enterococcus species isolated from different clinical samples at Black Lion Specialized Teaching Hospital, Addis Ababa, Ethiopia. BMC Res. Notes 2018, 11, 793. [Google Scholar] [CrossRef]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic resistance and biofilm formation in Enterococcus spp. isolated from urinary tract infections. Pathogens 2023, 12, 34. [Google Scholar] [CrossRef]
- Silago, V.; Moremi, N.; Mtebe, M.; Komba, E.; Masoud, S.; Mgaya, F.X.; Mirambo, M.M.; Nyawale, H.A.; Mshana, S.E.; Matee, M.I. Multidrug-resistant uropathogens causing community acquired urinary tract infections among patients attending health facilities in Mwanza and Dar es Salaam, Tanzania. Antibiotics 2022, 11, 1718. [Google Scholar] [CrossRef]
- Joya, M.; Aalemi, A.K.; Baryali, A.T. Prevalence and antibiotic susceptibility of the common bacterial uropathogen among uti patients in French Medical Institute for Children. Infect. Drug Resist. 2022, 15, 4291–4297. [Google Scholar] [CrossRef]
- Looney, A.T.; Redmond, E.J.; Davey, N.M.; Daly, P.J.; Troy, C.; Carey, B.F.; Cullen, I.M. Methicillin-resistant Staphylococcus aureus as a uropathogen in an Irish setting. Medicine 2017, 96, e4635. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Stoichitoiu, L.E.; Pinte, L.; Ceasovschih, A.; Cernat, R.C.; Vlad, N.D.; Padureanu, V.; Sorodoc, L.; Hristea, A.; Purcarea, A.; Badea, C.; et al. In-Hospital antibiotic use for COVID-19: Facts and rationales assessed through a mixed-methods study. J. Clin. Med. 2022, 11, 3194. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front. Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial resistance in the context of the sustainable development goals: A brief review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Philbin, M.; Hughes, G.; Bergin, C.; Fe Talento, A. Antimicrobial stewardship challenges and innovative initiatives in the acute hospital setting during the COVID-19 pandemic. J. Antimicrob. Chemother. 2021, 76, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Abdel Gawad, A.M.; Ashry, W.M.O.; El-Ghannam, S.; Hussein, M.; Yousef, A. Antibiotic resistance profile of common uropathogens during COVID-19 pandemic: Hospital based epidemiologic study. BMC Microbiol. 2023, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Khoshbakht, R.; Kabiri, M.; Neshani, A.; Khaksari, M.N.; Sadrzadeh, S.M.; Mousavi, S.M.; Ghazvini, K.; Ghavidel, M. Assessment of antibiotic resistance changes during the Covid-19 pandemic in northeast of Iran during 2020–2022: An epidemiological study. Antimicrob. Resist. Infect. Control 2022, 11, 121. [Google Scholar] [CrossRef]
- El Omari, L.; Sakhi, A.; Miloudi, M.; Elkamouni, Y.; Zouhair, S.; Arsalane, L. The impact of the COVID pandemic on the uropathogenic bacterial resistance profile: Experience of the bacteriology lab of the military hospital Avicenne in Marrakech. GSC Adv. Res. Rev. 2023, 14, 59–65. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Collection of Clinical Specimens during Field Investigation of Outbreaks; World Health Organization: Geneva, Switzerland, 2000; pp. 1–51. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Petca, R.C.; Popescu, R.I.; Mares, C.; Petca, A.; Mehedintu, C.; Sandu, I.; Maru, N. Antibiotic resistance profile of common uropathogens implicated in urinary tract infections in Romania. Farmacia 2019, 67, 994–1004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).