Clonal Complexes Distribution of Staphylococcus aureus Isolates from Clinical Samples from the Caribbean Islands
Abstract
1. Introduction
2. Results
2.1. Analysis of Clinical Isolates
2.2. Distribution of S. aureus Clonal Complexes and Strains
2.3. Observations Regarding Individual Clonal Complexes of S. aureus
2.4. Observations Regarding S. argenteus
2.5. Detection of PVL
2.6. Sequencing the Composite SCCmec Element in Clonal Complex 239 Isolates
| Gene ID | Gene Product/Explanation | Position in SCC of 2020-021_7037M | Position in SCC of 2020-048_8421A | Orientation in 2020-021_7037M and 048_8421A | Position in SCC of 2020-009_371M | Orientation in 2020-009_371M | Present in ATCC1228, M1, etc. |
|---|---|---|---|---|---|---|---|
| DR-SCC | direct repeat of SCC | 1 to 19 | 1 to 19 | n/a * | 1 to 19 | n/a | - |
| SCCterm07 | terminus of SCC towards orfX | 20 to 122 | 20 to 122 | n/a | 20 to 122 | n/a | X ** |
| hsdS | type I restriction-modification system site-specificity determinate | 256 to 1458 | 256 to 1458 | forward | 256 to 1458 | forward | X |
| tnp trnc. | transposase for IS1272 | 1513 to 1712 | 1513 to 1712 | trnc. | 1513 to 1712 | trnc. | X |
| speG | spermidine N-acetyltransferase | 1781 to 2278 | 1781 to 2278 | rev. compl. | 1781 to 2278 | rev. compl. | X |
| A9UFT0 | LPXTG protein homologue | 2531 to 2753 | 2531 to 2753 | rev. compl., trnc., frameshift | 2531 to 2753 | rev. compl., trnc., frameshift | X |
| PF11070 | putative PF11070 family protein | 2950 to 3360 | 2950 to 3360 | rev. compl. | 2950 to 3360 | rev. compl. | X |
| A9UFT0 | LPXTG protein homologue | 3523 to 3743 | 3523 to 3743 | rev. compl. | 3523 to 3743 | rev. compl. | X |
| Q9KX75 | putative protein | 3758 to 4261 | 3758 to 4261 | rev. compl. | 3758 to 4261 | rev. compl. | X |
| Q7A207 | putative protein | 4277 to 4588 | 4277 to 4588 | rev. compl. | 4277 to 4588 | rev. compl. | X |
| Q7A206 | putative protein | 4675 to 5025 | 4675 to 5025 | rev. compl. | 4675 to 5025 | rev. compl. | X |
| UTR_ccrB-4 | conserved 3’-untranslated region of ccrB | 5026 to 5525 | 5026 to 5525 | n/a | 5026 to 5525 | n/a | X |
| ccrB-4 | cassette chromosome recombinase B, type 4 | 5526 to 7154 | 5526 to 7154 | rev. compl. | 5526 to 7154 | rev. compl. | X |
| ccrA-4 | cassette chromosome recombinase A, type 4 | 7151 to 8512 | 7151 to 8512 | rev. compl. | 7151 to 8512 | rev. compl. | X |
| cch | cassette chromosome helicase | 8699 to 9334 | 8699 to 9334 | rev. compl., trnc. | 8699 to 9335 | rev. compl., trnc. | X |
| D2N398 | putative protein | 9787 to 10,146 | 9787 to 10,146 | rev. compl. | 9787 to 10,146 | rev. compl. | X |
| yozA | HTH-type transcriptional repressor | 10,353 to 10,679 | 10,353 to 10,679 | forward | 10,353 to 10,679 | forward | X |
| czrC | cadmium and zinc resistance gene C | 11,000 to 12,934 | 11,000 to 12,934 | forward | 11,000 to 12,934 | forward | X |
| SCCterm 02 | terminus of SCC towards orfX | 13,941 to 14,257 | 13,941 to 14,257 | n/a | 13,941 to 14,257 | n/a | - |
| Q2FKL3 | HNH endonuclease family protein | 14,258 to 14,624 | 14,258 to 14,624 | trnc., frameshift | 14,258 to 14,624 | trnc., frameshift | - |
| D1GU38 | putative protein | 14,689 to 15,552 | 14,689 to 15,552 | forward | 14,689 to 15,552 | forward | - |
| D2N370 | putative protein | 15,660 to 17,134 | 15,660 to 17,134 | forward | 15,660 to 17,134 | forward | - |
| Q4LAG3 | putative protein | 17,361 to 18,461 | 17,361 to 18,461 | forward | 17,361 to 18,461 | forward | - |
| Q3T2M7 | putative protein | 18,454 to 18,825 | 18,454 to 18,825 | forward | 18,454 to 18,825 | forward | - |
| ccrAA | cassette chromosome recombinase “AA” | 18,822 to 20,465 | 18,822 to 20,465 | forward | 18,822 to 20,465 | forward | - |
| ccrC | cassette chromosome recombinase C | 20,690 to 22,292 | 20,690 to 22,292 | forward, trnc. | 20,690 to 22,292 | forward, trnc. | - |
| tnp_IS200 | transposase of IS200 | 22,423 to 22,908 | 22,423 to 22,908 | forward | 22,422 to 22,907 | forward | - |
| ccrC | cassette chromosome recombinase C | 23,030 to 23,111 | 23,030 to 23,111 | forward, trnc. | 23,029 to 23,110 | forward, trnc. | - |
| Q4LAF9 | putative protein | 23,200 to 23,538 | 23,200 to 23,538 | forward | 23,199 to 23,537 | forward | - |
| Q7A206 | putative protein | 23,544 to 23,630 | 23,544 to 23,630 | forward, trnc. | 23,543 to 23,629 | forward, trnc. | - |
| Q7A207 | putative protein | 23,632 to 23,943 | 23,632 to 23,943 | forward | 23,631 to 23,942 | forward | - |
| Q9KX75 | putative protein | 23,959 to 24,465 | 23,959 to 24,465 | forward | 23,958 to 24,464 | forward | - |
| A5INT3 | putative protein | 24,486 to 24,803 | 24,486 to 24,803 | forward | 24,485 to 24,802 | forward | - |
| tnpA_Tn554 | transposase A of transposon Tn554 | 24,922 to 26,007 | 24,922 to 26,007 | forward | 24,921 to 26,006 | forward | - |
| tnpB_Tn554 | transposase B of transposon Tn554 | 26,004 to 27,896 | 26,004 to 27,896 | forward | 26,003 to 27,895 | forward | - |
| tnpC_Tn554 | transposase C of transposon Tn554 | 27,903 to 28,280 | 27,903 to 28,280 | forward | 27,902 to 28,279 | forward | - |
| ant9 | adenyltransferase AAd9 | 28,431 to 29,213 | 28,431 to 29,212 | forward | 54,251 to 55,033 | rev. compl. | - |
| erm(A) | rRNA adenine N-6-methyltransferase | 29,339 to 30,070 | 29,338 to 30,069 | rev. compl. | 53,394 to 54,125 | forward | - |
| lp_erm(A) | leader peptide of erm(A) | 30,128 to 30,187 | 30,127 to 30,186 | rev. compl. | 53,277 to 53,336 | forward | - |
| Q9KX74 | putative methyltransferase | 30,579 to 31,241 | 30,578 to 31,240 | forward | 52,223 to 52,885 | rev. compl. | - |
| Q4W1I0 | putative DNA binding regulator | 31,789 to 32,661 | 31,788 to 32,660 | forward | 50,803 to 51,675 | rev. compl. | - |
| D1GU55 | putative membrane protein | 32,709 to 33,008 | 32,708 to 33,007 | forward | 50,456 to 50,755 | rev. compl. | - |
| D1GU56 | putative protein | 33,024 to 33,512 | 33,023 to 33,511 | forward | 49,952 to 50,440 | rev. compl. | - |
| Q93IA1 | putative membrane protein | 33,647 to 34,174 | 33,646 to 34,173 | forward | 49,290 to 49,817 | rev. compl. | - |
| Q9KX75 | putative protein | 34,753 to 35,105 | 34,752 to 35,104 | forward trnc. | 48,358 to 48,711 | rev. compl., trnc., frameshift | - |
| A9UFT0 | LPXTG protein homologue | 35,119 to 35,340 | 35,118 to 35,339 | forward | 48,123 to 48,344 | rev. compl. | - |
| D1GU60 | putative protein | 35,490 to 36,056 | 35,489 to 36,055 | forward | 47,407 to 47,973 | rev. compl. | - |
| hsdR-SCC | trnc. fragment of hsdR | 36,136 to 38,349 | 36,135 to 38,348 | rev. compl. trnc. | 45,114 to 47,327 | forward, trnc. | - |
| IR_IS431 | inverted repeat of IS431 | 38,352 to 38,367 | 38,351 to 38,366 | n/a | 45,096 to 45,111 | n/a | - |
| Q7A213 | putative protein | 38,352 to 38,379 | 38,351 to 38,378 | forward trnc. frameshift | 45,084 to 45,111 | rev. compl., trnc., frameshift | - |
| tnp_IS431 | transposase for IS431 | 38,411 to 39,085 | 38,410 to 39,084 | rev. compl. | 44,378 to 45,052 | forward | - |
| IR_IS431 | inverted repeat of IS431 | 39,116 to 39,149 | 39,115 to 39,148 | trnc. | 44,322 to 44,337 | trnc. | - |
| Teg143 | trans-encoded RNA associated with tnpIS431 | 39,126 to 39,141 | 39,125 to 39,140 | n/a | 44,314 to 44,347 | n/a | - |
| mvaS-SCC | trnc. 3-hydroxy-3-methylglutaryl CoA synthase | 39,158 to 39,510 | 39,157 to 39,509 | forward, frameshift | 43,953 to 44,305 | rev. compl., frameshift | - |
| Q5HJW6 | putative protein | 39,608 to 39,911 | 39,607 to 39,910 | forward trnc. | 43,552 to 43,855 | rev. compl., trnc. | - |
| dru | SCC direct repeat units | 39,748 to 40,305 | 39,747 to 40,304 | n/a | 43,158 to 43,715 | n/a | - |
| ugpQ | glycerophosphoryl diester phosphodiesterase | 40,507 to 41,250 | 40,506 to 41,249 | forward | 42,213 to 42,956 | rev. compl. | - |
| ydeM | putative dehydratase | 41,347 to 41,775 | 41,346 to 41,774 | forward | 41,688 to 42,116 | rev. compl. | - |
| mecA | penicillin-binding protein 2a | 41,821 to 43,827 | 41,820 to 43,826 | rev. compl. | 39,637 to 41,642 | forward | - |
| mecR1 | methicillin resistance operon repressor 1 | 43,927 to 45,684 | 43,926 to 45,683 | forward | 37,779 to 39,537 | rev. compl. | - |
| mecI | methicillin resistance regulatory protein | 45,684 to 46,055 | 45,683 to 46,054 | forward | 37,408 to 37,779 | rev. compl. | - |
| psmMEC | phenol-soluble modulin from SCCmec | 46,140 to 46,208 | 46,139 to 46,207 | rev. compl. | 37,255 to 37,323 | forward | - |
| mecR2 | methicillin resistance operon repressor 2 | 46,528 to 47,676 | 46,527 to 47,675 | forward | 35,786 to 36,935 | rev. compl. | - |
| cstB-SCC | CsoR-like sulphur transferase-regulated gene B | 47,790 to 49,124 | 47,789 to 49,123 | rev. compl. | 34,338 to 35,672 | forward | - |
| cstA-SCC | CsoR-like sulphur transferase-regulated gene A | 49,156 to 50,220 | 49,155 to 50,219 | rev. compl. | 33,242 to 34,306 | forward | - |
| cstR-SCC | copper-sensing transcriptional repressor | 50,356 to 50,616 | 50,355 to 50,615 | forward | 32,846 to 33,106 | rev. compl. | - |
| DUF81-SCC | putative sulfite/sulfonate efflux | 50,616 to 51,259 | 50,615 to 51,258 | forward | 32,203 to 32,846 | rev. compl. | - |
| cadD_R35 | cadmium transport protein D | 51,527 to 52,144 | 51,526 to 52,143 | rev. compl. frameshift | 31,318 to 31,935 | forward | - |
| cadA_Tn554 | cadmium efflux adenosine triphosphatase | 52,225 to 54,639 | 52,224 to 54,638 | rev. compl. | 28,823 to 31,237 | forward | - |
| cadC_Tn554 | putative regulator of cadmium efflux | 54,632 to 54,997 | 54,631 to 54,996 | rev. compl. | 28,465 to 28,830 | forward | - |
| tnpC_Tn554 | transposase C of transposon Tn554 | 55,183 to 55,561 | 55,182 to 55,559 | rev. compl. | 55,184 to 55,561 | rev. compl. | - |
| tnpB_Tn554 | transposase B of transposon Tn554 | 55,568 to 57,460 | 55,566 to 57,458 | rev. compl. | 55,568 to 57,460 | rev. compl. | - |
| A5INT3 | putative protein | 57,655 to 57,978 | 57,653 to 57,976 | rev. compl. | 57,655 to 57,978 | rev. compl. | - |
| D1GUI6 | putative protein | 57,971 to 58,177 | 57,969 to 58,175 | rev. compl. | 57,971 to 58,177 | rev. compl. | - |
| Q9KX75 | putative protein | 58,179 to 58,700 | 58,177 to 58,698 | rev. compl. | 58,179 to 58,700 | rev. compl. | - |
| Q0P7G0 | putative protein | 58,719 to 59,030 | 58,717 to 59,028 | rev. compl. | 58,719 to 59,030 | rev. compl. | - |
| Q93IE0 | putative protein | 59,115 to 59,465 | 59,113 to 59,463 | rev. compl. | 59,115 to 59,465 | rev. compl. | - |
| ccrB-3 | cassette chromosome recombinase B, type 3 | 59,935 to 61,562 | 59,933 to 61,560 | rev. compl. | 59,936 to 61,563 | rev. compl. | - |
| ccrA-3 | cassette chromosome recombinase A, type 3 | 61,583 to 62,929 | 61,581 to 62,927 | rev. compl. | 61,584 to 62,930 | rev. compl. | - |
| D1GUJ2 | putative protein | 63,122 to 63,397 | 63,120 to 63,395 | rev. compl. | 63,123 to 63,398 | rev. compl. | - |
| cch | cassette chromosome helicase | 63,487 to 65,274 | 63,485 to 65,272 | rev. compl. | 63,488 to 65,275 | rev. compl. | - |
| DUF1413 | putative protein associated with ccr | 65,274 to 65,561 | 65,272 to 65,559 | rev. compl. | 65,275 to 65,562 | rev. compl. | - |
| Q2FKL7 | putative protein | 65,695 to 66,744 | 65,693 to 66,742 | forward | 65,696 to 66,745 | forward | - |
| Q933A2 | putative ADP-ribosyltransferase | 66,797 to 67,369 | 66,795 to 67,367 | forward | 66,798 to 67,370 | forward | - |
| DR-SCC | direct repeat of SCC | 67,459 to 67,477 | 67,457 to 67,475 | n/a | 67,460 to 67,478 | n/a | - |
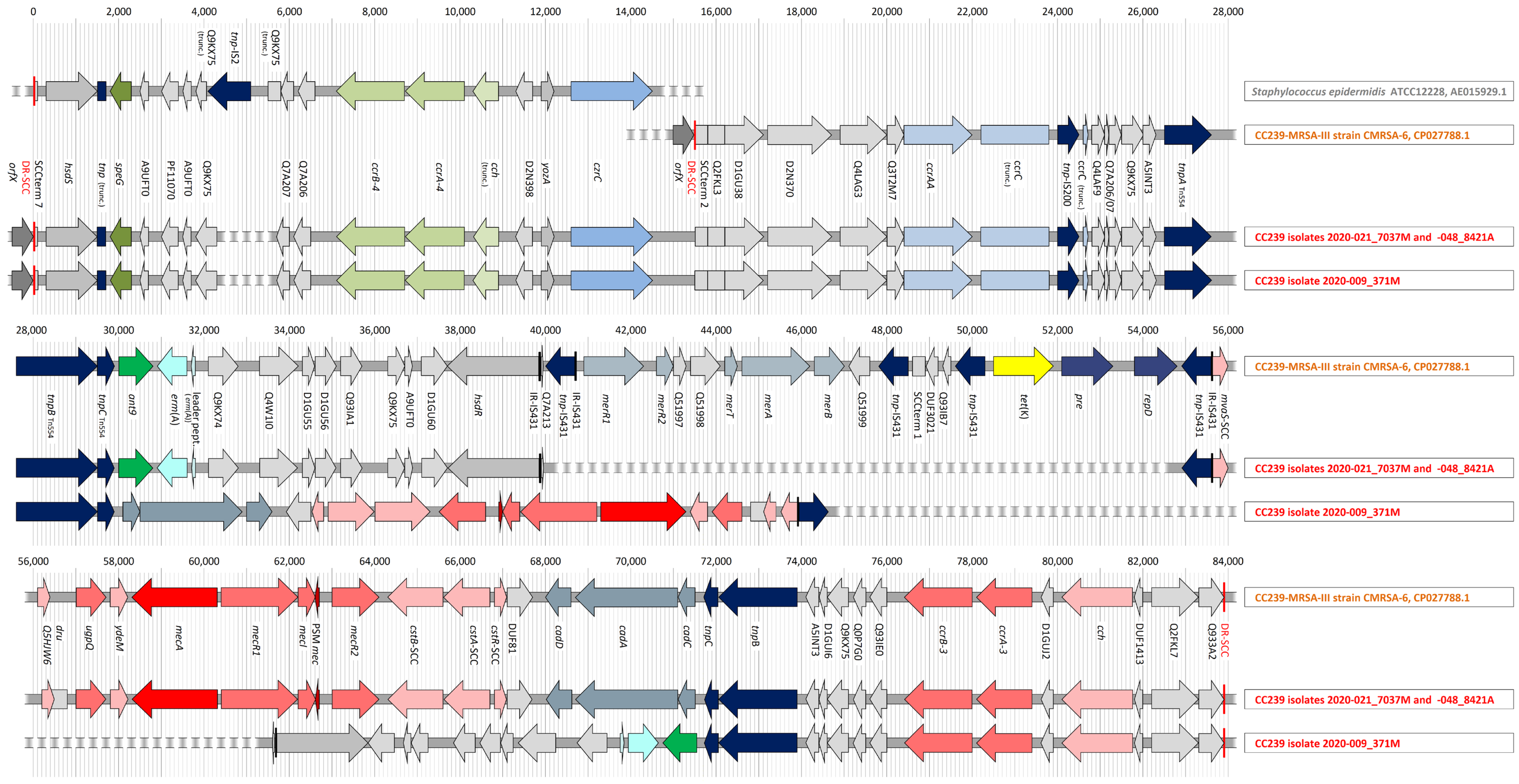
2.7. The sasX = sesI Gene in the Clonal Complex 239 Sequences
3. Discussion
4. Materials and Methods
4.1. Study Design and Eligibility
4.2. Laboratory Identification of Isolates
4.3. Microarray Analysis
4.4. Nanopore Sequencing
4.5. PVL Lateral Flow Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köck, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.E.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.L.; Struelens, M.J.; et al. Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in Europe. Eurosurveillance 2010, 15, 19688. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef]
- Mediavilla, J.R.; Chen, L.; Mathema, B.; Kreiswirth, B.N. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr. Opin. Microbiol. 2012, 15, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.G.; Hsu, L.-Y.; Kurt, K.; Weinert, L.A.; Mather, A.E.; Harris, S.R.; Strommenger, B.; Layer, F.; Witte, W.; de Lencastre, H.; et al. A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013, 23, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Community-associated MRSA: What makes them special? Int. J. Med. Microbiol. 2013, 303, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Medicine 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef]
- Ito, T.; Katayama, Y.; Asada, K.; Mori, N.; Tsutsumimoto, K.; Tiensasitorn, C.; Hiramatsu, K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1323–1336. [Google Scholar] [CrossRef]
- Ito, T.; Ma, X.X.; Takeuchi, F.; Okuma, K.; Yuzawa, H.; Hiramatsu, K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Chemother. 2004, 48, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, K.; Nonoguchi, R.; Matsuhashi, M.; Konno, M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J. Bacteriol. 1989, 171, 2882–2885. [Google Scholar] [CrossRef] [PubMed]
- Boyle-Vavra, S.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of Panton-Valentine leukocidin. Lab. Invest. 2007, 87, 3–9. [Google Scholar] [CrossRef]
- Buescher, E.S. Community-acquired methicillin-resistant Staphylococcus aureus in pediatrics. Curr. Opin. Pediatr. 2005, 17, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Bukharie, H.A. Increasing threat of community-acquired methicillin-resistant Staphylococcus aureus. Am. J. Med. Sci. 2010, 340, 378–381. [Google Scholar] [CrossRef]
- Coombs, G.W.; Monecke, S.; Pearson, J.C.; Tan, H.L.; Chew, Y.K.; Wilson, L.; Ehricht, R.; O’Brien, F.G.; Christiansen, K.J. Evolution and diversity of community-associated methicillin-resistant Staphylococcus aureus in a geographical region. BMC Microbiol. 2011, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Said-Salim, B.; Mathema, B.; Kreiswirth, B.N. Community-acquired methicillin-resistant Staphylococcus aureus: An emerging pathogen. Infect. Control Hosp. Epidemiol. 2003, 24, 451–455. [Google Scholar] [CrossRef]
- Vandenesch, F.; Naimi, T.; Enright, M.C.; Lina, G.; Nimmo, G.R.; Heffernan, H.; Liassine, N.; Bes, M.; Greenland, T.; Reverdy, M.E.; et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emergence. Emerg. Infect. Dis. 2003, 9, 978–984. [Google Scholar] [CrossRef]
- Alioua, M.A.; Labid, A.; Amoura, K.; Bertine, M.; Gacemi-Kirane, D.; Dekhil, M. Emergence of the European ST80 clone of community-associated methicillin-resistant Staphylococcus aureus as a cause of healthcare-associated infections in Eastern Algeria. Med. Mal. Infect. 2014, 44, 180–183. [Google Scholar] [CrossRef]
- Naas, T.; Fortineau, N.; Spicq, C.; Robert, J.; Jarlier, V.; Nordmann, P. Three-year survey of community-acquired methicillin-resistant Staphylococcus aureus producing Panton-Valentine leukocidin in a French university hospital. J. Hosp. Infect. 2005, 61, 321–329. [Google Scholar] [CrossRef]
- Takano, T.; Saito, K.; Teng, L.J.; Yamamoto, T. Spread of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) in hospitals in Taipei, Taiwan in 2005, and comparison of its drug resistance with previous hospital-acquired MRSA. Microbiol. Immunol. 2007, 51, 627–632. [Google Scholar] [CrossRef]
- Kourbatova, E.V.; Halvosa, J.S.; King, M.D.; Ray, S.M.; White, N.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am. J. Infect. Control 2005, 33, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Berglund, C.; Molling, P.; Sjoberg, L.; Soderquist, B. Predominance of staphylococcal cassette chromosome mec (SCCmec) type IV among methicillin-resistant Staphylococcus aureus (MRSA) in a Swedish county and presence of unknown SCCmec types with Panton-Valentine leukocidin genes. Clin. Microbiol. Infect. 2005, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Bonnstetter, K.K.; Wolter, D.J.; Tenover, F.C.; McDougal, L.K.; Goering, R.V. Rapid multiplex PCR assay for identification of USA300 community-associated methicillin-resistant Staphylococcus aureus isolates. J. Clin. Microbiol. 2007, 45, 141–146. [Google Scholar] [CrossRef]
- Ellington, M.J.; Perry, C.; Ganner, M.; Warner, M.; McCormick Smith, I.; Hill, R.L.; Shallcross, L.; Sabersheikh, S.; Holmes, A.; Cookson, B.D.; et al. Clinical and molecular epidemiology of ciprofloxacin-susceptible MRSA encoding PVL in England and Wales. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1113–1121. [Google Scholar] [CrossRef]
- Tristan, A.; Bes, M.; Meugnier, H.; Lina, G.; Bozdogan, B.; Courvalin, P.; Reverdy, M.E.; Enright, M.C.; Vandenesch, F.; Etienne, J. Global distribution of Panton-Valentine leukocidin--positive methicillin-resistant Staphylococcus aureus, 2006. Emerg. Infect. Dis. 2007, 13, 594–600. [Google Scholar] [CrossRef]
- Kaneko, J.; Kamio, Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 2004, 68, 981–1003. [Google Scholar] [CrossRef]
- Kaneko, J.; Kimura, T.; Kawakami, Y.; Tomita, T.; Kamio, Y. Panton-valentine leukocidin genes in a phage-like particle isolated from mitomycin C-treated Staphylococcus aureus V8 (ATCC 49775). Biosci. Biotechnol. Biochem. 1997, 61, 1960–1962. [Google Scholar] [CrossRef]
- Kaneko, J.; Kimura, T.; Narita, S.; Tomita, T.; Kamio, Y. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene 1998, 215, 57–67. [Google Scholar] [CrossRef]
- Zou, D.; Kaneko, J.; Narita, S.; Kamio, Y. Prophage, phiPV83-pro, carrying panton-valentine leukocidin genes, on the Staphylococcus aureus P83 chromosome: Comparative analysis of the genome structures of phiPV83-pro, phiPVL, phi11, and other phages. Biosci. Biotechnol. Biochem. 2000, 64, 2631–2643. [Google Scholar] [CrossRef]
- Chroboczek, T.; Boisset, S.; Rasigade, J.P.; Meugnier, H.; Akpaka, P.E.; Nicholson, A.; Nicolas, M.; Olive, C.; Bes, M.; Vandenesch, F.; et al. Major West Indies MRSA clones in human beings: Do they travel with their hosts? J. Travel. Med. 2013, 20, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, A.C.; Dumortier, C.; Hafer, C.; Taylor, B.S.; Sánchez, J.; Rodriguez-Taveras, C.; Leon, P.; Rojas, R.; Olive, C.; Lowy, F.D. Molecular characterization of Staphylococcus aureus from outpatients in the Caribbean reveals the presence of pandemic clones. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Moodley, A.; Williams, A.; Stegger, M.; Damborg, P.; Halliday-Simmonds, I.; Butaye, P. High Prevalence of USA300 Among Clinical Isolates of Methicillin-Resistant Staphylococcus aureus on St. Kitts and Nevis, West Indies. Front. Microbiol. 2019, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Hopman, J.; Peraza, G.T.; Espinosa, F.; Klaassen, C.H.; Velázquez, D.M.; Meis, J.F.; Voss, A. USA300 Methicillin-resistant Staphylococcus aureus in Cuba. Antimicrob. Resist. Infect. Control 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Leiva Peláez, O.; Stojanov, M.; Zayas Tamayo, A.M.; Barreras García, G.; González Aleman, M.; Martínez Ceballos, L.; Muñoz del Campo, J.L.; Bello Rodríguez, O.; Gonzalez Mesa, L.; Blanc, D.S. Molecular epidemiology of methicillin-resistant Staphylococcus aureus from 4 Cuban hospitals. Diagn. Microbiol. Infect. Dis. 2015, 81, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Baez, M.; Collaud, A.; Espinosa, I.; Perreten, V. MRSA USA300, USA300-LV and ST5-IV in pigs, Cuba. Int. J. Antimicrob. Agents 2017, 49, 259–261. [Google Scholar] [CrossRef]
- Gittens-St Hilaire, M.V.; Chase, E.; Alleyne, D. Prevalence, molecular characteristics and antimicrobial susceptibility patterns of MRSA in hospitalized and nonhospitalized patients in Barbados. New Microbes New Infect. 2020, 35, 100659. [Google Scholar] [CrossRef]
- Rosenthal, M.E.; Mediavilla, J.; Chen, L.; Sonnenfeld, J.; Pierce, L.; Shannon, A.; Boucher, H.; Pearlmutter, M.; Kreiswirth, B.; Kuo, Y.-H.; et al. Molecular epidemiology of Staphylococcus aureus in post-earthquake northern Haiti. Int. J. Infect. Dis. 2014, 29, 146–151. [Google Scholar] [CrossRef]
- Monecke, S.; Stieber, B.; Roberts, R.; Akpaka, P.E.; Slickers, P.; Ehricht, R. Population structure of Staphylococcus aureus from Trinidad & Tobago. PLoS ONE 2014, 9, e89120. [Google Scholar] [CrossRef]
- Akpaka, P.E.; Monecke, S.; Swanston, W.H.; Rao, A.C.; Schulz, R.; Levett, P.N. Methicillin sensitive Staphylococcus aureus producing Panton-Valentine leukocidin toxin in Trinidad & Tobago: A case report. J. Med. Case Rep. 2011, 5, 157. [Google Scholar] [CrossRef]
- Monecke, S.; Nitschke, H.; Slickers, P.; Ehricht, R.; Swanston, W.; Manjunath, M.; Roberts, R.; Akpaka, P.E. Molecular epidemiology and characterisation of MRSA isolates from Trinidad and Tobago. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1497–1500. [Google Scholar] [CrossRef]
- Akpaka, P.E.; Kissoon, S.; Rutherford, C.; Swanston, W.H.; Jayaratne, P. Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates from regional hospitals in Trinidad and Tobago. Int. J. Infect. Dis. 2007, 11, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Orrett, F.A.; Land, M. Methicillin-resistant Staphylococcus aureus prevalence: Current susceptibility patterns in Trinidad. BMC Infect. Dis. 2006, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Ngeno, C. Antimicrobial resistance in clinical isolates of Staphylococcus aureus from hospital and community sources in southern Jamaica. Int. J. Infect. Dis. 2007, 11, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.M.; Thorns, C.; Wint, H.; Didier, M.; Willis, R.; McMorris, N.; Castle, D.; Maharaj, N.; Orrett, F.A. The detection of mupirocin resistance and the distribution of methicillin-resistant Staphylococcus aureus at the University Hospital of the West Indies, Jamaica. West Indian Med. J. 2010, 59, 509–513. [Google Scholar]
- Swanston, W.H. Methicillin resistant Staphylococcus aureus. West Indian Med. J. 1999, 48, 20–22. [Google Scholar]
- Akpaka, P.E.; Kissoon, S.; Swanston, W.H.; Monteil, M. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolates from Trinidad & Tobago. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 16. [Google Scholar] [CrossRef]
- Bodonaik, N.C.; Nicholson, A. Methicillin resistance in Strains of Staphylococcus aureus at the University Hospital of the West Indies, Jamaica, 1980–1997. Int. Sci. Exc. 2002, 75. [Google Scholar]
- Monecke, S.; Jatzwauk, L.; Müller, E.; Nitschke, H.; Pfohl, K.; Slickers, P.; Reissig, A.; Ruppelt-Lorz, A.; Ehricht, R. Diversity of SCCmec elements in Staphylococcus aureus as observed in South-Eastern Germany. PLoS ONE 2016, 11, e0162654. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Hasman, H.; Stegger, M.; Andersen, P.S.; Skov, R.; Fluit, A.C.; Ito, T.; Aarestrup, F.M. Cloning and Occurrence of czrC, a Gene Conferring Cadmium and Zinc Resistance in Methicillin-Resistant Staphylococcus aureus CC398 Isolates. Antimicrob. Agents Chemother. 2010, 54, 3605–3608. [Google Scholar] [CrossRef]
- Li, M.; Du, X.; Villaruz, A.E.; Diep, B.A.; Wang, D.; Song, Y.; Tian, Y.; Hu, J.; Yu, F.; Lu, Y.; et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat. Med. 2012, 18, 816–819. [Google Scholar] [CrossRef]
- Holden, M.T.G.; Lindsay, J.A.; Corton, C.; Quail, M.A.; Cockfield, J.D.; Pathak, S.; Batra, R.; Parkhill, J.; Bentley, S.D.; Edgeworth, J.D. Genome Sequence of a Recently Emerged, Highly Transmissible, Multi-Antibiotic- and Antiseptic-Resistant Variant of Methicillin-Resistant Staphylococcus aureus, Sequence Type 239 (TW). J. Bacteriol. 2010, 192, 888–892. [Google Scholar] [CrossRef]
- Orrett, F.A. Methicillin resistance among Trinidadian isolates of community and hospital strains of Staphylococcus aureus and their patterns of resistance to non-beta-lactam antibiotics. Jpn. J. Infect. Dis. 1999, 52, 238–241. [Google Scholar]
- Akpaka, P.; Roberts, R.; Monecke, S. Molecular Analysis of Staphylococcus aureus Infections in Trinidad and Tobago. Br. Microbiol. Res. J. 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Ramdass, M.; Balliram, S.; Cadan, A.; Bhaggan, N.; Mohammed, B.; Singh, R.; Maharaj, J.; Boodram, A. Prevalence of methicillin-resistant Staphylococcus aureus in the surgical wards of the Port-of-Spain General Hospital, Trinidad and Tobago. West Indian Med. J. 2018, 67, 57–59. [Google Scholar] [CrossRef]
- Akpaka, P.E.; Roberts, R.; Monecke, S. Molecular characterization of antimicrobial resistance genes against Staphylococcus aureus isolates from Trinidad and Tobago. J. Infect. Public Health 2017, 10, 316–323. [Google Scholar] [CrossRef]
- Vire, F.P.; Akpaka, E.P.; Unakal, C. Prevalent virulent genes among methicillin resistant Staphylococcus aureus isolates from community settings in Trinidad and Tobago. Int. J. Infect. Dis. 2018, 73, 156–157. [Google Scholar] [CrossRef]
- Smyth, D.S.; McDougal, L.K.; Gran, F.W.; Manoharan, A.; Enright, M.C.; Song, J.H.; de Lencastre, H.; Robinson, D.A. Population structure of a hybrid clonal group of methicillin-resistant Staphylococcus aureus, ST239-MRSA-III. PLoS ONE 2010, 5, e8582. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Slickers, P.; Gawlik, D.; Müller, E.; Reissig, A.; Ruppelt-Lorz, A.; Akpaka, P.; Bandt, D.; Bes, M.; Boswihi, S.; et al. Molecular typing of ST239-MRSA-III from diverse geographic locations and the evolution of the SCCmec III element during its intercontinental spread. Front. Microbiol. 2018, 9, 1436. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.; Rossney, A.S.; Keane, C.T.; Enright, M.C.; Coleman, D.C. Seven novel variants of the staphylococcal chromosomal cassette mec in methicillin-resistant Staphylococcus aureus isolates from Ireland. Antimicrob. Agents Chemother. 2005, 49, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Larner-Svensson, H.; Worning, P.; Bartels, M.D.; Hestbjerg Hansen, L.; Boye, K.; Westh, H. Complete Genome Sequence of Staphylococcus aureus Strain M1, a Unique t024-ST8-IVa Danish Methicillin-Resistant S. aureus Clone. Genome Announc. 2013, 1, e00336-13. [Google Scholar] [CrossRef]
- Hau, S.J.; Bayles, D.O.; Alt, D.P.; Nicholson, T.L. Draft Genome Sequences of 14 Staphylococcus aureus Sequence Type 5 Isolates from California, USA. Genome Announc. 2017, 5, e00098-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Ren, S.X.; Li, H.L.; Wang, Y.X.; Fu, G.; Yang, J.; Qin, Z.Q.; Miao, Y.G.; Wang, W.Y.; Chen, R.S.; et al. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 2003, 49, 1577–1593. [Google Scholar] [CrossRef] [PubMed]
- Bowman, F.W. Test organisms for antibiotic microbial assays. Antibiot Chemother 1957, 7, 639–640. [Google Scholar]
- Hugh, R.; Ellis, M.A. The neotype strain for Staphylococcus epidermidis (Winslow and Winslow 1908) Evans 1916. Int. J. Syst. Evol. Microbiol. 1968, 18, 231–239. [Google Scholar] [CrossRef]
- Monecke, S.; Slickers, P.; Gawlik, D.; Müller, E.; Reissig, A.; Ruppelt-Lorz, A.; de Jäckel, S.C.; Feßler, A.T.; Frank, M.; Hotzel, H.; et al. Variability of SCCmec elements in livestock-associated CC398 MRSA. Vet. Microbiol. 2018, 217, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Hirose, M.; Ito, M.; Habadera, S.; Kobayashi, N. Clonal diversity of methicillin-resistant Staphylococcus aureus (MRSA) from bloodstream infections in northern Japan: Identification of spermidine N-acetyltransferase gene (speG) in staphylococcal cassette chromosomes (SCCs) associated with type II and IV SCCmec. J. Glob. Antimicrob. Resist. 2021, 24, 207–214. [Google Scholar] [CrossRef]
- Joshi, G.S.; Spontak, J.S.; Klapper, D.G.; Richardson, A.R. Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol. Microbiol. 2011, 82, 9–20. [Google Scholar] [CrossRef]
- Arias, C.A.; Rincon, S.; Chowdhury, S.; Martinez, E.; Coronell, W.; Reyes, J.; Nallapareddy, S.R.; Murray, B.E. MRSA USA300 clone and VREF--a U.S.-Colombian connection? N. Engl. J. Med. 2008, 359, 2177–2179. [Google Scholar] [CrossRef]
- Planet, P.J.; Diaz, L.; Rios, R.; Arias, C.A. Global Spread of the Community-Associated Methicillin-Resistant Staphylococcus aureus USA300 Latin American Variant. J. Infect. Dis. 2016, 214, 1609–1610. [Google Scholar] [CrossRef]
- Earls, M.R.; Coleman, D.C.; Brennan, G.I.; Fleming, T.; Monecke, S.; Slickers, P.; Ehricht, R.; Shore, A.C. Intra-Hospital, Inter-Hospital and Intercontinental Spread of ST78 MRSA From Two Neonatal Intensive Care Unit Outbreaks Established Using Whole-Genome Sequencing. Front. Microbiol. 2018, 9, 1485. [Google Scholar] [CrossRef]
- Roberts, M.C.; Joshi, P.R.; Greninger, A.L.; Melendez, D.; Paudel, S.; Acharya, M.; Bimali, N.K.; Koju, N.P.; No, D.; Chalise, M.; et al. The human clone ST22 SCCmec IV methicillin-resistant Staphylococcus aureus isolated from swine herds and wild primates in Nepal: Is man the common source? FEMS Microbiol. Ecol. 2018, 94, fiy052. [Google Scholar] [CrossRef]
- Roberts, M.C.; Joshi, P.R.; Monecke, S.; Ehricht, R.; Müller, E.; Gawlik, D.; Paudel, S.; Acharya, M.; Bhattarai, S.; Pokharel, S.; et al. MRSA Strains in Nepalese Rhesus Macaques (Macaca mulatta) and Their Environment. Front. Microbiol. 2019, 10, 2505. [Google Scholar] [CrossRef]
- Monecke, S.; Syed, M.A.; Khan, M.A.; Ahmed, S.; Tabassum, S.; Gawlik, D.; Müller, E.; Reissig, A.; Braun, S.D.; Ehricht, R. Genotyping of methicillin-resistant Staphylococcus aureus from sepsis patients in Pakistan and detection of antibodies against staphylococcal virulence factors. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 85–92. [Google Scholar] [CrossRef]
- Boswihi, S.S.; Verghese, T.; Udo, E.E. Diversity of clonal complex 22 methicillin-resistant Staphylococcus aureus isolates in Kuwait hospitals. Front. Microbiol. 2022, 13, 970924. [Google Scholar] [CrossRef]
- Senok, A.; Nassar, R.; Celiloglu, H.; Nabi, A.; Alfaresi, M.; Weber, S.; Rizvi, I.; Müller, E.; Reissig, A.; Gawlik, D.; et al. Genotyping of methicillin resistant Staphylococcus aureus from the United Arab Emirates. Sci. Rep. 2020, 10, 18551. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Müller, E.; Buechler, J.; Rejman, J.; Stieber, B.; Akpaka, P.E.; Bandt, D.; Burris, R.; Coombs, G.; Hidalgo-Arroyo, G.A.; et al. Rapid detection of Panton-Valentine leukocidin in Staphylococcus aureus cultures by use of a lateral flow assay based on monoclonal antibodies. J. Clin. Microbiol. 2013, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Breurec, S.; Fall, C.; Pouillot, R.; Boisier, P.; Brisse, S.; Diene-Sarr, F.; Djibo, S.; Etienne, J.; Fonkoua, M.C.; Perrier-Gros-Claude, J.D.; et al. Epidemiology of methicillin-susceptible Staphylococcus aureus lineages in five major African towns: High prevalence of Panton-Valentine leukocidin genes. Clin. Microbiol. Infect. 2011, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Egyir, B.; Guardabassi, L.; Esson, J.; Nielsen, S.S.; Newman, M.J.; Addo, K.K.; Larsen, A.R. Insights into nasal carriage of Staphylococcus aureus in an urban and a rural community in Ghana. PLoS ONE 2014, 9, e96119. [Google Scholar] [CrossRef] [PubMed]
- Egyir, B.; Guardabassi, L.; Sorum, M.; Nielsen, S.S.; Kolekang, A.; Frimpong, E.; Addo, K.K.; Newman, M.J.; Larsen, A.R. Molecular epidemiology and antimicrobial susceptibility of clinical Staphylococcus aureus from healthcare institutions in Ghana. PLoS ONE 2014, 9, e89716. [Google Scholar] [CrossRef]
- Okuda, K.V.; Toepfner, N.; Alabi, A.S.; Arnold, B.; Belard, S.; Falke, U.; Menschner, L.; Monecke, S.; Ruppelt-Lorz, A.; Berner, R. Molecular epidemiology of Staphylococcus aureus from Lambarene, Gabon. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1963–1973. [Google Scholar] [CrossRef]
- Shittu, A.O.; Oyedara, O.; Kenneth, O.O.; Raji, A.; Peters, G.; von Müller, L.; Schaumburg, F.; Herrmann, M.; Ruffing, U. An assessment on DNA microarray and sequence-based methods for the characterization of methicillin-susceptible Staphylococcus aureus from Nigeria. Front. Microbiol. 2015, 6, 1160. [Google Scholar] [CrossRef]
- Holt, D.C.; Holden, M.T.G.; Tong, S.Y.C.; Castillo-Ramirez, S.; Clarke, L.; Quail, M.A.; Currie, B.J.; Parkhill, J.; Bentley, S.D.; Feil, E.J.; et al. A very early-branching Staphylococcus aureus lineage lacking the carotenoid pigment staphyloxanthin. Genome Biol. Evol. 2011, 3, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lee, H.; Wang, X.M.; Lee, T.F.; Liao, C.H.; Teng, L.J.; Hsueh, P.R. High mortality impact of Staphylococcus argenteus on patients with community-onset staphylococcal bacteraemia. Int. J. Antimicrob. Agents 2018, 52, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Rautelin, H.; Kaden, R. Staphylococcus argenteus and Staphylococcus schweitzeri are cytotoxic to human cells in vitro due to high expression of alpha-hemolysin Hla. Virulence 2019, 10, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Schaumburg, F.; Kearns, A.; Larsen, A.R.; Lindsay, J.A.; Skov, R.L.; Westh, H. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: A position paper of members of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS). Clin. Microbiol. Infect. 2019, 25, 1064–1070. [Google Scholar] [CrossRef]
- Dupieux, C.; Blondé, R.; Bouchiat, C.; Meugnier, H.; Bes, M.; Laurent, S.; Vandenesch, F.; Laurent, F.; Tristan, A. Community-acquired infections due to Staphylococcus argenteus lineage isolates harbouring the Panton-Valentine leucocidin, France, 2014. Eurosurveillance 2015, 20, 21154. [Google Scholar] [CrossRef]
- McDonald, M.; Dougall, A.; Holt, D.; Huygens, F.; Oppedisano, F.; Giffard, P.M.; Inman-Bamber, J.; Stephens, A.J.; Towers, R.; Carapetis, J.R.; et al. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote aboriginal communities. J. Clin. Microbiol. 2006, 44, 3720–3727. [Google Scholar] [CrossRef]
- Tång Hallbäck, E.; Karami, N.; Adlerberth, I.; Cardew, S.; Ohlén, M.; Engström Jakobsson, H.; Svensson Stadler, L. Methicillin-resistant Staphylococcus argenteus misidentified as methicillin-resistant Staphylococcus aureus emerging in western Sweden. J. Med. Microbiol. 2018, 67, 968–971. [Google Scholar] [CrossRef]
- Monecke, S.; Slickers, P.; Ehricht, R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol. Med. Microbiol. 2008, 53, 237–251. [Google Scholar] [CrossRef]
- Senok, A.; Monecke, S.; Nassar, R.; Celiloglu, H.; Thyagarajan, S.; Müller, E.; Ehricht, R. Lateral Flow Immunoassay for the Detection of Panton-Valentine Leukocidin in Staphylococcus aureus from skin and soft tissue infections in the United Arab Emirates. Front. Microbiol. 2021, 939. [Google Scholar] [CrossRef] [PubMed]

| Strain | N (%) | W/Swab | Blood | Pus | Urine | Other |
|---|---|---|---|---|---|---|
| MSSA | 73 (86) | 41 | 9 | 7 | 8 | 8 |
| MRSA | 10 (12) | 6 | 2 | 0 | 0 | 2 |
| S. argenteus | 2 (2) | 1 | 1 | 0 | 0 | 0 |
| Total | 85 (100) | 48 (57) | 12 (14) | 7 (8) | 8 (9) | 10 (12) |
| Strain | N (%) | W/Swab | Throat | Blood |
|---|---|---|---|---|
| MSSA | 16 (100) | 13 | 1 | 2 |
| MRSA | 0 (0) | 0 | 0 | 0 |
| Total | 16 (100) | 13 (81) | 1 (6) | 2 (13) |
| Age Group (Years) | N (%) | MRSA | MSSA | S. argenteus |
|---|---|---|---|---|
| 0–9 | 15 (18) | 2 | 12 | 1 |
| 10–19 | 15 (18) | 1 | 14 | 0 |
| 20–29 | 4 (5) | 1 | 3 | 0 |
| 30–39 | 19 (22) | 1 | 18 | 0 |
| 40–49 | 9 (11) | 1 | 7 | 1 |
| 50–59 | 10 (12) | 3 | 7 | 0 |
| 60–69 | 7 (8) | 1 | 6 | 0 |
| 70–79 | 2 (2) | 0 | 2 | 0 |
| 80+ | 3 (3) | 0 | 3 | 0 |
| Other/unrecorded | 1 (1) | 0 | 1 | 0 |
| Total | 85 (100) | 10 (12) | 73 (86) | 2 (2) |
| Age Group (Years) | N (%) | MRSA | MSSA | S. argenteus |
|---|---|---|---|---|
| Male | 46 (54) | 4 | 41 | 1 |
| Female | 37 (44) | 6 | 30 | 1 |
| Not recorded | 2 (2) | 0 | 2 | 0 |
| Total | 85 (100) | 10 (12) | 73 (86) | 2 (2) |
| CC | Strain | SCCmec Type | N (%) for Trinidad and Tobago | N (%) for Jamaica |
|---|---|---|---|---|
| CC1 | CC1–MSSA | - | 1 (1.2%) | 4 (25%) |
| CC5 | CC5–MSSA | - | 1 (1.2%) | - |
| CC5–MSSA (PVL+) | - | 2 (2.4%) | - | |
| CC6 | CC6–MSSA | - | 4 (4.7%) | 1 (6.3%) |
| CC6–MSSA (PVL+) | - | 4 (4.7%) | 2 (12.5%) | |
| CC7 | CC7–MSSA | - | 5 (5.9%) | - |
| CC8 | CC8–MSSA | - | 2 (2.4%) | - |
| CC8–MSSA (PVL+) | - | 12 (14.1%) | - | |
| CC8–MSSA (ACME/PVL+) | ACME-I + speG + adhC + copA2 | 1 (1.2%) | - | |
| CC9 | CC9–MSSA | - | 1 (1.2%) | - |
| CC12 | CC12–MSSA | - | 1 (1.2%) | - |
| CC15 | CC15–MSSA | - | 3 (3.5%) | - |
| CC22 | CC22–MRSA–IV (PVL+/tst+) | SCCmec IVa | 1 (1.2%) | - |
| CC30 | CC30–MSSA | - | 1 (1.2%) | - |
| CC45 | CC45–MSSA | - | 2 (2.4%) | 2 (12.5%) |
| CC59 | CC59–MSSA | - | 1 (1.2%) | - |
| CC72 | ST72–MSSA | - | 2 (2.4%) | 2 (12.5%) |
| ST72–MRSA–[mecVT + fus] | SCCmec VT + fus | 1 (1.2%) | - | |
| CC88 | CC88–MRSA IV | SCCmec IVa | 1 (1.2%) | - |
| CC97 | CC97–MSSA | - | 9 (10.6%) | 1 (6.3%) |
| CC101 | CC101–MSSA | - | 1 (1.2%) | 1 (6.3%) |
| CC121 | CC121–MSSA | - | 1 (1.2%) | - |
| CC152 | CC152–MSSA | - | 1 (1.2%) | - |
| CC152–MSSA (PVL+) | - | 13 (15.3%) | 2 (12.5%) | |
| CC188 | CC188–MSSA | - | - | 1 (6.3%) |
| CC239 | CC239–MRSA–III, atypical “Southeast Asian Clade” * | Composite SCCmec III * | 5 (5.9%) | - |
| CC239–MRSA–[mec III + Cd/Hg + ccrC], “Southeast Asian Clade” | SCC [mec III + Cd/Hg + ccrC] (Bmb9393) | 2 (2.4%) | - | |
| CC398 | CC398–MSSA | - | 5 (5.9%) | - |
| S. argenteus CC2596 | CC2596–MSSarg | - | 2 (2.4%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monecke, S.; Akpaka, P.E.; Smith, M.R.; Unakal, C.G.; Thoms Rodriguez, C.-A.; Ashraph, K.; Müller, E.; Braun, S.D.; Diezel, C.; Reinicke, M.; et al. Clonal Complexes Distribution of Staphylococcus aureus Isolates from Clinical Samples from the Caribbean Islands. Antibiotics 2023, 12, 1050. https://doi.org/10.3390/antibiotics12061050
Monecke S, Akpaka PE, Smith MR, Unakal CG, Thoms Rodriguez C-A, Ashraph K, Müller E, Braun SD, Diezel C, Reinicke M, et al. Clonal Complexes Distribution of Staphylococcus aureus Isolates from Clinical Samples from the Caribbean Islands. Antibiotics. 2023; 12(6):1050. https://doi.org/10.3390/antibiotics12061050
Chicago/Turabian StyleMonecke, Stefan, Patrick Eberechi Akpaka, Margaret R. Smith, Chandrashekhar G. Unakal, Camille-Ann Thoms Rodriguez, Khalil Ashraph, Elke Müller, Sascha D. Braun, Celia Diezel, Martin Reinicke, and et al. 2023. "Clonal Complexes Distribution of Staphylococcus aureus Isolates from Clinical Samples from the Caribbean Islands" Antibiotics 12, no. 6: 1050. https://doi.org/10.3390/antibiotics12061050
APA StyleMonecke, S., Akpaka, P. E., Smith, M. R., Unakal, C. G., Thoms Rodriguez, C.-A., Ashraph, K., Müller, E., Braun, S. D., Diezel, C., Reinicke, M., & Ehricht, R. (2023). Clonal Complexes Distribution of Staphylococcus aureus Isolates from Clinical Samples from the Caribbean Islands. Antibiotics, 12(6), 1050. https://doi.org/10.3390/antibiotics12061050






